Bakau minyak
"One of the most common mangrove tree species"
Table of Contents

Features of R. apiculata. Top-left: Flowers on short bracts. Bottom-left 1: Eye-shaped leaves. Bottom-left 2: Stilt roots and aerial roots. Top-right: Grey bark with vertical fissures. Bottom-right: Cylindrical propagule. (Photo: Wild Singapore)
Basic Information
Name
Binomial: Rhizophora apiculata Blume, 1827Vernacular: Bakau minyak, Bakau putih, Bakau tandok (Tan, 2008), Corky stilt mangrove (Duke, 2006a), Tall stilt-root mangrove (Wightman, 2006), Tall-stilted mangrove (Lovelock, 1993)


First description of R. apiculata (Blume, 1827).
Etymology
Rhizo-phora means 'root bearing' in Greek, referring to the stilt roots characteristic of the genus. apiculata means 'to end abruptly' in Latin, referring to the leaf apex (Duke, 2006; Wightman, 2006).

R. apiculata has numerous stilt roots emerging from the stem. (Photo: Wild Singapore)
 |
| Leaf - � Pierre GRARD - IFP |
The leaf apex of R. apiculata is abrupt and pointed. (Photo: Checklist of Mangrove species of South East India and Sri Lanka)
Taxonavigation
Kingdom: Plantae
Phylum: Tracheophyta
Class: Magnoliopsida
Order: Malpighiales
Family: Rhizophoraceae
Genus: Rhizophora
Species: R. apiculata
Distribution
R. apiculata is found in south Asia including Bangladesh, Brunei Darussalam, Cambodia, India, Indonesia, Malaysia, Myanmar, Philippines, Singapore, Sri Lanka, Thailand, southern Viet Nam, and China (Hainan Island). It is also found in the Northern Maldives. In Australasia, its range includes Northwest Australia, Northeast Australia, Federated States of Micronesia, Guam, New Caledonia, Palau, Papua New Guinea, Solomon Islands, and Vanuatu.
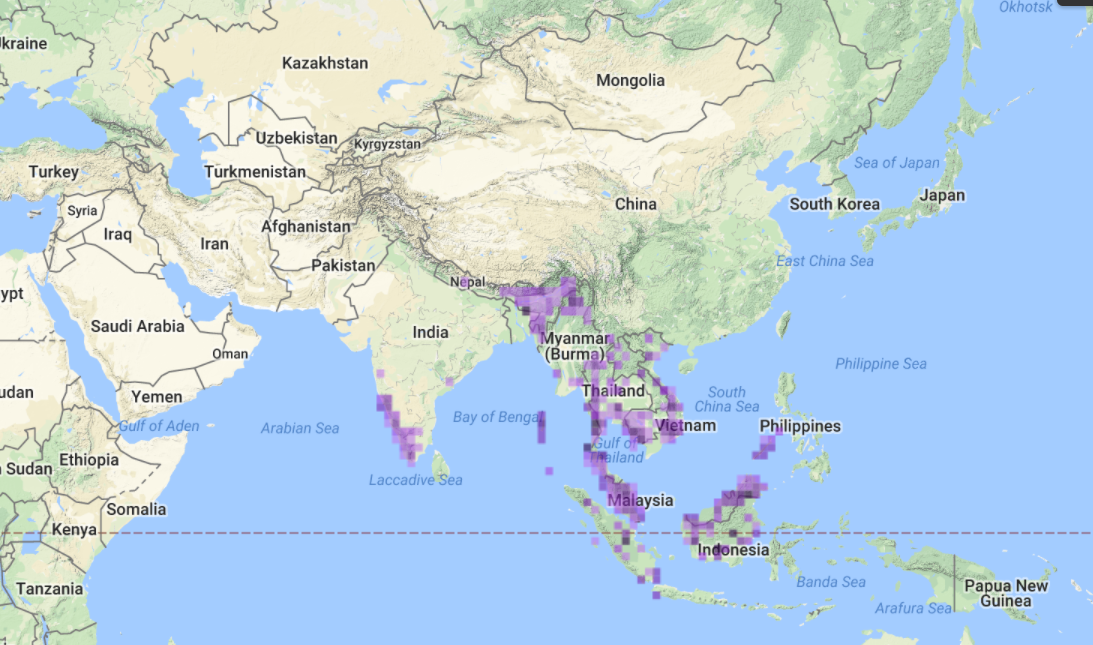
Global distribution of R. apiculata. Adapted from the IUCN list of threatened species.
Description
Overall
- Trees 20-30m tall
- Bark dark grey and chequered
- Conspicuous arching stilt roots that can extend 5m up the stem
- Often with lots of aerial roots emerging from the branches

R. apiculata along the coast of Thailand. (Photo: Britannica Online for Kids)
Leaves
- Leaves 8-15cm long
- Eye-shaped
- Glossy green
- Stiff
- Tiny evenly distributed black spots on the underside
- Stipule (i.e., outgrowths on the base of leafstalk) usually (but not always) red
 |
| Leaves - � Pierre GRARD - IFP |
Leaves of R. apiculata. (Photo: Checklist of Mangrove species of South East India and Sri Lanka)
Flowers
- Flowers 1-2cm in diameter
- In pairs
- Grow on short and swollen bracts
- Calyx globular, hard, thick, brown on the outside yellow inside
- Petals yellow to white, flat membranous and hairless, falling off soon after blossoming

Flowers of R. apiculata grow on very short bracts so that they appear to be stuck directly onto the branch. (Photo: Checklist of Mangrove species of South East India and Sri Lanka)
Fruits
- About 2cm in length
- The fruit looks like a brown, upside down pear
- Crowned by short persistent sepals
- The cylindrical hypocotyl can be up to 40cm long, smooth, green ripening purple

Pear-like fruits of R. apiculata with long hypocotyls. (Photo: Wild Singapore)
 |
| Hypocotyl - � Pierre GRARD - IFP |
Propagules of R. apiculata turn purple when ripening. (Photo: Checklist of Mangrove species of South East India and Sri Lanka)
Diagnosis
Rhizophora in Indo-West Pacific region consists of 3 species, R. apiculata, R. mucronata and R. stylosa. They can be distinguished in the field by some easily observed characters. R. mucronata and R. stylosa have slender (i.e., length much greater than the width) bracts at the base of mature buds and fruits as distinguished from R. apiculata that have bracts almost as wide, or wider than the length (Duke, 2006b). In addition, the bark of R. apiculata is grey, almost smooth, with vertical fissures, whereas the bark of R. mucronata is nearly black or reddish, rough or sometimes scaly (Hanum & Van der Maesen, 1997).

Left: R. apiculata. Middle: R. mucronata. Right: R. stylosa. R. mucronata and R. stylosa have slender bracts at the base of fruits, whereas R. apiculata have short bracts. (Photo: John Yong)
 |
| Bark - � Pierre GRARD - IFP |
 |
| Bark - � Pierre GRARD - IFP |
Left: Grey bark of R. apiculata. Right: Black bark of R. mucronata. (Photo: Checklist of Mangrove species of South East India and Sri Lanka 1, 2)
Biology
Habitat
R. apiculata is found in the intermediate estuarine zone in the mid-intertidal region. This species tolerates a maximum salinity of 65 ppt and a salinity of optimal growth of 8-15 ppt (Robertson & Alongi, 1992). It grows gregariously on deep, soft and muddy soils that are flooded by normal high tides, often consolidated and sheltered from surf and currents by pioneer species of Avicennia L. and Sonneratia L.f. It avoids hard soils and develops well in per-humid regions where it can form almost pure stands, sometimes in association with Bruguiera spp. or R. mucronata. It does not occur in freshwater swamps. It is killed by frost and extended periods of near-freezing temperatures (Hanum & Van der Maesen, 1997).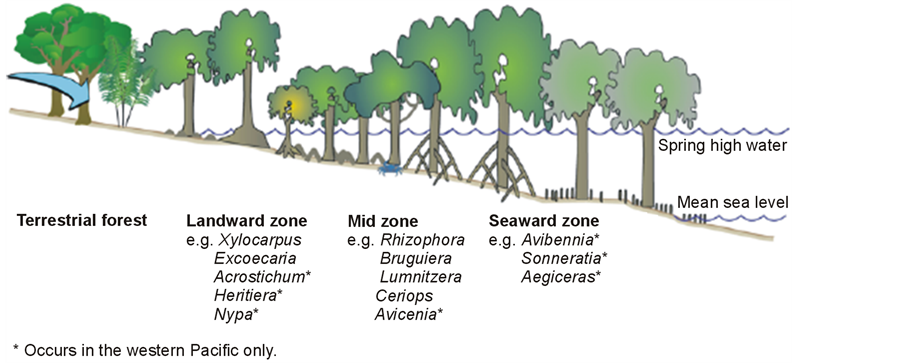
Rhizophora habits the mid-zone sheltered by pioneer species(Waycott et al., 2011).
Reproduction
The tiny flowers are wind-pollinated, producing lots of powdery pollen and no fragrance or nectar. They are also self-pollinating. Insects have occasionally been observed foraging for pollen. One-seeded fruits start to germinate when still hanging on the tree. The root protrudes from the fruit, producing a green, spindle-shaped rod (hypocotyls) of up to 40cm long. Eventually, the seedling falls from the fruit, floats with the high tide and establishes if it reaches a suitable site (Hanum & Van der Maesen, 1997). Seedlings may retain their viability for several months. Rhizophora seedlings grow rapidly to avoid being submerged at high tide. They can grow by 60cm in the first year (Tan, 2008).

R. apiculata propagules. (Photos: John Yong)

Development of seedling (Photo: A Guide to Mangroves of Singapore)
Adaptations
- Prop and Stilt Roots
In Rhizophora, branched, looping roots arise from the trunk and lower branches. At this stage, they are known as prop roots.They become stilt roots only when they take the function of flying buttresses when the tree is older and the bottom of the trunk becomes upside down conical and may even lose contact with the ground. The stilt roots improve the stability of the tree by providing a broader base and support in the soft and unstable mud. They also help in aeration as they are exposed for at least most of the day between tides.
 |
| Stilt and prop roots - � Pierre GRARD - IFP |
Prop and stilt roots of R. apiculata. (Photo: Checklist of Mangrove species of South East India and Sri Lanka)
- Ultrafiltration of salt
- Vivapary
Economical Values
The wood is heavy and hard and requires careful seasoning to prevent splitting. It is used for foundations in piling, beams and the outriggers of dugout canoes, as well as furniture and interiors of houses. Branched stilt roots are used for making anchors. It is also a valuable fuelwood species and is often commercially planted for charcoal production. It is sometimes planted to protect bunds and dykes. It is also the preferred species for mangrove silviculture used in mangrove rehabilitation and plantation forestry (Tan, 2008).
Charcoal making industry in Malaysia. Adapted from Youtube.
Ecological Values
- Refuge
- Food
- Natural water filter
- Coast stabilization
Conservation Status
R. apiculata is widespread and common within its range. It is threatened by the loss of mangrove habitat throughout its range, primarily due to extraction and coastal development, and there has been an estimated 20% decline in mangrove area within this species range since 1980. Mangrove species are more at risk from coastal development and extraction at the extremes of their distribution, and are likely to be contracting in these areas more than in other areas. It is also likely that changes in climate due to global warming will further affect these parts of the range. Although there are overall range declines in many areas, they are not enough to reach any of the threatened category thresholds. This species is listed as Least Concern (Duke et al., 2010).
Phylogeny
The combined Bayesian phylogeny based on chloroplast and ITS data provided strong support to taxon relationships (Lo et al., 2014). Rhizophora taxa were divided into three strongly supported clades namely NW, RA, and RMS, corresponding to three groups of taxa. All individuals of R. apiculata belong to Clade RA. This clade is further divided into two subclades – one contains individuals from Australia, islands of the NW Pacific (Guam and Micronesia), and subtropical Asia (Japan); and the other contains individuals from Southeast Asia (Malaysia, North Sulawesi, Philippines, and Thailand) and Sri Lanka.
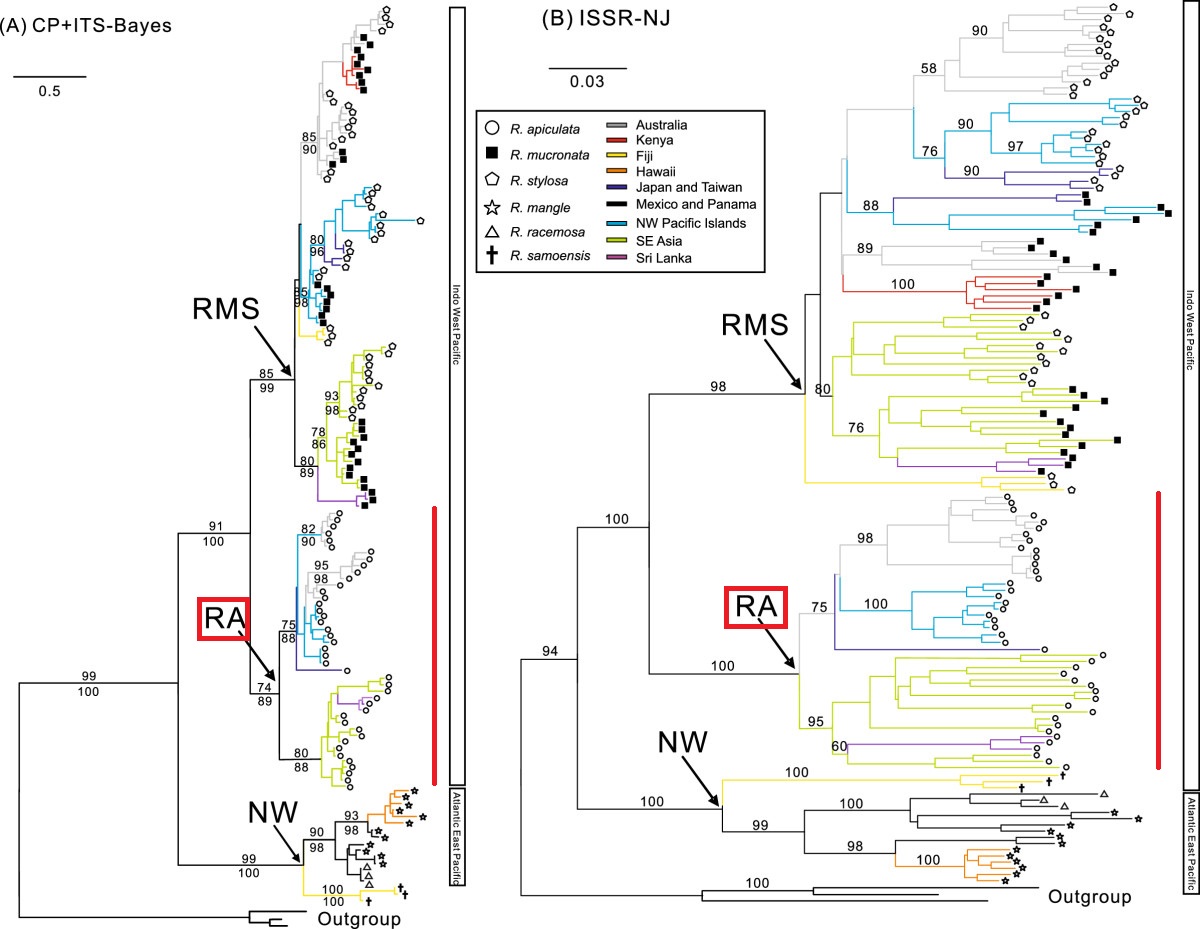
Genetic relatedness among population samples of Rhizophora. (A) Bayesian tree based on combined chloroplast and nuclear ribosomal ITS data using the GTR + I + G model. Bootstrap (BS; above branch) and posterior probability (PP; below branch) values >50% are indicated. Individuals of Bruguiera gymnorrhiza were used for rooting purposes. (B) Neighbour-joining tree based on Jaccard distances, showing relatedness among population samples of Rhizophora species. Bootstrap values >50% are indicated. (Figure from Lo et al., 2014)
In the Indo-West Pacific, the divergence of R. apiculata from R. mucronata and R. stylosa likely occurred during the Eocene (~38.9 ± 12Ma), although these species co-occur in many areas of the Indo-West Pacific and have a similar wide distribution range. Within Clade RA, the split between {Southeast Asia, Sri Lanka} and the {Australia, Kenya, Northwest Pacific Islands, subtropical Asia} lineages was dated to the Oligocene-Miocene boundary (~29-24 Ma).
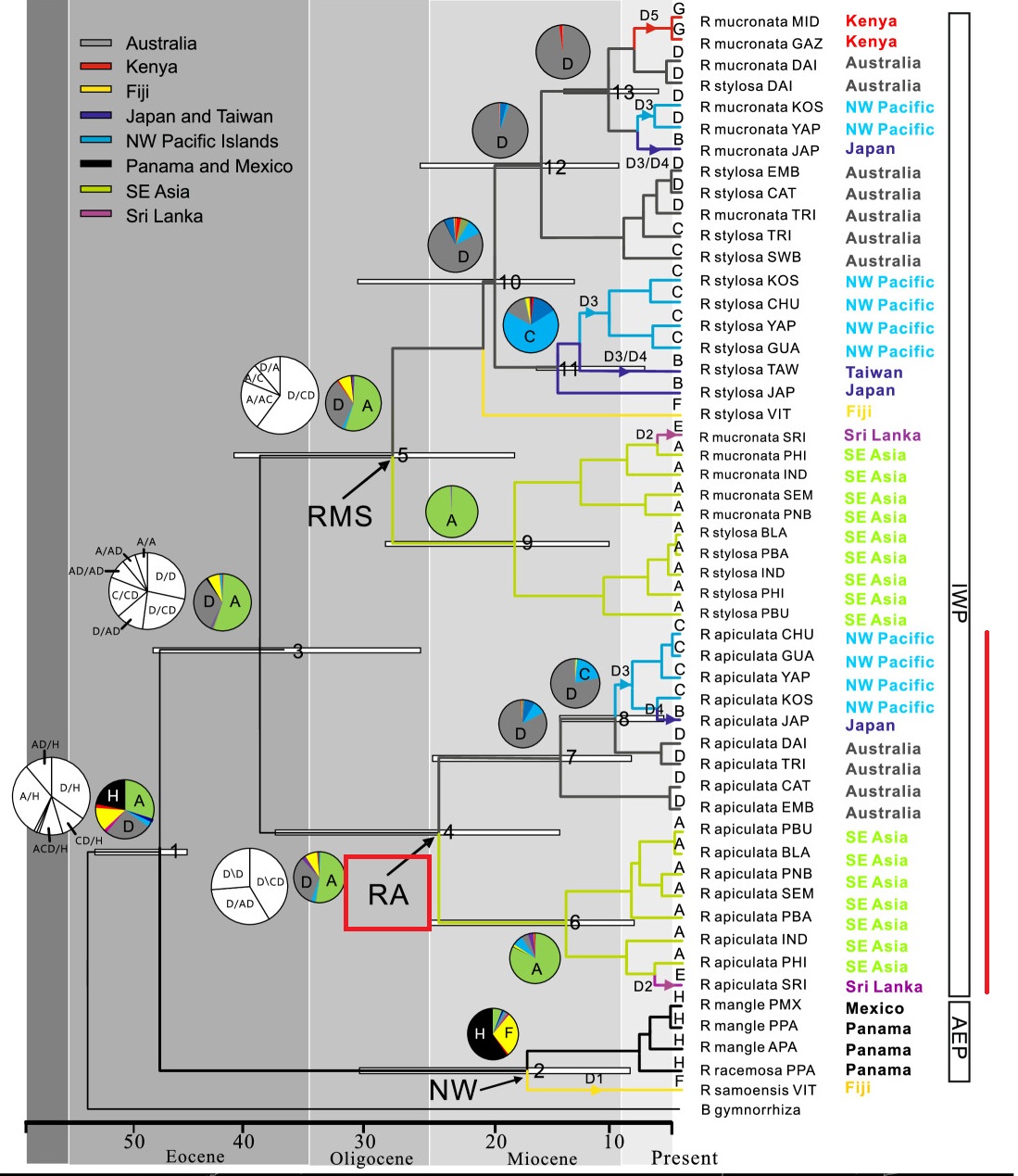
Chronogram of Rhizophora based on BEAST analyses of the combined chloroplast and ITS data. White bars indicate confidence interval of the estimated time of divergence of the respective nodes. Pie charts indicate the probable ancestral areas based on Lagrange (black and white) and Mesquite (color) analyses for the clade of interest. (Figure from Lo et al., 2014)
Type Information
R. apiculata was first described in Java. Lectotypes can be found in Nationaal Herbarium Nederland, Leiden University branch (L), L0009917 and L0009918.
Literature and References
Blume, C. L. (1827). Enumeratio plantarum Javae et insularum adjacentium: minus cognitarum vel novarum ex herbariis Reinwardtii, Kohlii, Hasseltii et Blumii. apud JW van Leeuwen.Duke, N.C. (2006a). Australia's Mangroves. The authoritative guide to Australia's mangrove plants. University of Queensland, Brisbane.
Duke, N. C. (2006b). Rhizophora apiculata, R. mucronata, R. stylosa, R.× annamalai, R.× lamarckii (Indo-West Pacific stilt mangrove), ver. 2.1. Species profiles for pacific island agroforestry. Permanent Agriculture Resources (PAR), Holualoa, Hawaii.
Duke, N., Kathiresan, K., Salmo III, S.G., Fernando, E.S., Peras, J.R., Sukardjo, S. & Miyagi, T. 2010. Rhizophora apiculata. The IUCN Red List of Threatened Species. Version 2014.3. <www.iucnredlist.org>. Downloaded on 24 November 2014.
Hanum, I. F., & Van der Maesen, L. J. G. (1997). PROSEA: Plant Resources of South-East Asia 11, Auxiliary Plants. Yayasan Obor Indonesia.
Lo, E. Y., Duke, N. C., & Sun, M. (2014). Phylogeographic pattern of Rhizophora (Rhizophoraceae) reveals the importance of both vicariance and long-distance oceanic dispersal to modern mangrove distribution. BMC evolutionary biology, 14(1), 83.
Lovelock, C. (1993). Field Guide to the Mangroves of Queensland. Australian Institute of Marine Science.
Robertson, A. I., & Alongi, D. M. (1992). Tropical mangrove ecosystems (Vol. 41, pp. 1-330). American Geophysical Union.
Waycott, M., McKenzie, L.J., Mellors, J.E., Ellison, J., Sheaves, M.T., Coller, C., Schwarz, A-M., Webb, A., Johnson, J.E. and Payri, C.E. (2011). Vulnerability of mangroves, seagrasses and intertidal flats in the tropical Pacific to climate change.
Wightman, G. (2006). Mangroves of the Northern Territory, Australia: identification and traditional use. Northern Territory. Dept. of Natural Resources, Environment and the Arts, Palmerston.
Useful links:
R. apiculata in Wild Singapore
R. apiculata on the NParks Flora and Fauna website.
R. apiculata in Guide to the mangroves of Singapore.