Greater Coucal
Table of Contents
 |
| Figure 1. Photo of C. sinensis. Source: Sumit Sengupta (Permission Pending) |
1. Overview
Centropus sinensis (Stephen 1815), also commonly known as Greater Coucal or Crow Pheasant, is a large crow-like bird up to 50cm in length including the tail[1]. It belongs to the Cuculidae family but is not a brood parasite, raising its chicks on their own. They are non-migratory, residents found in Asia, inhibiting a range of habitats[2][3] and can be found in different parts of Singapore[4], from jungle and plantation to urban garden[5] though it may be hard to spot due to its shy and retiring nature[6].Brood ParasiteBrood Parasite refers to a species that depends on another species to look after its young. It is an interaction between two different species, where the parasitic species will lay its egg in the nest of its host. The host species will then, unknowingly, look after the young of the parasitic species. As this will lowers the reproductive success of the host species, the host species will develop mechanism to deter parasitic species[44].
2. Description
2.1 Adults
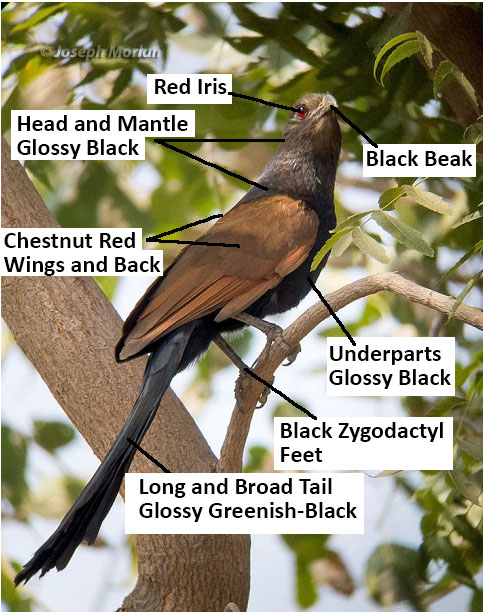 |
| Figure 2. Photo and Description of C. sinensis Adult. (Photo © JosephMorlan 2016, used by permission) edited by Lau Jun Jie. |
Both the male and female are similar in appearances and they can grow up to 45-50 cm in length, including the tail[10]. They have black, glossed plumage on the head, mantle and underparts. The gloss may varies from glossy blue or purple black to dull sooty black. Wings and back are chestnut red in color while under wing coverts are black in color. Their tails are long and broad; and are glossy greenish-black in color. Feet and beaks are black in color while their iris varies from red to brown. There is no seasonal variation in plumage[11][1]. Like all cuckoos,Greater Coucal has zygodactyl feet[12]. To know more about the body structure or topology of bird, click here.
Zygodactyl Feet
 |
| Figure 3. Different type of Bird feet.Taken from Wikimedia Commons, by Cburnett, C. M. L. licensed under Creative Common. |
2.2 Juvenile
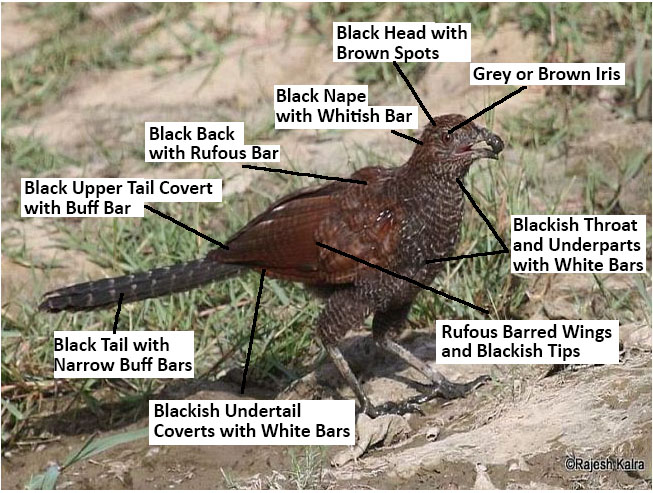 |
| Figure 4. Photo and Description of C. sinensis Juvenile. Source: Rajesh Kalra, 2015 (Permission Granted) and edited by Lau Jun Jie |
Juveniles have dull black head with brown spots. Nape is black with whitish bar while back is black with with rufous bars. Upper tail coverts are black with buff bars while tail is black with narrow buff bars. They have wings where the coverts, inner secondaries and tips of outer flight feather that are rufous barred and blackish tips. The underparts, from throat to undertail coverts are blackish barred dull white. The iris varies from grey to brown[11].
2.3 Nestlings
 |
| Figure 5. Photo and Description of C. sinensis Nestling. Source: Devendra Bhardwaj, 2014 (Permission Granted) and edited by Lau Jun jie |
Upon hatching, nestling has black skin and long white hair-like down, called trichoptiles[13]. Center of its belly is pinkish while the upper mantle is black with pink edges and egg tooth. Their gape is yellow in color while the iris is brown and feet are brownish[11].
Horrifying fact: Coucal OilIn Indo-Malayan region, Greater Coucal chicks are also used to make an emulsion called Crow Pheasant Oil or Minyak buring butbut,
which is believed to help treat urns, sprains, fracture and broken legs[42]. However, it is important to note that there are no scientific basis for these 'medicine'
and should not buy them as harm are caused to the chicks to produce these oil.
3. Diagnosis
3.1 Greater Coucal versus Lesser Coucal
 |
| Figure 6. Photo and Description of C. bengalensis. Source: Daisy O'Neill, 2015 (Permission Granted) and edited by Lau Jun Jie |
Centropus bengalensis, or commonly known as Lesser Coucal, is similar in appearance to Greater Coucal. However, Lesser Coucal is smaller in size compared to Greater Coucal. Lesser Coucal also has tiny white streaks in its duller plumage[6]. Lesser Coucal also has glossy shafts on the head feathers, making it scruff looking compared to
Greater Coucal[11]. Greater Coucal can also be distinguished from their vocalization and habitats. Both Greater Coucal and Lesser Coucal have similar calls but Lesser Coucal's calls is more rapidly and at a higher pitch than Greater Coucal[19]. While the range of Lesser Coucal and Greater Coucal overlap, Lesser Coucal is often found in grassy habitats and not in forest[11].
Call of Lesser Coucal
Source: Filip Verbelen, 2009 [14] (Under Creative Commons License)
3.2 Subspecies of Greater Coucal
There are six known subspecies of Greater Coucal and differ slightly in their appearances.3.2.1 Centropus sinensis sinensis (Stephen, 1815)
The appearance is as described above under section 3 Description and is the nominate form[11] .3.2.2 Centropus sinensis parroti Stresemann, 1913
The upper back of its body is black in color[11] while underparts are glossed blue. Forehead, face and throat are also more brownish. The wings of juvenile are not barred and wings have a darker, chestnut color[2] .3.2.3 Centropus sinensis intermedius Hume, 1873
It is smaller in size compared to other subspecies[11] .3.2.4 Centropus sinensis bubutus Horsfield, 1821
It has wings that are of paler rufous in color and is larger in size compared to other subspecies[11] .3.2.5 Centropus sinensis anonymus Stresemann, 1913
It has shorter wings which are of darker brown compared to C. sinensis bubutus [11] .3.2.6 Centropus sinensis kangeangensis (Vordeman, 1893) [2]
They have two different plumage phase.1. Their plumage are paler with head and body buffy white in color. The wings are brown in color while the tail is whitish above and rufous below.
2. Plumage is darker in color with head and back brown in color. The wings are brownish purple with darker brown tips. Tail is bronzy purple in color and the iris varies from red to yellow [11] .
4. Distribution Range and Habitats
Greater Coucal has an extremely large range, and can be found throughout Southern Asia, from India East to South China and Indonesia [16]. However, different subspecies are found in different places, though there may be overlap in range, as seen in the table 1 below.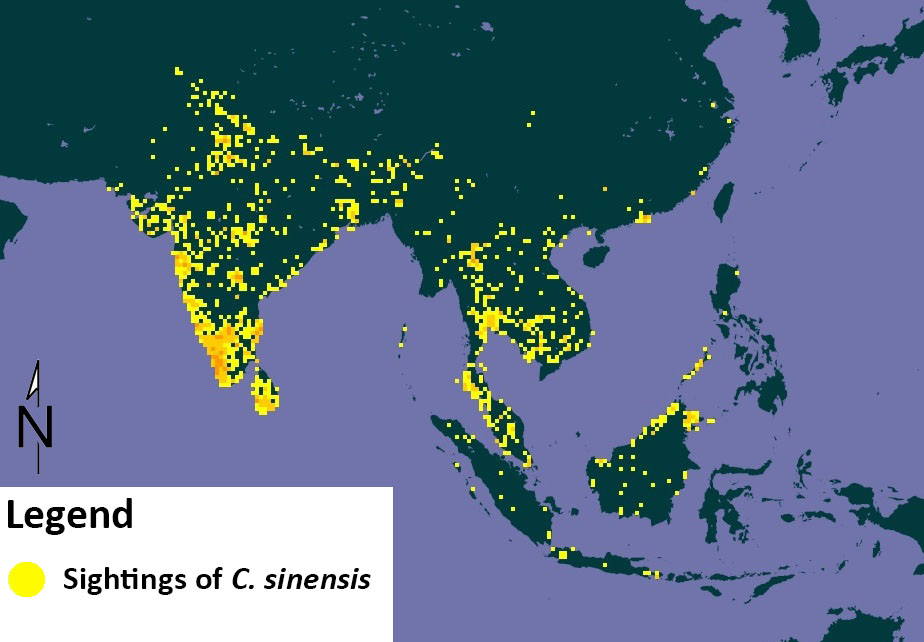 |
| Figure 7. Distribution of C. sinensis. Source: Global Biodiversity Information Facility[17] and edited by Lau Jun Jie |
| Subspecies |
Location |
| Centropus sinensis sinensis (Stephen, 1815) |
Pakistan to North India and South China |
| Centropus sinensis parroti Stresemann, 1913 |
South Peninsular India and Sri Lanka |
| Centropus sinensis intermedius Hume, 1873 |
Bangladesh to Myanmar, South Thailand, Indochina and northern Malay Peninsular |
| Centropus sinensis bubutus Horsfield, 1821 |
Southern Malay Peninsular, Sumatra, Nias, Mentawai Islands, Java, Bali, Borneo and western Philippines |
| Centropus sinensis anonymus Stresemann, 1913 |
Southwestern Philippines |
| Centropus sinensis kangeangensis (Vordeman, 1893) |
Kangean Island |
Greater Coucal are able to inhabit large range of habitats. They can be found in found in plantations[18] , forest[19] , secondary scrub, mangrove, river banks[1] , marshes, reed beds[15] , bamboo, paddyfields, garden, streams, lakes[11] , grassland, shrubbery and near human habitation[16].
4.1 Greater Coucal in Singapore
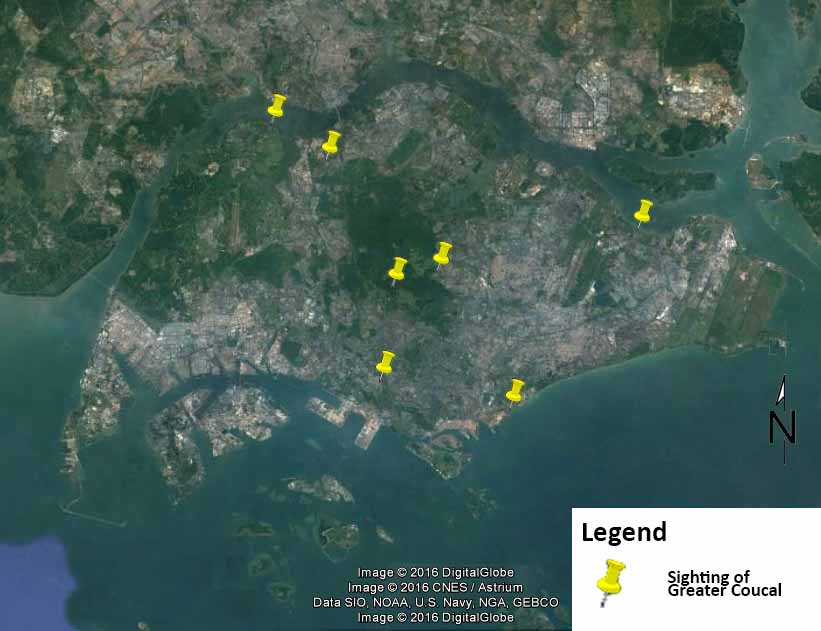 |
| Figure 8. Sightings of Greater Coucals in Singapore. Source: Google Earth (Edited by Lau Jun Jie) |
Greater Coucals have been spotted around Singapore, including, but not limited to, Central Catchment, Pasir Ris, Sungei Buloh, Venus Dr[47], Kranji, Portsdown[48] and Marina South (per. obs.). While sightings of the bird is rather uncommon, they are not listed as an endangered species in Singapore Red Data Book[49].
5. Ecology and Behaviour
5.1 Biology
5.1.1 Sexual Size Dimorphism
There is sexual size dimorphism in Greater Coucals where the female is larger than the male. The difference in size may be due to a few reasons.1. As female Greater Coucal puts on attractive behavior to attract mate, having a larger body size may be advantageous as it favors sexual competition.
2. Larger body size may be advantageous for female in egg laying ability. Larger body size may better allows resource accumulation as resource storage capability increases more rapidly than metabolic cost with increases in body size.
3. Similar to bird of preys, as male Greater Coucal gathers food for offspring, having a smaller body size is more economical in terms of foraging and transport energetics[23].
5.1.2 Single Atrophied Testis in Male
Testes in male Coucals are asymmetrical in size where the right testis is always larger than the left testis which is atrophied. The left testis is never firm or oval, or increase in size during the breeding season even when the right testis is at its maximum size[20] . It was originally thought that Coucals having a single functional testis is attributed to paternal care, as one functional testis would produce less testosterone, promoting male parental care[50]. However, research shows that non-parasitic cuckoos do not have smaller testes than parasitic cuckoos. In fact, the total testis mass for non-parasitic cuckoos is much larger than parasitic cuckoos where the enlarged right testes more than compensate for the atrophied left testis[20]. While it is not unusual for birds to have asymmetrical size testes, the left testis is usually the larger of the two, in contrast to Coucals where the right testis is bigger than the left testis[22].5.1.3 Nictitating Membrane
 |
| Figure 9. Photo of Nictitating Membrane in C. sinensis (right) and without (left). Source: Jaysukh Parekh “Suman”, 2012 (Permission Pending) and edited by Lau Jun Jie |
5.2 Reproduction
5.2.1 Courtship
 |
| Figure 10. Photo of C. sinensis Mating. Source: Amar-Singh HSS, 2014 (Permission Granted) |
Video 2: Video of a pair of Greater Coucal Mating
Source: Mate, 2014 (Permission Pending)
Larger female tends to compete for mates, by perching on a prominent spots and showing advertisement behavior[2]. As male Greater Coucal are more involved in nesting duties, this allows the female to have greater opportunities to compete for better quality mate[2]. Both male and female may put up courtship display which involves the male bringing a food present and hopping after the female. The female may then run for a short distance on the ground, with wings partly open and vibrating. If the female accepts the male, she will lowers her tail and droops her wings to signal her willingness to copulate[24] . The female may also emit a harsh cry. Both the male and female will then fly back up to the tree, hopping from branch to branch. The male will then mounts the female and copulate. The male may feed the female during copulation[25] .
5.2.2 Breeding
Greater Coucals, unlike most other cuckoos, are non-parasitic and build their own nests and care for their nestlings. They form monogamous pair for at least a season, with the male providing most of the parental care. Greater Coucals are monsoon breeders, where breeding season may lasts from June to September, though it may varies in different range[11] . The nests, mostly built by the male, are usually domed-shaped and made of leaves, twigs and other materials[24] . Territory range varies, from 0.9 to 7.2 hectare[23] . The clutch size is large (6-7 eggs) although egg laying is irregular, with intervals of 2-3 days between eggs. Egg incubations, carried out by the male, starts from the first egg and lasts over a long period of time. Incubation period is 14-18 days[24] . Eggs are ovaloid in shape and chalky white in color, turning yellowish overtime during incubation[24] .5.2.3 Nestling
Video 1: Video of Greater Coucal Nestlings
Source: IsaanHippo, 2011 (Permission Granted)
Newly hatched nestlings are black in color with eyes closed and covered with trichoptiles[24] . Chicks are altricial and are able to leave the nest after 14-18 days. However, they may still continue to receive parental care. When threatened, nestlings may also exhibit anti-predatory strategies. This includes escaping from the nest via a rear exit, excreting a foul smelling stick fluid or produce a hissing sound similar to a snake[2].
Altricial vs PrecocialAltricial chicks refer to chicks that remain in the nest for most or the entire period of their development. They are usually hatched with closed eyes and incapable of most activity other than begging. In contrast, precocial chicks are independent of their parents, some capable of flight from the first day of their postnatal life [46].
5.3 Diet
Greater Coucals are opportunistic predators and feeds on a large variety of food.They feed on a range of insects like caterpillars, grasshoppers and stick-insects as well as other invertebrates like slugs, snails, spiders and millipedes. They are also able to feed on small vertebrates like snakes[15] , young mice[2] , bird nestlings[26] as well as bird eggs[27][5][2][28], fruits and seeds[11]. Greater Coucals employ a variety of techniques to hunt for food. This includes probing holes in tree trunks, gliding or jumping to catch insects; and chasing and flying after lizard, snakes and insects on the ground[11] .5.4 Vocalization
Call of Greater Coucal
Source: Deepal Warakagoda, 2007[29] (Under Creative Commons License)
Greater Coucals have very distinctive calls describe as deep, low-pitched 'coop-coop-coop'[15][2] . Male and female Greater Coucals often calls in duet, where their calls overlap in time, with the female at a lower pitch than the male[11] . Other calls include hissing threatening call by the juvenile, an alarm or threaten call 'k'wisss' by the adult, a 'tch-truu' call by the female and juvenile when they spot male with food[11] .
Fun Fact: Superstition and BeliefsGreater Coucals are associated with many superstitious beliefs. It is believed that sighting of Greater Coucal will bring good luck,
especially for people who are embarking on a journey[40] . The deep and booming calls are also associated with spirits and omens[41].
5.5 Habits
 |
| Figure 11. Photo of C. sinensis Sunbathing. Source: Rajesh Kalra , 2013 (Permission Granted) |
Greater Coucals are most active in the morning and late afternoon. They may sunbathe, as a pair or singly, in the morning or after rain with their wings spread out [5]. Greater Coucal is a shy and secretive bird and tends to stay under cover in dense vegetation[6] . It hunts for prey on the ground by skulling in, through and out of dense undergrowth[15][11]. C sinensis may walk,stalk and run in pursuit for its prey before flushing them by flashing its wings forward[11] .
Flight of Greater Coucal is slow and weak where they alternate between flapping and gliding with heavy wing beats.They tend to fly at low elevation. To fly over greater distances, Greater Coucal may jump from branches to branches to get to the canopy before taking off from the treetop. Their roosts are commonly found in reedbeds[11][15].
6. Ecolgical Roles
Greater Coucals may play an important role in their environment. Firstly, as they feed on fruits, they may help to disperse seeds over large distance due to their rather sizeable range (0.9 to 7.2 hectare). Secondly, as feed on a large variety of food, they serves as natural predator to a large number of pests including caterpillars and snails. Lastly, they aid in nutrient cycling by disposing their organic waste as they move from places to places. When they die, their bodies are also broken down by decomposers into humus[38] .7. Conservation Status
 |
| Figure 12. C. sinensis Conservation Status. Source: IUCN, n.d.[39] |
8. Nomenclature
Binomial name: Centropus sinensisVernacular name: C. sinensis has been given numerous vernacular name which includes but not limited to:
| Language |
Name |
Pronounciation |
| English |
Greater Coucal, Crow-Pheasant |
|
| Japanese |
オオバンケン |
ō ban-ken |
| Malay |
Bubut Besar |
|
| Chinese |
大红毛鸡 |
da hong mao ji |
| Thai |
นกกระปูดใหญ่ |
nók kà-pùut yày |
One reason for the large number of vernacular names given to Greater Coucals may be due to their large distribution range throughout Asia, where different cultures have different names for Greater Coucals. Hereafter, the common name Greater Coucal will be use to refer to the species.
8.1 Sinensis: Chinese Coucal?
The species epithet, sinensis, is derived from Ancient Greek Σῖναι (Sînai), which in turn may be derived from Sanskrit चीन (Cina), Arabic اَلصِّين (aṣ-ṣīn) and Old Chinese 秦 (Dzin). However, they all refer to China or Chinese[8]. The species epithet sinensis may have been coined by James Francis Stephens back in 1815 as the first specimen described was collected in China, Ning Po[9].9. Taxonomy and Systematic
9.1 Taxonomic Classification
| Animalia |
| Chordata |
| Aves |
| Cuculiformes |
| Cuculidae |
| Centropodidae |
| Centropus Illiger, 1811 |
| Centropus sinensis (Stephen,1815) |
Classification of Greater Coucal is listed in Table 3 above, with the most inclusive taxon at the top, and least inclusive at the bottom. Taxonomic ranks have been omitted as they do not represent meaningful taxonomic information.
9.2 Original description and type
| Figure 13. Original Description of Centropus Sinensis [8] |
No type information for Greater Coucals was found online, although syntype specimen of the subspecies C. sinensis kangeanensis Voderman, 1893 can be found at Naturalis Biodiversity Centre [30] .
9.3 Phylogeny
Greater Coucal belongs to the order of cuckoos, Cuculiforme, and the family, Cuculidae[12] . While Cuckoos are known to form a monophyletic group through molecular data[11] , higher level relationship such as inter-ordinal relationship are not clear[11][12]. However, researchs have been carried out to try to resolve the phylogenetic relationship of birds. We will look at some of these studies to understand the relationship of rds.Centropus in relation to other birds.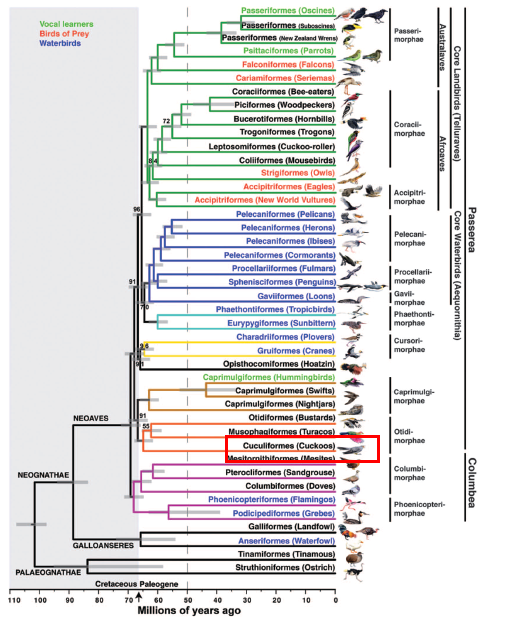 |
| Figure 14. Genome-scale Phylogeny of BirdsSource: Jarvis et al., 2014[31] and edited by Lau Jun Jie |
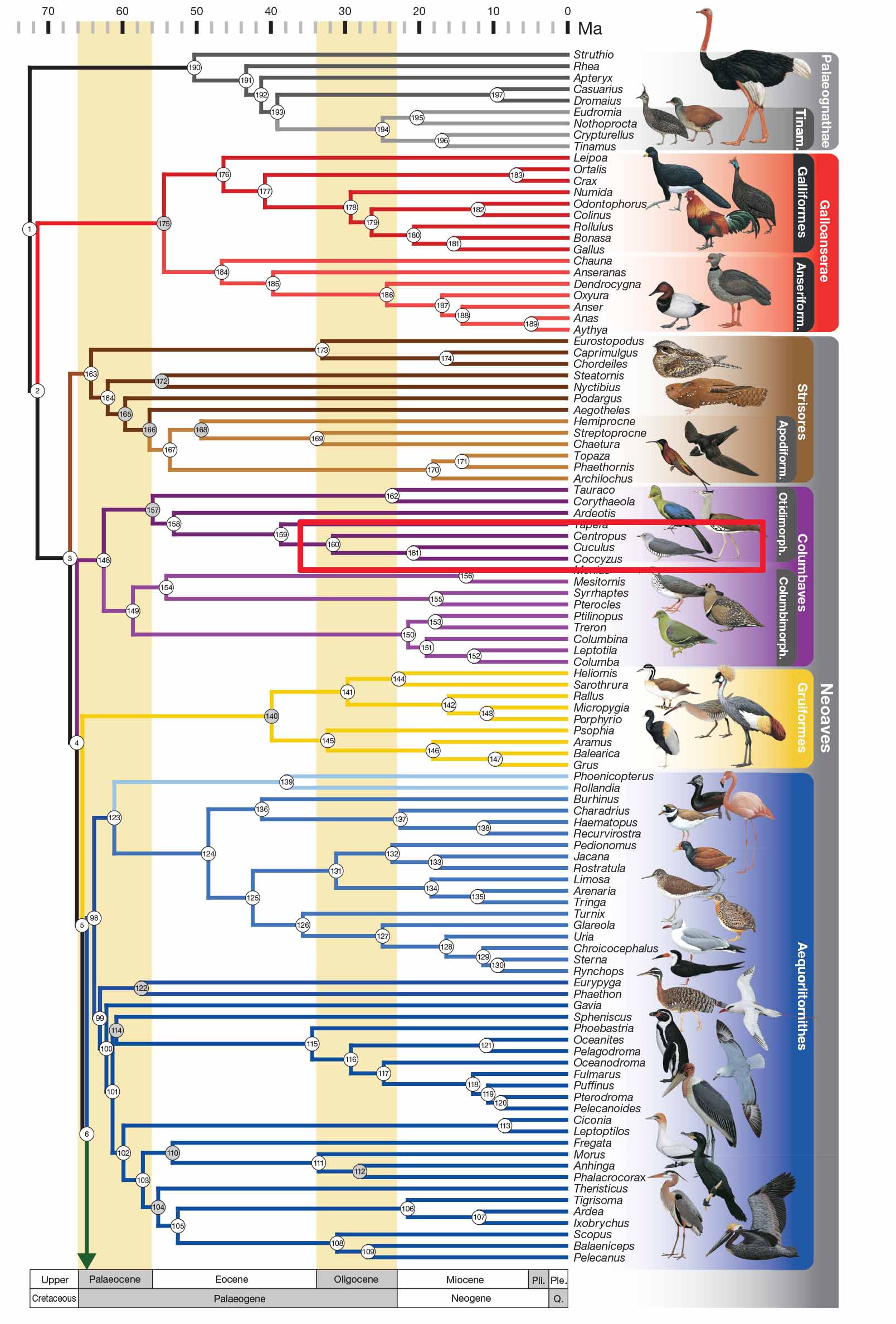 |
| Figure 15. Phylogenetic Tree of Birds by Prum et al. (2015) [52] edited by Lau Jun Jie |
Within Cuculifores, Cuculidae is the only taxon, which in turn contains Cuculinae (Old World Cuckoos), Coccyzinae (New World Cuckoos) and Centropodidae. A study was conducted by Prum et al. (2015) where DNA sequences of 198 species of birds were used for phylogenetic analysis. Both Bayesian and Maximum Likelihood Analysis were carried out, both methods resulting in very identical plylogenetic trees for all major avian lineages [52]. Figure 14 is based on Bayesian Analysis where all nodes are supported with posterior probabilities (PP) of 1.0. Figure 14 shows that Cuculinae (Old World Cuckoos) diverged from Coccyzinae (New World Cuckoos) 22 million years ago while Centropodidae diverge from the combined clade of Old and New World Cuckoos 32 million years ago. However, it is important to note that while the nodes analysed by Bayesian method are supported with maximum PP of 1.0, the result of analysis may change as more information are made available. Hence, it is important to analyse the same data with different analysis method. In the case of Prum et al., Maximum Likelihood Analysis was also carried out where the result is almost identical to the Bayesian Analysis tree; and almost all nodes of the Maximum Likelihood tree have maximum support of 1.00 based on bootstrap method [52].
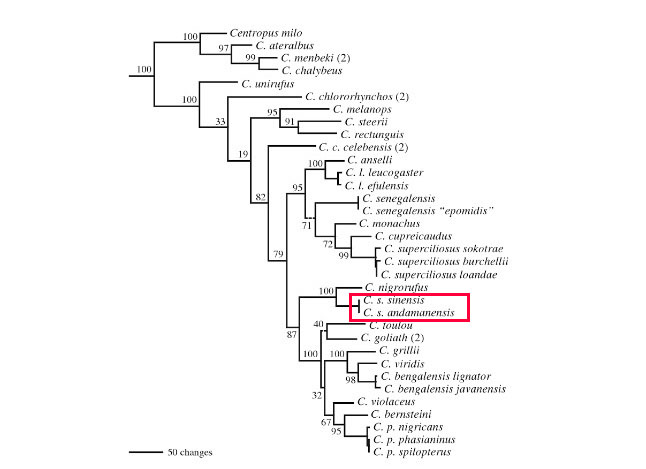 |
| Figure 16. Phylogeny of Centropodinae. Source: Payne, 2005 [11] and edited by Lau Jun Jie |