Plants, animals, or rocks?
Many people don't know what corals actually are. Well, they're not plants, and they're certainly not rocks. In fact, they are ANIMALS!It's understandable that some think corals are rocks, or even plants - they do look like rocks, and they're also "stuck" to the substrate.
Where's the animal you speak of?Each coral colony is a combination of many, many, MANY, polyps. Each polyp is an individual animal which lives in the "hole" you see in the picture below. This "hole" is also known as a corallite cup. Coral polyps resembles a lot like sea anemones, and that's because they belong to the phylum Cnidaria, and are most closely related to other marine organisms such as sea anemones, jellyfish and hydroids. Read more about Cnidaria here.
 |
| C. aspera on an intertidal seawall. Photo by Dayna Hui. |
Table of Contents
1. Overview
Scientific name: Coelastrea aspera (Verrill, 1866)
Common names: Lesser star coral, though this commonly refers to many other Goniastrea species[1] ; Honey-comb hexagonal coral[2]
Coelastrea aspera, formerly known as Goniastrea aspera, is widespread across the Indo-Pacific[3] . In Singapore, Goniastrea species are one of the most dominant scleractinian corals on reefs and seawalls on the Southern offshore islands[4] . They are commonly found in shallow waters such as intertidal habitats, but can also be found up to 15 metres in depth[5] [6] . Goniastrea species are usually small to medium-sized, but individual colonies on reef flats may adjoin to form expanses up to 5 metres long[7] . They are known to be one of the most resilient species of corals, especially at tolerating aerial exposure during spring tides, which explains their dominance in intertidal communities[8] .
Coelastrea aspera dominating on intertidal habitats. Photo from Corals AIMS[9] (Creative commons)
.
2. Biology
2.1 Symbiotic relationship with zooxanthellae
Ever wondered where corals get their (usually brown) colour from? It does not come from the coral itself, but from their symbiotic relationship with an algae: zooxanthellae (Symboidinium sp.)! Zooxanthellae are unicellular dinoflagellates, and have symbiotic relationships with many other marine invertebrates, such as sea anemones, jellyfish, sponges, flatworms[10] and giant clams. Symbiotic relationships are long-term interactions between two species, and they essentially can't live without each other. Not all corals have zooxanthellae (also known as azooxanthellae corals), though most reef corals do.
How do they depend on each other?
Zooxanthellae cells reside in coral tissue, giving them their dull and brown colour in general. The other fluorescent pigments come from coral proteins. Apart from providing them a home, the coral polyps provide carbon dioxide to the zooxanthellae for photosynthesis when they undergo respiration. In return, zooxanthellae photosynthesizes and provide food and oxygen to the coral polyps, which the polyps use to produce proteins and carbohydrates to build their calcium carbonate skeleton. Without corals, zooxanthellae don't have a home to live in. Without zooxanthellae, corals have no food!
vs.
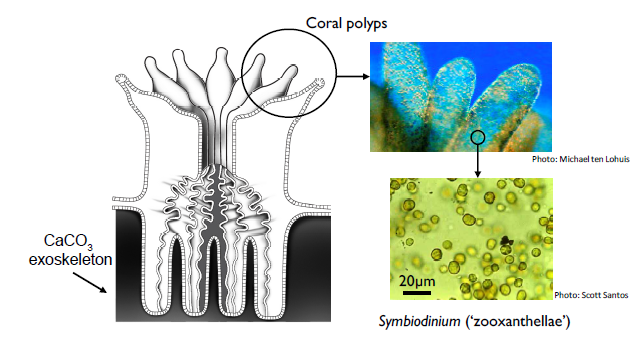
Location of zooxanthellae in coral polyps. Photo taken from Woolridge (2013)[11] (Fair use)
Zooxanthellae as an indication of coral health (Coral bleaching)
Their highly dependent relationship makes zooxanthellae density a proxy for coral health. When corals face unfavourable conditions, such as changes in ocean temperature, pollution or extreme sunlight, the coral polyps expel their zooxanthellae, which explains the loss of colour[12] . A common phenomenon is temperature-induced bleaching events, where sea temperatures become way to high, causing corals reefs to become bleached, which looks really depressing.
More about coral bleaching:

A common misconception is that bleached corals are dead, but corals can still remain alive for a short period of time without their symbiotic partner. If conditions become favourable again, zooxathellae can return to these bleached corals again and the corals' pigmentation will return. However, if temperatures remain high and unfavourable conditions persist, the zooxanthellae are unlikely to return and the coral will eventually die due to the lack of food. Apart from stress due to rise in temperatures, zooxanthellae density is commonly used as a proxy for coral health due to other stressors. it was found that a species of coral, Montastrea faveolata (Scleractinia), had reduced zooxanthellae densities when in contact with mixed turf algae[13] . An ongoing study is also investigating if mixed turf algae on intertidal seawalls in Singapore have a similar effect on C. aspera.
2.2 Behaviour
Stinging tentacles
Corals may seem lifeless in the day, but all the action happens at night! While most corals retract their polyps during the day and let their symbiotic zooxanthellae do the work, they extend their tentacles at night to capture food for feeding, for competition with other corals or organisms, and also for defence!| C. aspera in-situ with tentacles extended at night. Photo by Dayna Hui. |
 |
| Close-up of C. aspera with tentacles extended. Photo by Dayna Hui. |
Their tentacles contain stinging cells (nematocysts) that can poison and kill smaller organisms, such as plankton, which they subsequently capture for feeding. Don't worry - the nematocysts are not harmful to humans. They also use it to poison and kill their competitors when competing for space. The video below shows a Hydnophora sp. attacking a Platygyra sp. over a period of six days in Hong Kong - you can see that a part of the Platygyra sp. clearly died at the end of it video, with only its' bare skeleton left.
Hydnophora sp. attacking a Platygyra sp., video by Anna Depetris on Youtube.
Mucus secretion
If you've ever seen a coral in real life, especially when they are exposed during low spring tides on intertidal habitats, you might notice a layer of mucus on the surface of the colony. Corals excrete this mucus to protect themselves from various stressors, such as pathogens, dessication, sedimentation and pollutants[14] . It also helps them to capture food more efficiently, and defend themselves against other corals when they are competing for space
[15] .
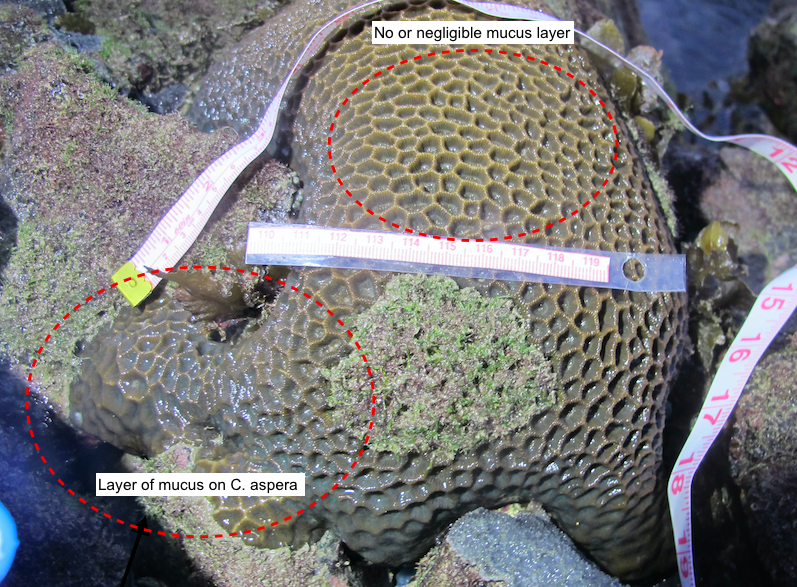 |
| C. aspera during low spring tide, with edges showing a mucus layer, while the centre of the colony seems to be desiccated. Photo by Dayna Hui. |
Corals also retract their polyps to defend themselves when they sense danger. For example, if you sweep your finger across a coral with its' tentacles extended, they will retract back in, just like how a mimosa leaf would!
| C. aspera with tentacles partially extended. Photo by Dayna Hui. |
2.3 Reproduction
Corals reproduce in 3 ways - asexually by budding, and sexual reproduction by the release of eggs, sperms or well-developed planulae, and fragmentation.
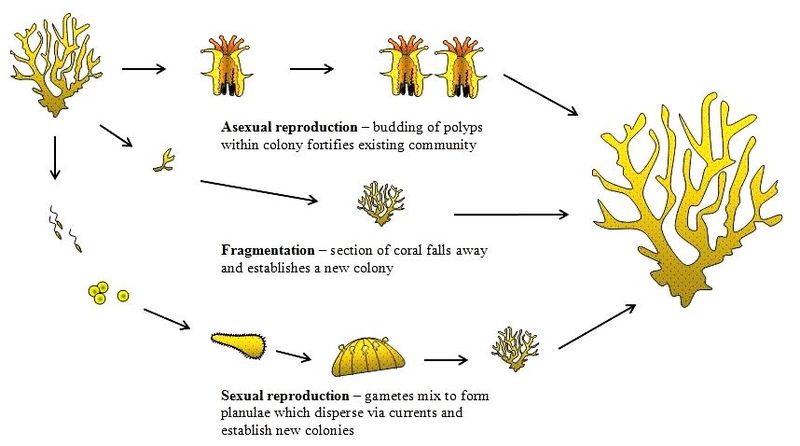
Coral reproduction. Photo taken from the coraldigest (Fair use)
Asexual reproduction
Corals grow or reproduce asexually by budding of clonal polyps within the parent colony. This usually happens when conditions are relatively stable. Budding can either be intratentacular or extratentacular[16] . During intratentacular budding, the parent polyp divides into two or more daughter polyps - similar to the mitosis process. During extratentacular budding, a daughter polyp emerges adjacent to the parent polyp, and is usually smaller in size. This only occurs for corals with separate walls. C. aspera undergoes intratentacular budding. The type of budding is sometimes used to distinguish between different genera.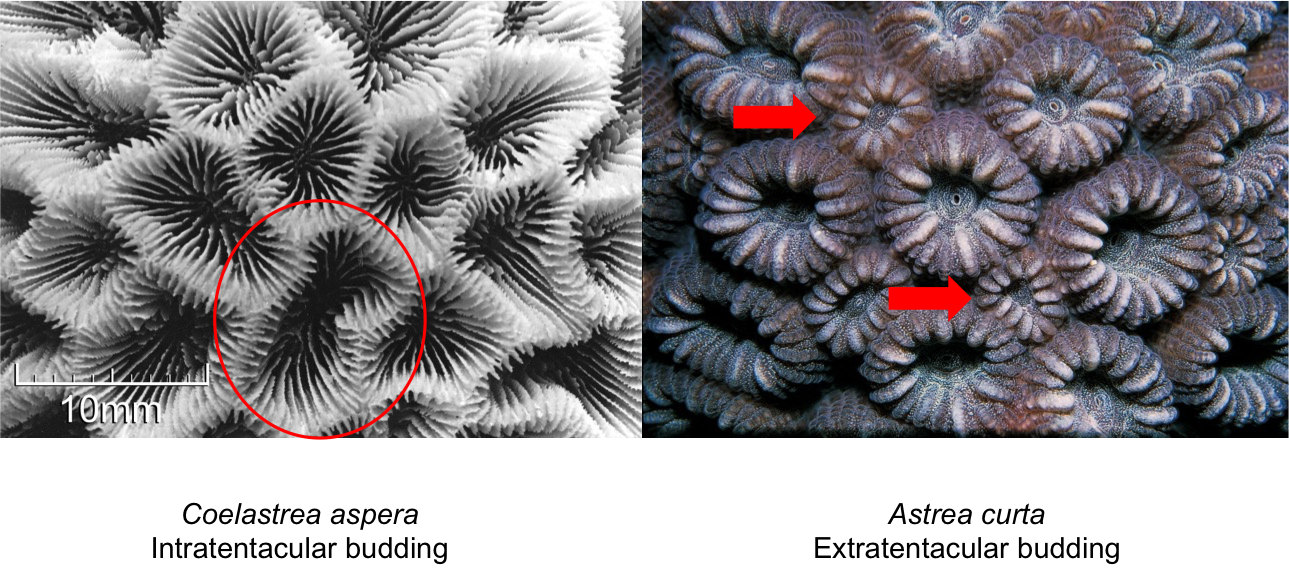
Comparison between intratentacular and extratentacular budding. Photos adapted from Corals AIMS (Creative commons). Annotations by Dayna Hui.
Sexual reproduction
Mass coral spawning - nature's most spectacular phenomenon
Coral spawning footage from Jonathan Bird's Blue World. Video from Youtube.
Most corals undergo external fertilization by spawning eggs and sperms. This phenomena is termed as a mass spawning event. Different colonies on the reef synchronize their release of eggs and sperms all at the same time. They get their cues from water temperatures and the lunar cycle[17] . The sperms and eggs float to the surface, where fertilization occurs and form planulae (free-swimming larvae). Some corals also undergo internal fertilization and release well-developed planulae, which often has a higher chance of survivability and better settlement rates.
Planulae are naturally attracted to light, and hence swim towards the surface water where they are transported by the currents. When they sense a suitable substrate nearby (usually from chemical cues by coralline algae[18] ), they swim towards it and settle down. If it isn't, they can bounce off the substrate, swim back to the surface, and continue on their journey to finding a home.
Why synchronize their release?
As sessile organisms, corals that are distant from each other cannot come into contact with each other to mate. Thus, synchronizing their release of eggs and sperms is crucial in order to increase the success rate of fertilization. The released eggs and sperms can only last for a short-time in the water, but once fertilized, the planula larvae can swim in the waters for several days to months, depending on the species. Mass spawning also helps to increase genetic diversity. Some also suggest that this mass release could serve to satiate predators. The predator satiation is an anti-predatory mechanism, in which the organisms (prey) occur at extremely high densities, increasing the chances of survivability because their predator can only eat so much before they get saturated with food! Apart from coral spawning, a similar phenomenon occurs in our terrestrial forests, where trees also synchronize their release of seeds (nuts) all at the same time. You can read more here.
What about Coelastrea aspera?
Coelastrea aspera colonies are simultaneous hermaphordites[19] , this means they have both male and female organs and they can function simultaneously. They are both broadcast spawners and planula larvae brooders[20] [21] . At the Great Barrier Reef, they release floating eggs and sperms that are expelled in packets, which rise to the surface rapidly. Although most species release floating eggs, a (previously) closely-related species, G. favulus, release sticky eggs that sink to the bottom, and sperms are expelled separately[22] . It has been suggested that releasing sticky eggs or well-developed planulae is one way corals retain their larvae within the adult's vicinity, where survivability rates are higher because of suitable conditions, while broadcast spawning helps to maintain gene flow and genetic diversity[23] . Researchers have found that the distribution of C. aspera is generally aggregated and clumped, which is likely due to the release of brooded planulae rather than spawned gametes[24] . These two different strategies adopted by C. aspera could explain their wide distribution in the Indo-Pacific.

Fragmentation
Coral fragmentation, also known as propagation, occurs when fragments of corals break off naturally from their parent colony and drift off elsewhere to continue their lives. More recently, coral fragmentation or propagation has become a popular tool to cultivate corals. This process is very similar to plant propagation, where the parent colony or plant are cut into smaller pieces that are genetically similar. Researchers have found that the growth rates of propagated corals are much faster than the parent colonies, and that fragmented corals can even fuse together very quickly[27] ! This is important for coral conservation and restoration, especially in light of more frequent coral bleaching events that lead to massive losses of coral reefs.
3. Conservation status
 |
| IUCN status, image taken from IUCN Red List |
Although C. aspera is of "Least Concern"[29] , it still faces threats from habitat loss due to human modification to coastlines and rising sea temperatures due to climate change.
3.1 Conserving coral reefs
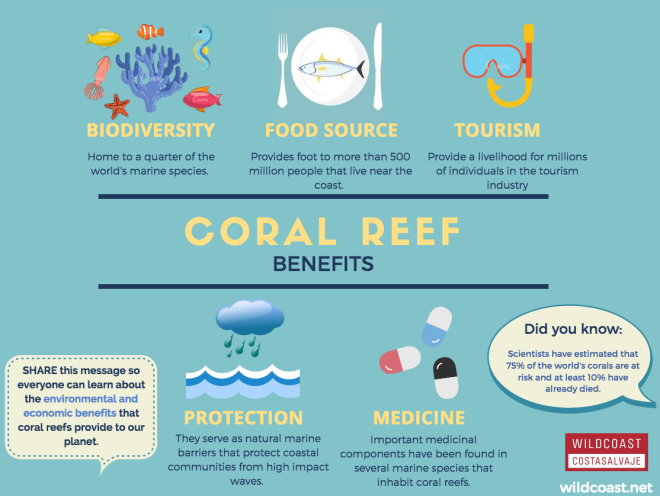
The benefits of coral reefs. Picture from The WildCoast Blog (Free use)
Coral reefs are one of the most important and diverse ecosystems on the planet. They help to buffer strong wave actions and protect our coastlines, they provide habitats to many marine organisms, recycle nutrients, and they fix carbon and nitrogen too! We humans depend on coral reefs too. They generate revenue for the economy in the fishing industry (often a fishing ground as many fish spawn on coral reefs) and tourism industry.
Threats to coral reefs[30]
- Unsustainable fishing practices e.g. cyanide bombing, overfishing
- Use of sunscreen
- Unsustainable tourism practices
- Climate change
- Dredging
- Human impacts: sedimentation, nutrient enrichment (pollution)
What YOU can do to help
As an individual, you can help to protect coral reefs by being a responsible tourist. If you do snorkel or dive, choose reef-safe sunscreen, or don't use sunscreen at all! Wear arm sleeves and long pants to protect yourself from UV radiation instead. Also, pick an environmentally-friendly ecotourism agency that is committed to protecting coral reefs. Lastly, DO NOT LEAVE LITTER BEHIND!
3.2 Corals have memories
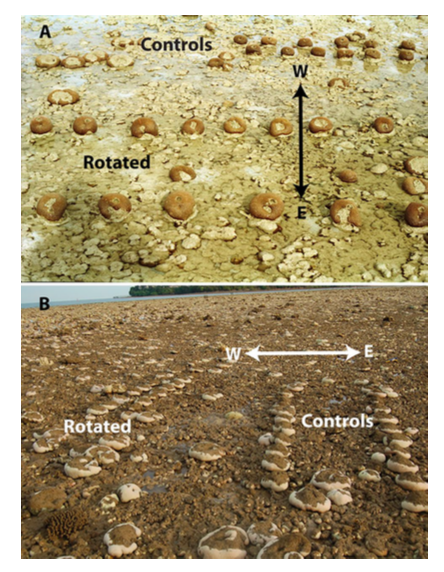
Multiple studies have been done regarding the thermo-tolerance of C. aspera at on an intertidal reef flat in Phuket, Thailand[32] [33] [34] [35] [36] . Researchers found that corals can develop thermo-tolerance from exposure and acclimatization to high irradiance, and that they can display within-colony variation in response to bleaching. Solar bleaching occurs annually from January to March at the study site, where skies are clear and tides are low. Only west sides of the colonies are subjected to high irradiance during solar bleaching, while the east sides are generally shaded. In May, mean temperatures are the highest and temperature-induced bleaching in C. aspera colonies were observed in the years 1991 and 1995. However, what's interesting is that the east sides of the colonies were more frequently bleached, while west sides of the colonies remained highly pigmented[37] ! In 2000, the zooxanthellae and chlorophyll a concentrations of these colonies were investigated, and it was found that the west side of colonies had consistently higher concentrations than the east[38] . 10 years later in 2010, another major bleaching event occurred at the study site due to unprecedentedly high temperatures. Researchers analysed the zooxanthellae densities of the colonies and again, found higher densities in the west side of colonies than the east[39] ! This is of notable interest for future conservation work as it suggests that corals can retain their 'memory' for over a decade, and that corals can develop thermo-tolerance after exposure to regular high solar irradiance. This will be extremely relevant in light of rising sea temperatures due to climate change, and perhaps more studies can be done on other species.
3.3 Coral conservation in Singapore
Since the 1960s, many of Singapore's coral reefs have been destroyed for development and reclamation, and the few remaining ones are shallow and unstable. In addition, high sedimentation rates are characteristic of Singapore's water, due to frequent dredging by ships. This is quite expected since Singapore is a major shipping hub. Despite that, Singapore hosts over 250 species of corals, making up about one-third of the world's scleractinian diversity[40] . However, our remaining reefs are still threatened by anthropogenic impacts, in particular - high sedimentation rates and loose substrate and rubble. Loose substrate and rubble means that they get shifted around easily and are susceptible to abrasion and burying, hence lowering coral larvae's chances of settling successfully. High sedimentation rates are a constant problem, but successful transplantation project suggests that the coral population in Singapore have been able to overcome this. More recently, Singapore is focusing on the role of coral nurseries and coral transplantation on our seawalls in increasing our diversity and coral population[41] .Coral nurseries and transplantation to seawalls
Coral nurseries play important roles in protecting diversity. Nurseries can occur in situ and ex situ - i.e. in the sea and in aquariums. Coral nubbins or propagules are attached to trays made from PVC pipes and plastic mesh, where they live and grow in sheltered environments until they become large enough to be transplanted to degraded reefs. Their larger size helps to increase their chances of survivability. In Singapore, corals are often transplanted onto our seawalls, considering that seawalls make-up more than 60% of our coastlines. Survivorship of transplanted corals has been quite successful, with about 80% still attached 3 months after transplantation[42] . However, note that the types of corals that are transplanted have to be considered. Since seawalls are subjected to high energy waves, corals that are "flatter", such as encrusting corals, will definitely have higher survivorship than branching corals, which would break easily. With increasing development and modifications to coastlines, many countries will soon experience similar problems and environmental conditions (high sedimentation rates and loose substrate), along with many seawalls along the coastline. Thus, Singapore could serve as a model for coral conservation for highly sedimented waters.
Take a look at the video below to get an idea of how coral nurseries look like!
"Coral gardens could save ocean life". Video published by Cable News Network (CNN) on Youtube.
Contribute to coral conservation in Singapore
"Plant-A-Coral, Seed-A-Reef Programme" - adopt a coral or enhance a reef through donations
Reef monitoring with Blue Water Volunteers - help to monitor coral reefs' health in the Southern Islands of Singapore
International Coastal Clean-up (ICCS) - though not directly related to coral conservation, cleaning up our shores will definitely help to conserve our marine habitats!
For more opportunities to contribute to marine conservation, check out wildsingapore - you will definitely find something suitable for you!
4. Morphology
4.1 General anatomy and skeleton of corals
General anatomy
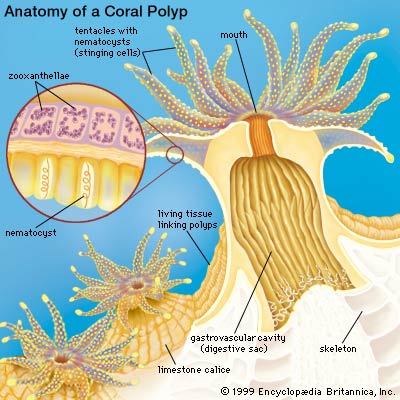
Each coral colony typically consists of several polyps. A polyp is an individual (animal!) and they resemble alot like anemones! Each polyp has an upward-facing mouth surrounded by retractable tentacles with nematocysts (stinging cells), and they "create" their own home by secreting a calcareous exoskeleton in a cup-shaped structure, also known as a corallite. Corals get bigger in size as they continue to excrete this calcareous exoskeleton, growing upwards and outwards.
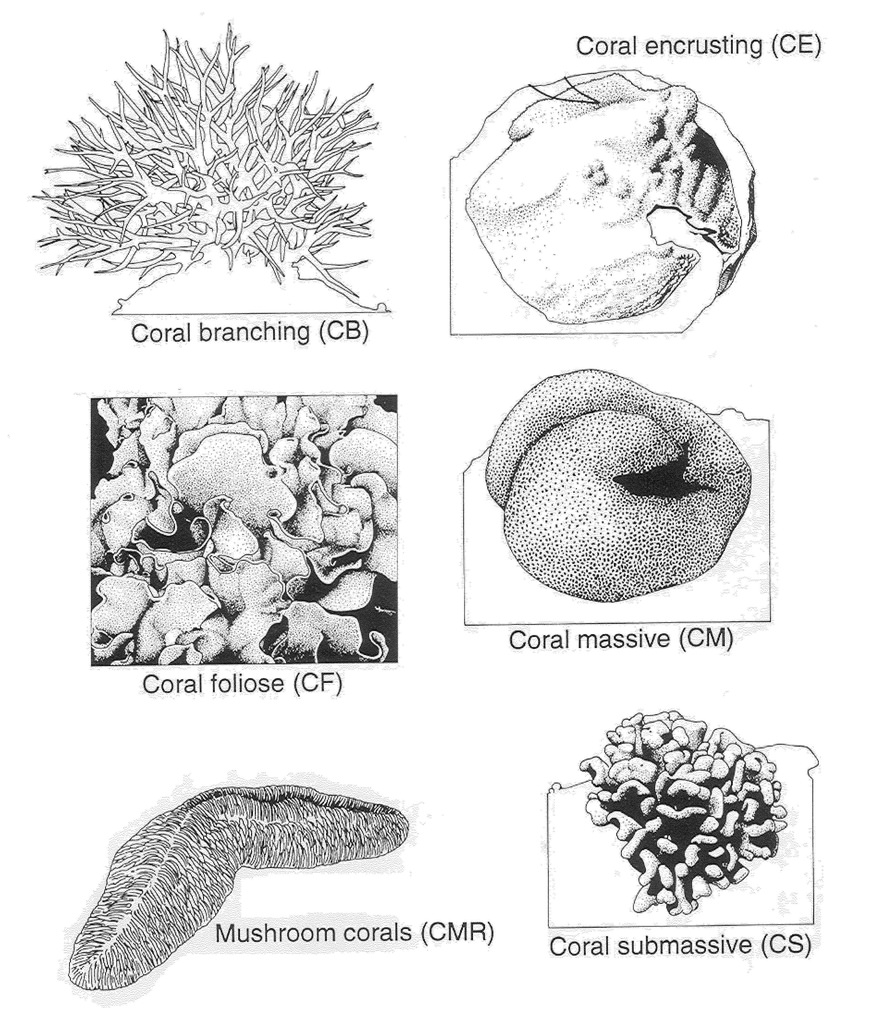
Growth form
Corallite arrangement
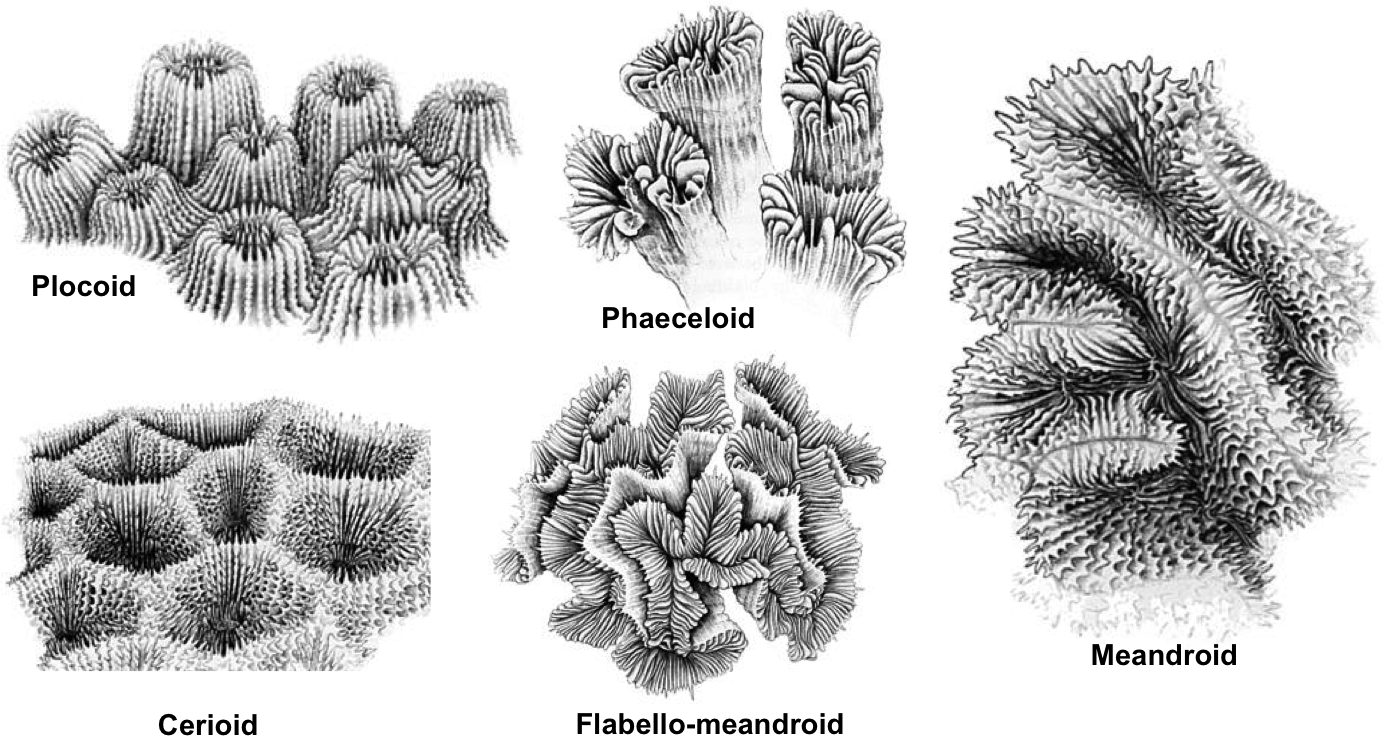 |
| Corallite arrangement in corals. Plocoid: Corallites with own walls; Phaeceloid: Corallites with own walls, but corallites are long and tubular; Cerioid: Corallites with shared walls; Flabello-meandroid: Valleys without shared walls; Meandroid: Valleys with shared walls. Photos from Coral AIMS (Creative commons). |
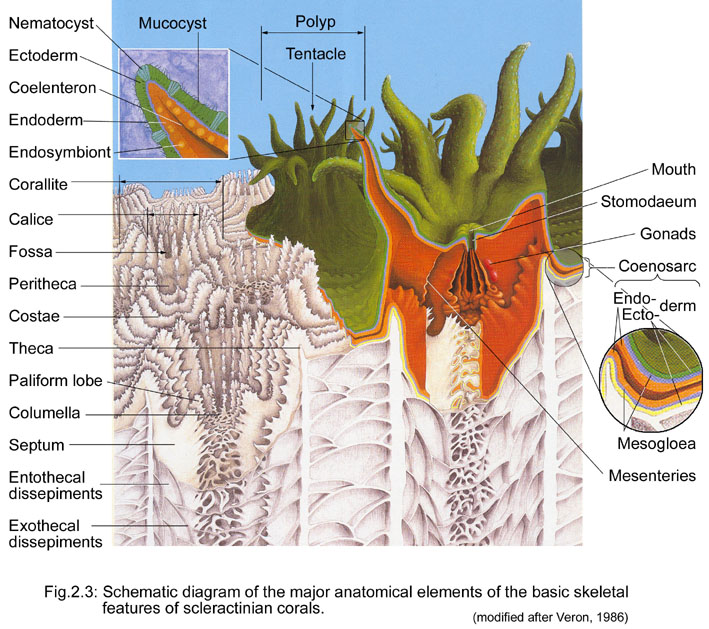
4.2 Description of Coelastrea aspera
Description of Coelastrea aspera[43]
- Massive or encrusting growth form
- Generally pale brown in colour, but can be dark in turbid waters
- Corallites have shared walls (cerioid)
- Corallites have an angular and/or hexagonal appearance
- Presence of distinct paliform lobes (though this may be absent in exposed waters)
- Corallite size generally between 5-10mm
- Septa are regularly spaced out and neat-looking, with long and short septa alternating
 |
| Overall features of C. aspera. Photo and annotations by Dayna Hui. |
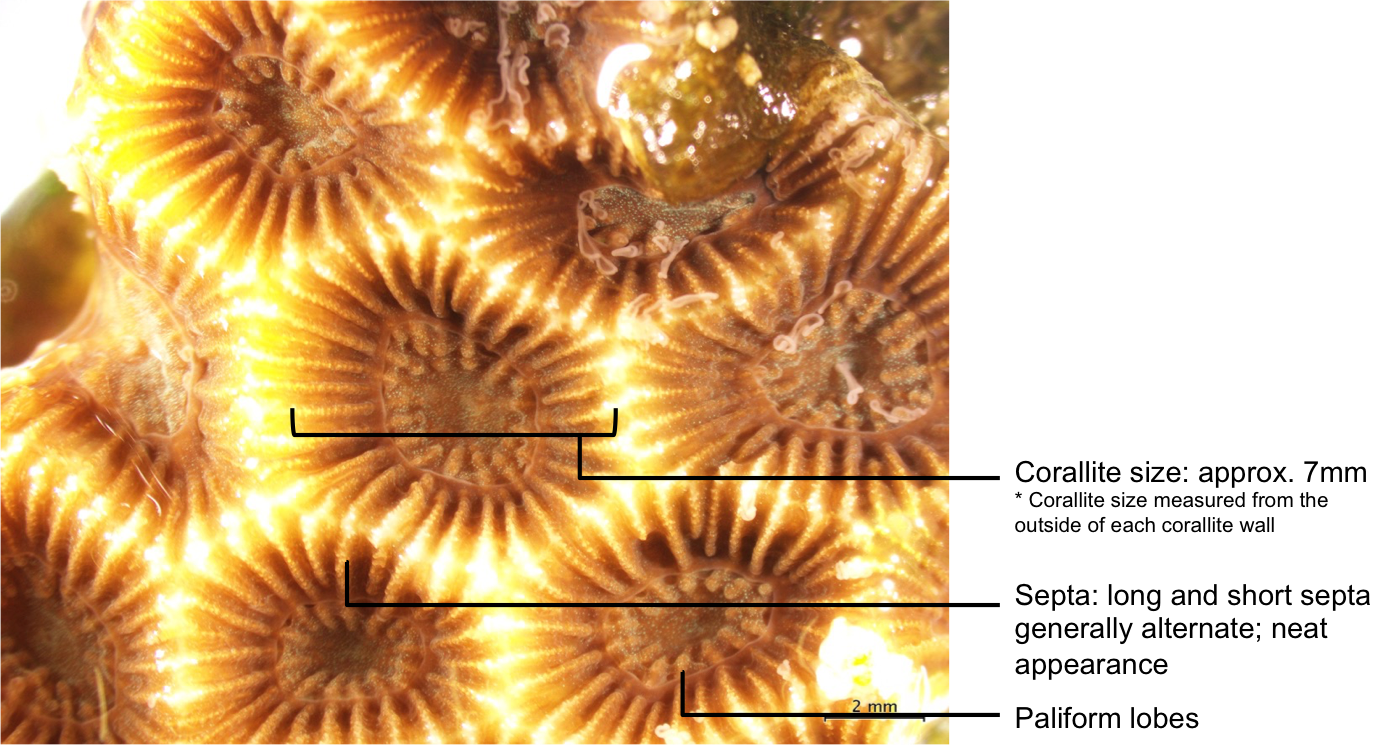 |
| Skeletal features of C. aspera. Photo and annotations by Dayna Hui. |
4.3 Differentiating between similar-looking genera and species
This section will be described based on Coelastrea aspera being in its' previous genus, Goniastrea. Comparisons are largely based on descriptions found at Coral AIMS (2011) [44] .
Comparison between similar-looking genera
Goniastrea and Favites
Both taxa share similar skeletal characteristics such as cerioid corallites, angular corallites, shared walls, and similar colouration, but they can be differentiated by:
- Presence of distinct paliform lobes in Goniastrea sp., which is usually absent in Favites sp.
| Favites abidta |
Coelastrea aspera |
||||
|
|
||||
| No paliform lobes |
Distinct paliform lobes |
Goniastrea and Platygyra (meandering)
It is straightforward to distinguish between Goniastea colonies with cerioid corallites from Platygyra colonies, which are almost always meandering. However, there are some Goniastrea colonies that are meandering too, and can easily be mistaken as a Platygyra colony. Here's how to differentiate them:
- Presence of distinct paliform lobes in Goniastrea sp., which is usually absent or weakly developed in Platygyra sp.
- Presence of columella centres in Goniastrea sp., which are usually indistinguishable in Platygyra sp.
Comparison between similar-looking species
Goniastrea species can be grouped into 3 groups:
- Monocentric with corallite diameter less than 5mm
- Predominantly monocentric with corallite diameter over 5mm
- Predominantly meandroid
Goniastrea aspera and G. palauensis
Goniastrea aspera and G. palauensis belong to the second group, where colonies are predominantly monocentric with corallite size more than 5mm. These are the only 2 species that belong to this group that are found in Singapore. These 2 species look extremely similar, but can be distinguished by:
- Smaller corallites in G. aspera (usually between 5-10mm), and larger corallites in G. palauensis (usually more than 10mm)
- Thin walls in G. aspera and thick walls in G. palauensis
| Goniastrea palauensis |
Goniastrea aspera |
||||
|
|
||||
|
|
5. Taxonomy and systematics
5.1 Taxonavigation
Scientific classification[45]Kingdom: Animalia
Phylum: Cnidaria
Class: Anthozoa
Sub-class: Hexacorallia
Order: Scleractinia
Family: Merulinidae
Genus: Coelastrea Verrill, 1866
Species: Coelastrea aspera (Verrill, 1866)
5.2 Nomenclature
Original description
Coelastrea aspera was originally described as Goniastrea aspera by Verrill in 1866[46] , but have recently been placed under Coelastrea by Huang et al. in 2014[47] .
 |
| Original description of Coelastrea asepera by Verrill in 1866 |
Synonyms[48]
- //Astraea (Fissicella) magnifica// (Dana, 1846) (synonym)
- //Favites aspera// (Verrill, 1866) (previous combination)
- //Goniastrea aspera// Verrill, 1866 (original combination, basionym)
- //Goniastrea equisepta// Nemenzo, 1959 (synonym)
- //Goniastrea incrustans// Duncan, 1889 (synonym)
- //Goniastrea mantonae// Crossland, 1952 (synonym)
- //Goniastrea spectabilis// (Verrill, 1872) (synonym)
- //Prionastraea spectabilis// Verrill, 1872 (synonym)
5.3 Type information
Type information for Coelastrea
The type species for Coelastrea is Coelastrea tenuis Verrill 1866. However, there is much ambiguity about whether C. tenuis is a valid species because of the lack of physical specimen since its' original description. Earlier explorers stated that the locality of C. tenuis was from Hawai'i, but is unlikely because no Goniastrea species are known to be present there. Reports by CITES stated that living specimens of C. tenuis were exported out of the eastern Pacific and an unspecified locality in the USA between 1996 and 1997 - however, these were not supported by voucher collections, and are likely to be misidentifications. Huang et al. argue that C. tenuis may have been misidentified as G. aspera more recently.
Type information of Coelastrea aspera [49]
The type information of C. aspera are based on 2 syntypes: USNM 402 and 403 (2 dry specimens, Fig. 8B-D), and its' type locality is in Ryukyu Islands, Japan. Other morphological features are also shown in the figure below (Fig. 8E-F).
More information about the types can be found at the USNM Invertebrate Zoology Cnidaria Collection.
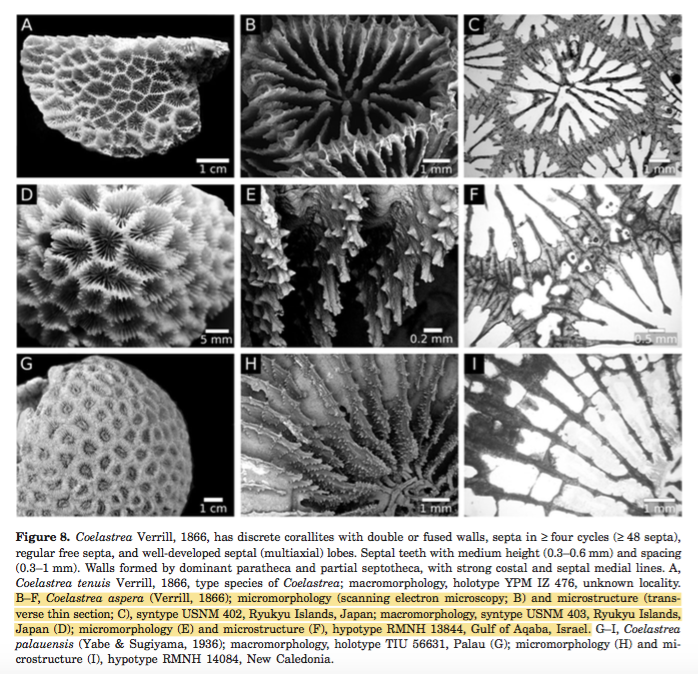 |
| Figure adapted from Huang et al. (2014) (Fair use) |
5.4 DNA information
To Genbank (19 nucleotide, 9 protein)
To Barcode of Life (2 barcodes)
5.5 Phylogeny
This section is largely based on a paper by Huang et al. (2014)[50] , who reconstructed phylogeny trees for the families Diploastraeidae, Montastraeidae, Merulinidae, Lobophylliidae, and Mussidae. Tree A was constructed based on nuclear and mitochondrial markers, while Tree B was constructed based on 44 morphological characters at 3 different scales - macromorphology, micromorphology and microstructure.
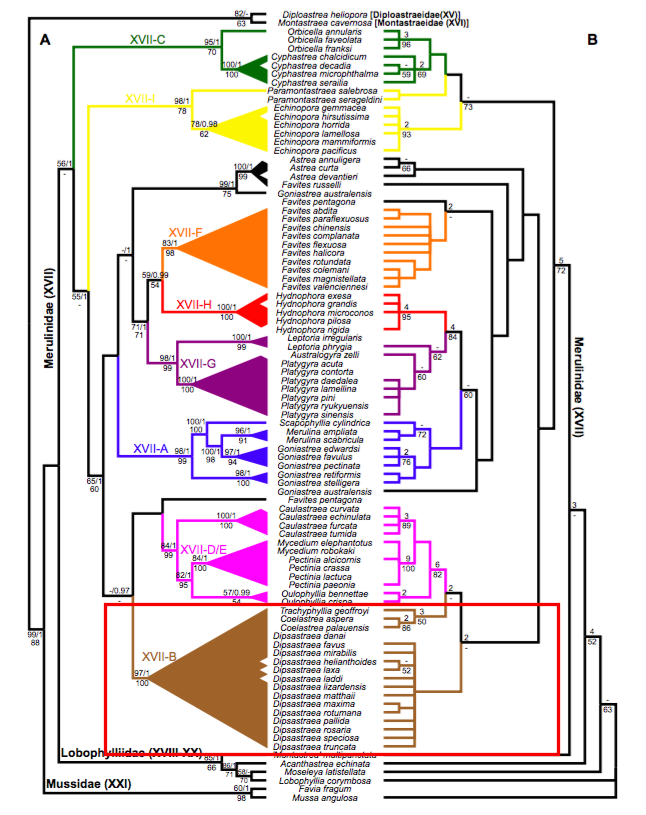 |
| Tree A: Maximum likelihood tree based on three nuclear and two mitochondrial markers. The numbers on the branches show support values: upper value indicates maximum likelihood bootstrap with Bayesian posterior probability, lower value indicates maximum parsimony bootstrap. Tree B: One of eight parsimony trees, based on the analysis of 44 morphological characters. The numbers on the branches show support values: upper value indicates Bremer decay index, lower value indicates maximum parsimony bootstrap. Tree adapted from Huang et al. (2014). Annotations by Dayna Hui. |
 |
| A section of the reconstructed phylogeny trees, showing the clade that consists of Trachyphillia, Coelastrea and Dipsastraea. Annotation by Dayna Hui. |
The move from Goniastrea to subclade XVII-B (primarily Dipsastraea)
Clearly, Coelastrea aspera and C. palauensis are genetically distant from their previous genus, Goniastrea. They are, however, genetically well-supported in the clade XVII-B that primarily consists of Dipsastraea (formerly Favia) (Tree A: maximum likelihood bootstrap = 97; maximum parsimony bootstrap = 100). Despite this, Huang et al. accorded Trachyphyllia geoffroyi and the two former Goniastrea species (G. aspera and G. palauensis) their own genera because of obvious differences in morphology, sharing few characters with Dipsastraea. Additionally, based on the long molecular branch lengths in T. geoffroyi and the two former Goniastrea species, which is a symptom of long-branch attraction problem even in maximum likelihood analyses (usually more prominent in maximum parsimony analyses), Huang et al. accorded them their own genera, considering that some Dipsastraea species are more distinct[51] .
Differences in morphology between Coelastrea and Dipsastraea
As mentioned, there are obvious morphological differences between the 2 former Goniastrea species and Dipsastraea species. Two synapomorphies were identified for Coelastrea: (1) fused (shared) walls or limited coenosteum, and (2) presence of regular free septa. These apomorphies are used to differentiate from closely-related genera, especially Dipsastraea and Trachyphyllia, though these characters are partially present amongst Goniastrea. The figure below shows the obvious difference between shared walls in Coelastrea and separate walls in Dipsastraea.
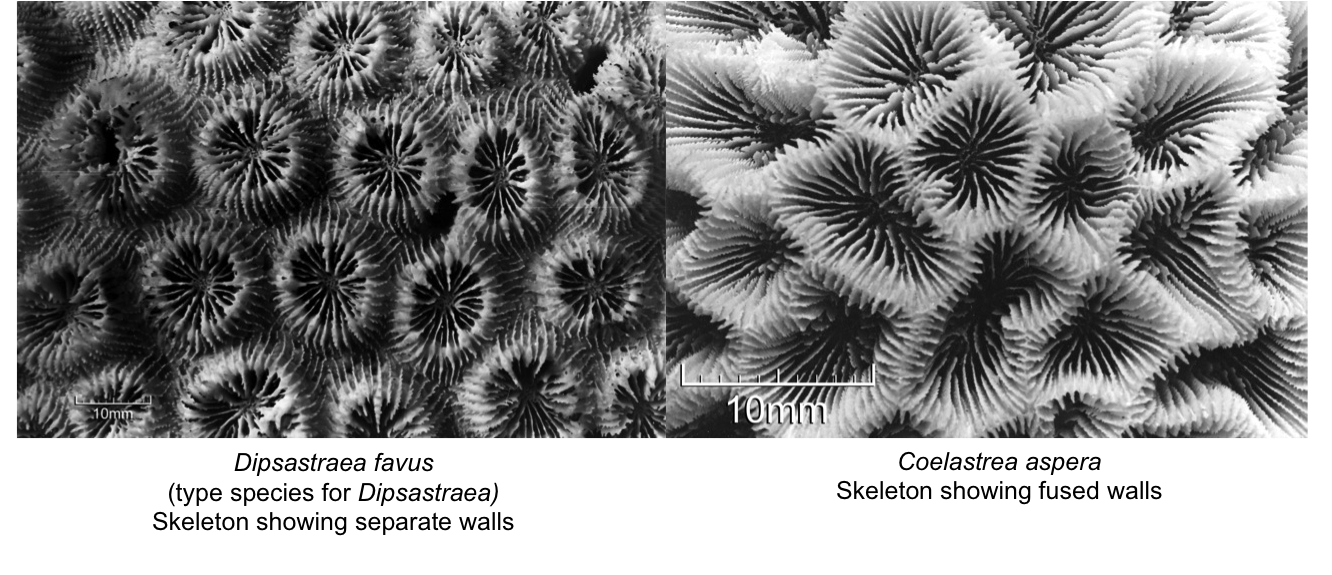
High support for the clade Coelastrea
The clade Coelastrea is well-supported on the morphology tree (bootstrap support = 86). Huang et al. resurrected Coelastrea and placed the G. aspera and G. palauensis in this genus as their macromorphological charaters matched almost all of Coelastrea tenuis (except the lack of columellae in C. tenuis).
5.6 A note on coral taxonomy and systematics
This section is largely based on Huang et al. (2011)[52] .
Traditional coral taxonomy has largely been based on morphology, but this is clearly problematic because of high intra-specific variation of skeletal structures - often influenced by varying environmental conditions. Variations can even occur within-colony: for example, the basal corallites of Pocillopora damicornis colonies are usually more similar to other Pocillopora species than their own peripheral corallites within the same colony. This begs the question of how reliable morphology is in coral taxonomy? Like most other fauna, molecular analyses have been becoming a popular tool in taxonomy. Fukami et al.[53] found that majority of the taxa are non-monophyletic - in particular, 11 out of 16 conventional families are polyphyletic, which is a severe problem for taxonomists.
This problem is apparent in the informal group 'Bigmessidae', which consists of Faviidae, Merulinidae, Pectiniidae and Trachyphyllidae. Based on morphological characters, many 'Bigmessidae' species display homoplasious characters; genetically, they are scattered amongst these four families. According to the molecular and morphology phylogenetic reconstructions by Huang et al. (2011), the authors found that several clades were well-supported by various optimality criterion (maximum likelihood, maximum parsimony and Bayesian likelihood) while the Neighbour-joining method produced a rather unresolved tree, which is expected. Generally, the neighbour-joining method is only used to make quick approximations, but should not be used for analyses because it only produces one tree, which may not be the most optimal. It also does not provide information about character states, making it meaningless. Between maximum likelihood and maximum parsimony, maximum likelihood is generally preferred over maximum parsimony because results are supported by statistical significance and maximum parsimony is known to have long-branch attraction problems. Nevertheless, both tend to work in tandem when bootstrap support values are high - though they do conflict each other when support values are low. In the case of Coelastrea, both maximum parsimony and maximum likelihood for the molecular and morphology tree show that the clade is well-supported, and hence little room for debate.
The example of Coelastrea aspera and C. palauensis, as well as Trachyphyllia geoffreyi, being accorded their own genus, rather than being lumped in Dipsastraea, is a clear example of the insufficiencies of molecular phylogeny. Despite the controversies of using morphology in coral taxonomy, they are clearly still important in grouping species into relevant and meaningful groups. Thus future taxonomy work on corals should consider both molecular and morphology in phylogeny work.
Are these relationships meaningful?
At this point, despite the high support values for both molecular and morphology trees, I personally struggle to find the link in how, for example, the Coelastrea clade is more closely related to Dipsastraea and Trachyphyllia, compared to their former genus, Goniastrea. Intuitively, one would not group them together based because of their very dissimilar morphological characters. For example, Trachyphyllia geoffroyi is a free-living coral, which is very different from Dipasastraea and Coelastrea species! In contrast, C. aspera and C. palauensis share similar characters with Goniastrea - e.g. fused walls and regular free septa. Intuitively, these new-found relationships do not seem meaningful in terms of morphology because there is no intuitive "pattern recognition" that humans tend to lean towards in grouping things together. However, if the phylogeny based on the molecular tree could be explained based on other life history strategies, such as reproductive biology and breeding systems, or even cytogenetic and biochemical characteristics, perhaps these relationships would be meaningful.
6. Additional Information
To World Register of Marine SpeciesTo Corals of the World
To Coral Trait Database
To Coral Finder 3.0
7. References
- ^ Myers, P., R. Espinosa, C. S. Parr, T. Jones, G. S. Hammond, and T. A. Dewey. 2017. The Animal Diversity Web (online). Accessed from <http://animaldiversity.org> on 12 November 2017
- ^ Tan, R., 2008. Honey-comb hexagonal corals. WildSingapore. Accessed from <http://www.wildsingapore.com/wildfacts/cnidaria/coralhard/faviidae/hexa/hexahoneycomb.htm> on 12 November 2017
- ^ Huang, D., F. Benzoni, H. Fukami, N. Knowlton, N. D. Smith, A. F. Budd, 2014. Taxonomic classification of the reef coral families Merulinidae, Montastraeidae, and Diploastraeidae (Cnidaria: Anthozoa: Scleractinia). Zoological Journal of the Linnean Society, 171: 277-355.
- ^ Ng, C. S. L., D. Chen & L. M. Chou, 2012. Hard coral assemblages on seawalls in Singapore. Contributions to Marine Science, 2012: 75-79.
- ^ Brown, B. E., C. A. Downs, R. P. Dunne, R. P. & S. W. Gibb, 2002. Exploring the basis of thermotolerance in the reef coral Goniastrea aspera. Marine Ecology Progress Series, 242: 119-129.
- ^ DeVantier, L., Hodgson, G., Huang, D., Johan, O., Licuanan, A., Obura, D.O., Sheppard, C., Syahrir, M. & Turak, E. 2014. Goniastrea aspera. The IUCN Red List of Threatened Species 2014: e.T133452A54263428. Accessed from <http://dx.doi.org/10.2305/IUCN.UK.2014-1.RLTS.T133452A54263428.en> Downloaded on 24 October 2017.
- ^ DeVantier, L., Hodgson, G., Huang, D., Johan, O., Licuanan, A., Obura, D.O., Sheppard, C., Syahrir, M. & Turak, E. 2014. Goniastrea aspera. The IUCN Red List of Threatened Species 2014: e.T133452A54263428. Accessed from <http://dx.doi.org/10.2305/IUCN.UK.2014-1.RLTS.T133452A54263428.en> Downloaded on 24 October 2017.
- ^ Australian Institute of Marine Science (AIMS), 2011. Accessed from <https://www.aims.gov.au/>
- ^ Australian Institute of Marine Science (AIMS), 2011. Goniastrea aspera. Accessed from <http://coral.aims.gov.au/factsheet.jsp?speciesCode=0187>
- ^ Ocean Portal (2017). Zooxanthellae and Coral Bleaching. Accessed from <http://ocean.si.edu/slideshow/zooxanthellae-and-coral-bleaching> on 20 November 2017
- ^ Woolridge, S. A., 2013. Breakdown of the coral-algae symbiosis: towards formalising a linkage between warm-water bleaching thresholds and the growth rate of the intracellular zooxanthellae. Biogeosciences, 10: 1647-1658.
- ^ Buchheim, J., n.d.. Coral reef bleaching. Odyssey Expeditions: Marine Biology Learning Center Publications. Assessed from <http://www.marinebiology.org/coralbleaching.htm> on 20 November 2017
- ^ Quan-Young, L. I., J. Espinoza-Avalos, 2006. Reduction of zooxanthellae density, chlorophyll a concentration, and tissue thickness of the coral Montastraea faveolata (Scleractinia) when competing with mixed turf algae. Limnology Oceanography, 51: 1159-1166.
- ^ Brown, B. E., J. C. Bythell, 2005. Perspectives on mucus secretion in reef corals. Marine Ecology Progress Series, 296: 291-309.
- ^ Brown, B. E., J. C. Bythell, 2005. Perspectives on mucus secretion in reef corals. Marine Ecology Progress Series, 296: 291-309.
- ^ Australian Institute of Marine Science (AIMS), 2011. Colony Formation. Accessed from <http://coral.aims.gov.au/info/reproduction-sexual.jsp> on 12 November 2017
- ^ Australian Institute of Marine Science (AIMS), 2011. Sexual reproduction. Accessed from <http://coral.aims.gov.au/info/reproduction-sexual.jsp> on 12 November 2017
- ^ Harrington, L., K. Fabricuis, G. Ath, A. Negri, 2004. Recognition and selection of settlement substrata determine post-settlement survival in corals. Ecology, 85(12): 3428-3437.
- ^ Babcock, R.C. 1984. Reproduction and distribution of two species of Goniastrea (Scleractinia) from the Great Barrier Reef Province. Coral Reefs, 2: 187-195.
- ^ Babcock, R.C. 1984. Reproduction and distribution of two species of Goniastrea (Scleractinia) from the Great Barrier Reef Province. Coral Reefs, 2: 187-195.
- ^ Nozawa, Y., P. L. Harrison, 2005. Temporal settlement patterns of larvae of the broadcast spawning reef coral Favites chinensis and the broadcast spawning and brooding reef coral Goniastrea aspera from Okinawa, Japan. Coral Reefs, 24: 274-282.
- ^ Babcock, R.C. 1984. Reproduction and distribution of two species of Goniastrea (Scleractinia) from the Great Barrier Reef Province. Coral Reefs, 2: 187-195.
- ^ Babcock, R.C. 1984. Reproduction and distribution of two species of Goniastrea (Scleractinia) from the Great Barrier Reef Province. Coral Reefs, 2: 187-195.
- ^ Babcock, R.C. 1984. Reproduction and distribution of two species of Goniastrea (Scleractinia) from the Great Barrier Reef Province. Coral Reefs, 2: 187-195.
- ^ Babcock, R.C. 1984. Reproduction and distribution of two species of Goniastrea (Scleractinia) from the Great Barrier Reef Province. Coral Reefs, 2: 187-195.
- ^ Babcock, R.C. 1984. Reproduction and distribution of two species of Goniastrea (Scleractinia) from the Great Barrier Reef Province. Coral Reefs, 2: 187-195.
- ^ Tasoff, H. March 30, 2017. The fight to save coral. Scienceline. Accessed from <http://scienceline.org/2017/03/fight-save-coral/> on 29 November 2017
- ^ Forsman, Z. H., C. A. Page, R. J. Toonen, D. Vaughan, 2015. Growing coral larger and faster: micro-colony-fusion as a strategy for accelerating coral cover. PeerJ, DOI 10.7717/peerj.1313.
- ^ DeVantier, L., Hodgson, G., Huang, D., Johan, O., Licuanan, A., Obura, D.O., Sheppard, C., Syahrir, M. & Turak, E. 2014. Goniastrea aspera. The IUCN Red List of Threatened Species 2014: e.T133452A54263428. Accessed from <http://dx.doi.org/10.2305/IUCN.UK.2014-1.RLTS.T133452A54263428.en> on 24 October 2017
- ^ National Oceanic and Atmospheric Administration (NOAA). Anthropogenic threats to corals. Accessed from
<https://oceanservice.noaa.gov/education/kits/corals/coral09_humanthreats.html> on 29 November 2017 - ^ Brown, B. E., R. Dunne, A. J. Edwards, M. J. Sweet, N. Phongsuwan, 2014. Decadal environmental 'memory' in a reef coral? Marine Biology, 162(2): 479-483.
- ^ Brown, B. E., C. A. Downs, R. P. Dunne, R. P. & S. W. Gibb, 2002. Exploring the basis of thermotolerance in the reef coral Goniastrea aspera. Marine Ecology Progress Series, 242: 119-129.
- ^ Martin, D. A., B. E. Brown, 1996. Dynamics of solar bleaching in the intertidal reef coral Goniastrea aspera at KO Phuket, Thailand. Marine Ecology Progress Series, 136: 235-244
- ^ Brown, B. E., R. Dunne, M. Goodson, A. Douglas, 2002. Experience shapes the susceptibility of a reef coral to bleaching. Coral Reefs, 21(2): 119-126.
- ^ Brown, B. E., R. Dunne, M. E. Warner, I. Ambarsari, W. K. Fitt, S. W. Gibb, D. G. Cummings, 2000. Damage and recovery of Photosystem I1 during a manipulative field experiment on solar bleaching in the coral Goniastrea aspera. Marine Ecology Progress Series, 195: 117-124.
- ^ Brown, B. E., R. Dunne, A. J. Edwards, M. J. Sweet, N. Phongsuwan, 2014. Decadal environmental 'memory' in a reef coral? Marine Biology, 162(2): 479-483.
- ^ Martin, D. A., B. E. Brown, 1996. Dynamics of solar bleaching in the intertidal reef coral Goniastrea aspera at KO Phuket, Thailand. Marine Ecology Progress Series, 136: 235-244
- ^ Brown, B. E., C. A. Downs, R. P. Dunne, R. P. & S. W. Gibb, 2002. Exploring the basis of thermotolerance in the reef coral Goniastrea aspera. Marine Ecology Progress Series, 242: 119-129.
- ^ Brown, B. E., R. Dunne, A. J. Edwards, M. J. Sweet, N. Phongsuwan, 2014. Decadal environmental 'memory' in a reef coral? Marine Biology, 162(2): 479-483.
- ^ Huang, D., 2009. An inventory of zooxanthellae Scleractinian corals in Singapore, including 33 new records. The Raffles Bulletin of Zoology 2009, 22: 69-80.
- ^ Ng, C. S. L, T. C. Toh, L. M. Chou, 2013. Current status of Coral Reef Restoration in Singapore. The Asian Conference on Sustainability, Energy & the Environment 2013. Pg. 546-558.
- ^ Ng, C. S. L, T. C. Toh, L. M. Chou, 2013. Current status of Coral Reef Restoration in Singapore. The Asian Conference on Sustainability, Energy & the Environment 2013. Pg. 546-558.
- ^ Australian Institute of Marine Science (AIMS), 2011. Goniastrea aspera. Accessed from <http://coral.aims.gov.au/factsheet.jsp?speciesCode=0187> on 20 November 2017
- ^ Australian Institute of Marine Science (AIMS), 2011. Accessed from <https://www.aims.gov.au/>
- ^ Hoeksema, B. (2014). Coelastrea aspera (Verrill, 1866). Accessed from World Register of Marine Species at <http://www.marinespecies.org/aphia.php?p=taxdetails&id=762427> on 14 November 2017
- ^ Verrill, A. E. 1866. Synopsis of the polyps and corals of the North Pacific Exploring Expedition, under Commodore C. Ringgold and Captain John Rodgers, U.S.N., from 1853 to 1856. Collected by Dr. Wm. Stimpson, naturalist to the expedition. With descriptions of some additional species from the west coast of North America. Proceedings of the Essex Institute, 5: 17–50. Accessed from <https://www.biodiversitylibrary.org/item/103663#page/270/mode/1up>
- ^ Huang, D., F. Benzoni, H. Fukami, N. Knowlton, N. D. Smith, A. F. Budd, 2014. Taxonomic classification of the reef coral families Merulinidae, Montastraeidae, and Diploastraeidae (Cnidaria: Anthozoa: Scleractinia). Zoological Journal of the Linnean Society, 171: 277-355.
- ^ Hoeksema, B. (2014). Coelastrea aspera (Verrill, 1866). Accessed from World Register of Marine Species at <http://www.marinespecies.org/aphia.php?p=taxdetails&id=762427> on 14 November 2017 on 2017-11-14
- ^ Huang, D., F. Benzoni, H. Fukami, N. Knowlton, N. D. Smith, A. F. Budd, 2014. Taxonomic classification of the reef coral families Merulinidae, Montastraeidae, and Diploastraeidae (Cnidaria: Anthozoa: Scleractinia). Zoological Journal of the Linnean Society, 171: 277-355.
- ^ Huang, D., F. Benzoni, H. Fukami, N. Knowlton, N. D. Smith, A. F. Budd, 2014. Taxonomic classification of the reef coral families Merulinidae, Montastraeidae, and Diploastraeidae (Cnidaria: Anthozoa: Scleractinia). Zoological Journal of the Linnean Society, 171: 277-355.
- ^ Arrigoni, R., F. Stefani, M. Pichon, P. Galli, F. Benzoni, 2012. Molecular phylogeny of the Robust clade (Faviidae, Mussidae, Merulinidae, and Pectiniidae): An Indian Ocean perspective. Molecular Phylogenetics and Evolution, 65: 183.193.
- ^ Huang, D., W. Y. Licuanan, A. H. Baird, H. Fukami, 2011. Cleaning up the ‘Bigmessidae’: Molecular phylogeny of scleractinian corals from Faviidae, Merulinidae, Pectiniidae and Trachyphylliidae. BMC Evolutionary Biology, 11: 37.
- ^ Fukami, H., C. A. Chen, A. F. Budd, A. Collins, C. C. Wallace, Y. Y. Wallace, C. F. Dai, K. Iwao, C. R. C. Sheppard, N. Knowlton, 2008. Mitochondrial and nuclear genes suggest that stony corals are monophyletic but most families of stony corals are not (Order Scleractinia, Class Anthozoa, Phylum Cnidaria). PLoS ONE, 3: e3222.