Coenobita cavipes Stimpson, 1858
 |
| Figure 1(Left): Brown Land Hermit Crab (C. cavipes) wearing a sinistral land snail shell from Jakarta, Indonesia. Note that the shell is not part of the hermit crab. Source: Wikimedia Creative Commons; by HappyCrabbie, available for use by public domain. Figure 2 (right): A photo of Coenobita cavipes without a shell. Source: Scary Israel. |
1. WHERE CAN I FIND THIS SPECIES OF HERMIT CRAB?
Table of Contents
1.1 Local Distribution
Coenobita cavipes cannot be found on the mainland of Singapore. These hermit crabs were thought to found in Pulau Ubin and probably Pulau Tekong, as well as the Southern islands of Singapore [1]. In 2016, a new species called Coenobita lila was discovered in Singapore because it was misidentified as C. cavipes. Therefore, there has been a concern on whether what has been called "C. cavipes" from Singapore and adjacent areas are all C. lila [2]. It is nevertheless interesting to find out about C. cavipes and their unique features which distinguish themselves from other Coenobitoid species.
C. cavipes are generally found in shores and sheltered lagoons, where they have access to both land and water. They are also found in mangroves where they use trees as shelter [3] or at mangroves where there are sandier substrates. C. cavipes, just like other land hermit crabs, reproduce and spend their early stages in water. Even though C. cavipes is a land hermit crab, it has to enter the sea or brackish water regularly to replenish the water in their shells, It requires regular access to water with high salinity, which binds it to the shore.
 |
| Hermit crabs on a rocky shore. By: Steve Simonsen (permission pending) |
1.2 Global Distribution
Globally, they can be found in the eastern part of Africa, China, Japan, Taiwan, Phillippines and Malaysia, the Indo-Pacific region.2. Facts
2.1 Name
Binomial name:Coenobita cavipes
Vernacular name:
オカヤドカリ (in Japanese)
Etymology:
Its vernacular name in Japanese means a hermit crab living on land, a natural treasure in Ogasawara archipelago.
 |
| Hermit crab peeping. GIF Powered By tenor. (Permission granted) |
Coenobita cavipes are Decapods under the family Coenobitidae and are considered as land hermit crabs. The name 'hermit crab' is derived from human hermits, because human hermits are always hiding from others, especially when faced with problems. This is similar to the hermit crabs, as they are always carrying gastropod shells wherever they go. Yet, they are different from the human hermits as the shells that they carry are essential for their survival. Gastropod shells are required for hermit crabs because only the first half of the crab is covered with hard exoskeleton, leaving their soft abdomen vulnerable to predators. Therefore, gastropod shells are always required to protect the hermit crabs.
Land hermit crabs live close to the shoreline and must have access to both land and water [4]. Unlike aquatic hermit crabs, an adult land hermit crab will die if completely submerged in water for long periods of time. It was thought to have three species of land hermit crabs in the offshore islands of Singapore, and are not easily distinguishable in the field [5]. However, the number of Coenobita species that can be found in Singapore is yet to be confirmed as more studies are required.
3. Characteristics
3.1 Anatomy
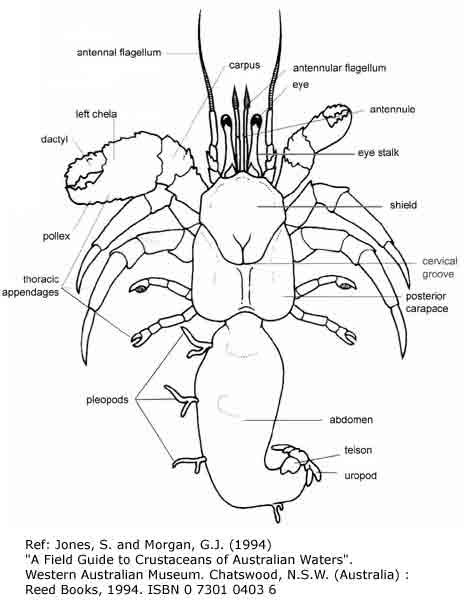 |
| Figure 3: Anatomy of a land hermit crab. Source: Biology of Land Hermit Crabs. Cambridge University Press, 1988. |
Abdomen: A hermit crab does not have exoskeleton on its abdomen. This makes it vulnerable, which is why it has to cover its abdomen with a shell.
Antennae: The antennae of a hermit crab allows it to sense information from its surroundings.
Cephalothorax: It is the part where the head and thorax fused together. The cephalothorax is covered with a hard exoskeleton.
Exoskeleton: It is made up of layers of protein-chitin, making the exoskeleton hard and therefore protecting the hermit crab from predators.
Eyes: A hermit crab's eyes are attached to eyestalks, which can move.
Gills: A hermit crab has gills that are attached to the branchial chamber. Its gills have to remain moist. This is because they take in oxygen either through water or moisture in the air.
Hair: The crab has hairs between the joint of their legs. They are actually extensions of the exoskeleton and not true hairs for sensory.
Legs: A hermit crab has five pairs of legs with different functions. The first pair has large claws for climbing and fighting. The second pair is used for walking and detecting food. The third pair is used for walking and the fourth and fifth pair are used to hold itself inside the shell.
Maxillipeds: Maxillipeds are found around the crab's mouth, which is used to hold and tear food, as well as for grooming.
Pleopods: Small appendages that are found on the left side of the abdomen, where females can attach their eggs on to small hairs on pleopods.
Uropods: They are used for hermit crabs to hook onto the shell and help hermit crabs to grip the shell.
3.2 Morphology
Coenobita cavipes are easily confused with other Coenobita species. Hence, it is very important to know their differences and pay more attention to their distinguishing features. The two other species that are often confused with C. cavipes are C, lila and C. violascens.
| Figure 4: A–C, Coenobita lila n. sp. A, holotype male (sl 16.7 mm) (ZRC); B, paratype ovigerous female (sl 10.8 mm) (ZRC); C, female (sl 11.8 mm) (ZRC); D–F, C. cavipes Stimpson, 1858, Houwan, Kenting, Pingtung, Taiwan (NCHUZOOL 13637). D, female (sl 13.8 mm); E, female (sl 13.5 mm); F, female (sl 14.9 mm). By: Rahayu, Shih & Ng, 2016. |
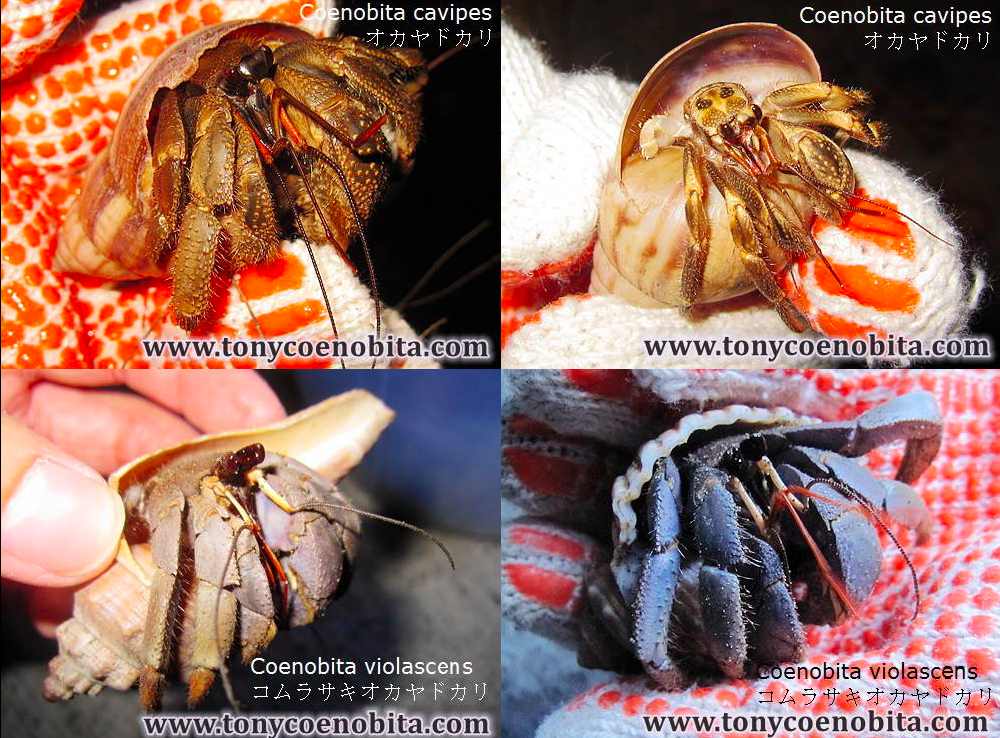 |
| Figure 5: Images of C. cavipes & C. violescens. The main distinguishable feature is their colour. C. cavipes is usually brown whereas C. violescens is violet. By: Tony, 2003 (permission pending). |
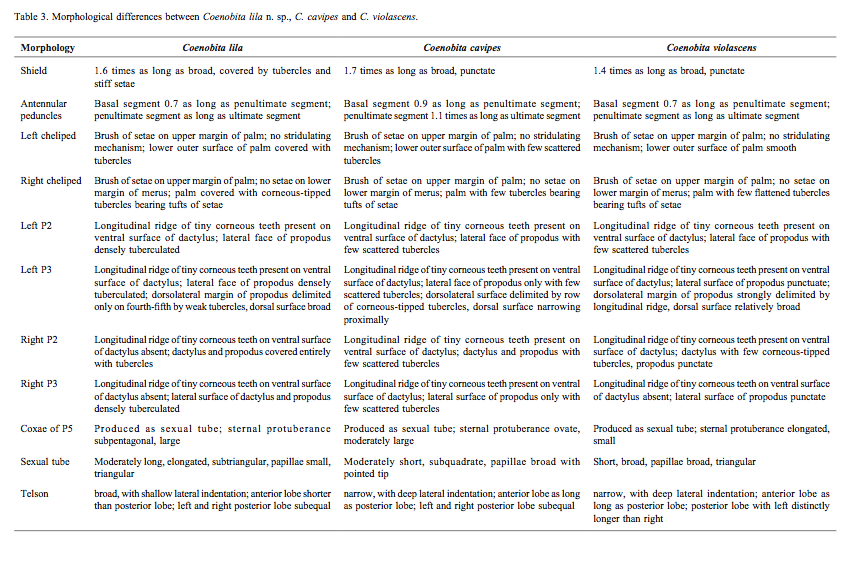 |
| Figure 6: Table of the morphological differences between C. cavipes, C. lila and C. violescens. By: Rahayu, Shih & Ng, 2016. |
Colour & eyes
Coenobita cavipes are usually mottled-brown or blue-grey. Body is generally brown, with lighter colour at the pincer. Their eyes are elongated and the stalks are black on adults and comma-shaped. The bottom section of the second antenna is orange [3]. The colour of the body, as well as the orange antenna, are the distinguishing features between C. cavipes and other Coenobita species.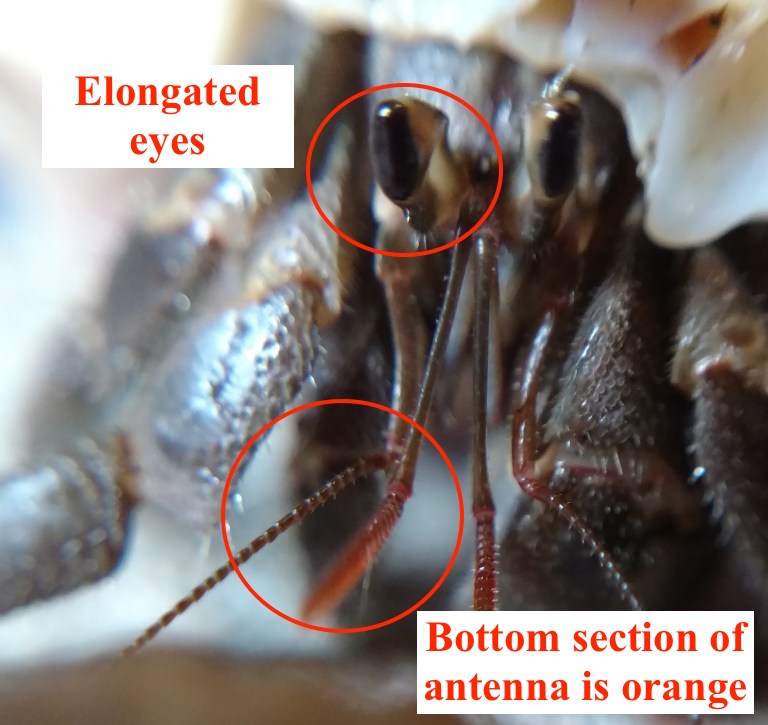 |
| Figure 7: Frontal view of C. cavipes. Adapted from: Sean Caroll, The Crab Street Journal 2017 (Permission granted). |
Abdomen, legs & claws
A hermit crab has to press its abdomen, fourth and fifth pairs of legs as well as uropods against the inner wall of the shell [4]. They have large left claws (A), usually for defence and for tree climbing. Their smaller right claws (B) and the rest of the appendages are for collecting and passing food and water to the mouth [4].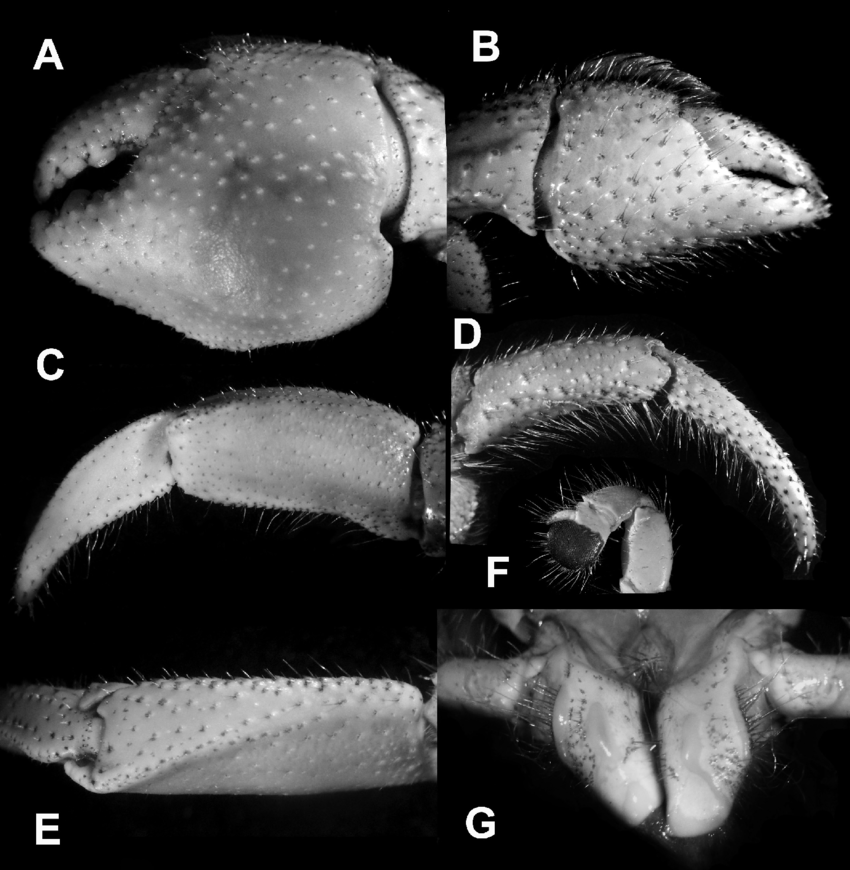 |
| Figure 8: C. cavipes. A, left cheliped (claw), outer view; B, right cheliped, outer view; C, dactylus and propodus of left P3, lateral view; D, dactylus and propodus of right P3, lateral view; E, dorsal surface of propodus of left P3; F, left P4, lateral view; G, sternite and coxae of male P5. By: Rahayu, Shih & Ng, 2016. |
Gills
Land hermit crabs have reduced gills and their moist gill chambers have highly vascularized gill chambers for gas exchange [4]. Its gill chambers are large and hold water to keep the gill filaments [5]. |
| Figure 9: Image of the gills of C. cavipes. Adapted from: University of New South Wales. (Permission granted) |
Size
Hermit crabs vary in their sizes, depending on the availability of food and gastropod shells, as well as competition among populations [9]. Their sizes can range from a fraction of an inch (a few millimetres) to a maximum reported a size of 16.0mm[4]. |
| Figure 10: Relative size of a C. cavipes to a hand. By: Tony, 2003. (Permission pending) |
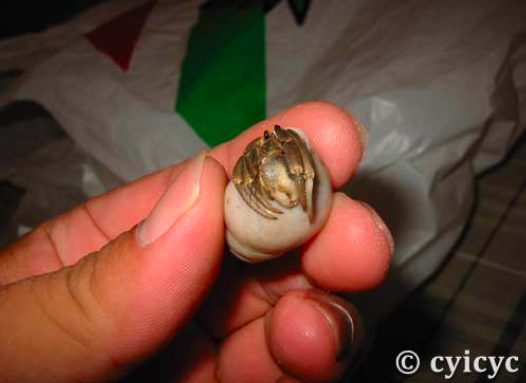 |
| Figure 11: Relative size of a C. cavipes. By: Tony, 2003. (Permission pending) |
C. cavipes usually find shells that are turbo-shaped [4].
 |
| Figure 12: Turbo-shaped shells that are commonly used by hermit crabs. Source: Wikimedia Creative Commons; By H. Zell, 'Turbo Imperalis', available for use by public domain. |
3.3 Feeding & Behaviour
Hermit crabs have a wide range of feeding mechanisms, including deposit-feeding, suspension-feeding, predation and scavenging [10]. Often, what these hermit crabs eat depends on the habitat that they live in. These hermit crabs are food for animals, such as larger crabs or birds and hence plays an important role in the shore ecosystem. C. cavipes are also seen cannibalizing other smaller C. cavipes and C. rogsus. They also feed on human faeces, bird droppings, dead fish, rotting vegetation etc.C. cavipes feed at low tide, where more animals are exposed when there is a low tide. In addition, they prefer to climb and seek shelter in mangrove trees during high tide. One reason for these hermit crabs to have a wide range of feeding mechanisms and habit is because they are more efficient in the olfactory food-detection system [8], hence enabling them to be more versatile in the search of food.
3.4 Reproduction & Life Cycle [9]
Reproduction is similar to other crustaceans, is through copulation. Mating rituals of hermit crabs can contain fighting over mates. It is noted that their characteristic shell plays a certain role in mating. During mating, both the males and females will partially remove their body from their shells to allow copulation to take place. The male releases spermatophores to the female and fertilizes the eggs. Once copulation is over, they return their bodies back to their shells.Although Coenotibids are fully terrestrial as adults, their eggs hatch at the sea [8][10][8]. Both eggs and larval undergo planktonic larval development in the sea. The mature hermit crabs then move to land. Their lifecycle is similar to marine hermit crabs [9].
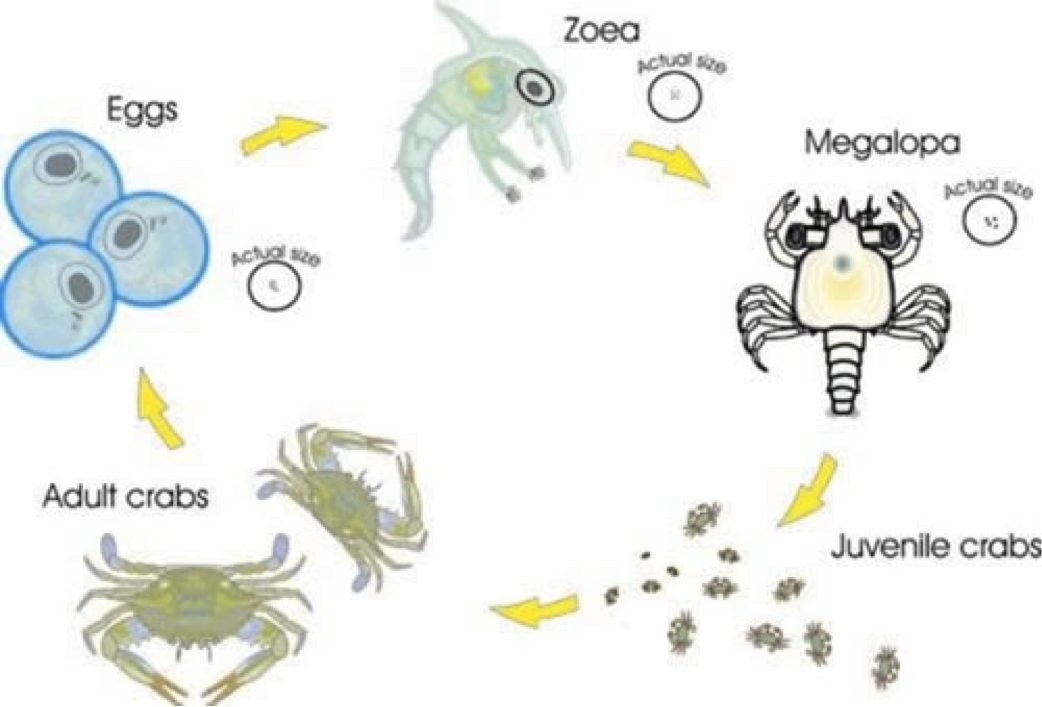 |
| Figure 13: Life cycle of a land hermit crab. By: Brodie & Harvey, 2001. |
Male land hermit crab mates with the female hermit crab and the eggs are laid and held on the pleopods.
The female hermit crab holds on the eggs until they mature (~1 month).
The female hermit crab goes down to the ocean during high tide and lays her eggs in the ocean.
These new hatchlings are called Zoea, and they float along the ocean as planktons.
Each zoea passes through 4-6 stages, each stage lasting for 40-60 days as it grows bigger.
The zoea eventually metamorphosized into megalopa, which looks like a combination of a crab and a lobster.
At the end of the megalopa stage, the land hermit crab will start looking for gastropod shell to hide.
The megalopa then buries itself in the sand and metamorphosized into a juvenile hermit crab. The metamorphosis changes the hermit crab's gills so they can no longer breathe in water.
The juvenile hermit crab will have to constantly moult to grow a larger exoskeleton, hence constantly finding new gastropod shells to fit its abdomen.
 |
| Figure 14: A female hermit crab with eggs. By: Alaska Hermit blog (permission pending). |
3.5 Moulting [9]
Hermit crabs moult because their exoskeleton does not grow with their body. When they moult, they are vulnerable and there have to dig into the sand to protect themselves. They hence have to wait for ten days or more for their new skin to harden up.Hermit crabs normally eat their old exoskeleton as it contains chitin, which helps their new exoskeleton to harden up. For moulting to happen, several conditions have to be fulfilled. It has to be dark, and with no other hermit crabs around which may be a threat. This is because the moulting hermit crab is vulnerable, and requires moisture and heat. If the conditions are just right, the hermit crab will secrete a moulting hormone and dig down to bury himself while moulting. However, if the conditions are not favourable, a different hormone will be secreted to delay moulting, but cannot delay for long. If the hermit crab cannot moult in time, or moult under stressful conditions, it will die.
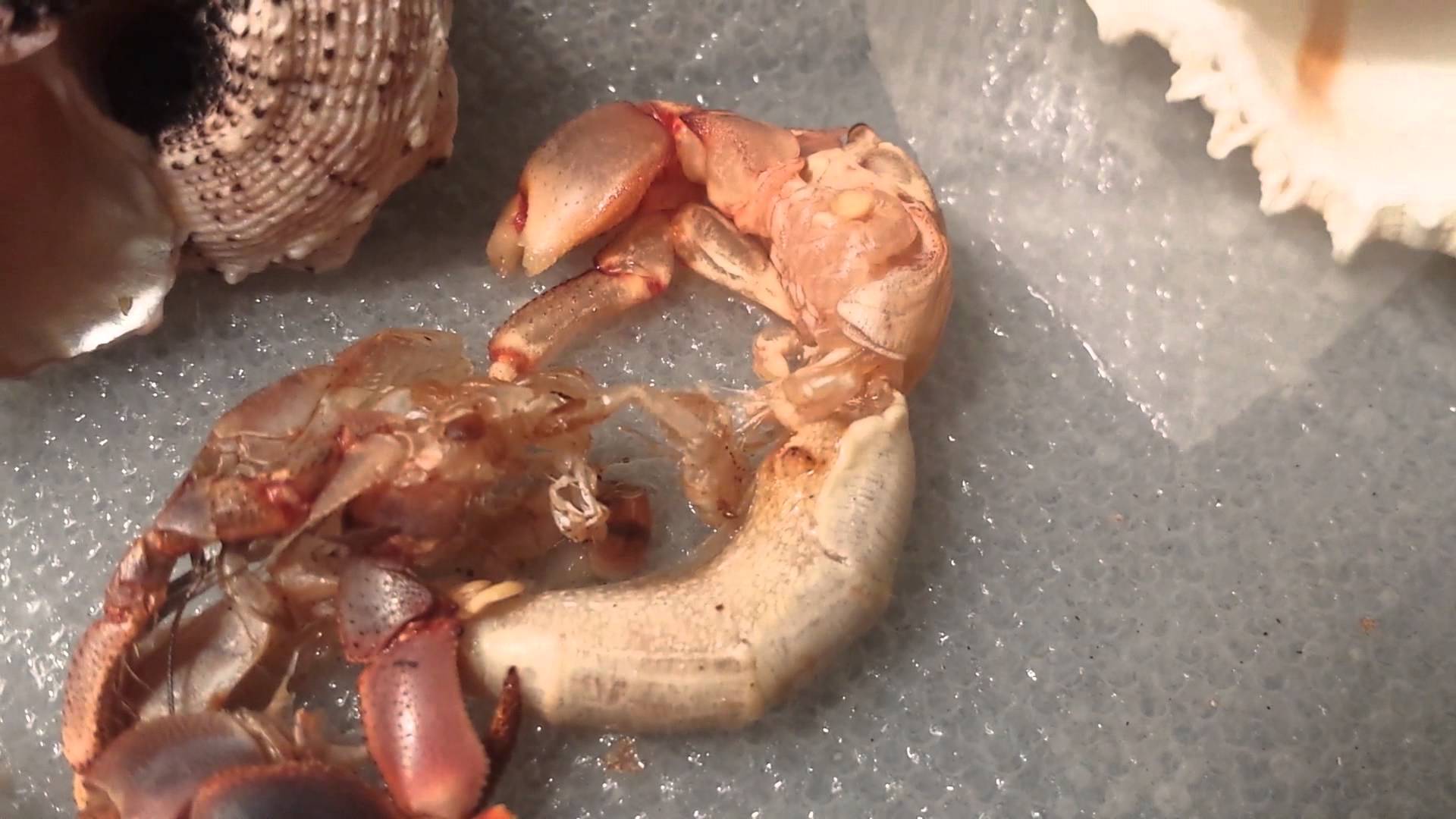 |
| Figure 15: Image of a moulting hermit crab. Source: YouTube video, by Plantexperts. |
4. Ecology
Coenobita cavipes Inhabitat the supralittoral zones of the beaches, and immediately adjacent vegetations or forests, where they forage for dead plant and animal matter. They are well adapted to terrestrial living and are able to breathe through the atmospheric air with their modified gill chambers.
Even though hermit crabs depend heavily on gastropod shells for protection and desiccation, it is very rare that they will procure shells from living gastropods [11] or dig up shells that are empty but buried [12]. It is therefore expected to have a heavy intraspecific competition for gastropod shells [13]. Therefore, such intense competition is usually the limiting factor in many hermit crab populations and assemblages [14].
Land hermit crabs such as C. cavipes are atypical, such that they live on land and not in the sea, yet very successful. They appear in numerous number and dominate the fringe of the environments where they occur [14]. They are also proven to be the most successful macrofauna in the terrestrial-marine interface as well as the most successful invaders [15].
Their success is probably due to the presence of their shells, which is not just for protection and water storage, but also for clustering, migration, and temporary refuge-seeking [16]. All these help in their shell exchanges, to obtain food, shells and water. In addition, they also help in preventing predators, conserving water and temperature [16].
5. Status & Threats
C. cavipes is not listed in IUCN.
Even so, their habitats are frequently disturbed as shores are easily accessible and regularly cleaned of any debris that washed up on the high tide mark. However, this is exactly the place where the land hermit crabs find shelter, food and new shells. Without their habitat, land hermit crabs such as C. cavipes will seldom be found on the beaches.
6. Aquarium trade
Of the approximately 16 terrestrial species of genus Coenobita in the world, C. cavipes is one of the species that is growing in popularity as pets [32]. As C. cavipes cannot be found on the mainland of Singapore, it is not traded as pets in Singapore. A video on several C. cavipes in captivity is shown below.
7. Importance of shells
The way hermit crab uses discarded gastropod shells is a type of symbiotic relationship - called commensalism. This is such that the hermit crab benefits from the gastropod shell, whereas the gastropod does not benefit or harm by the hermit crab.
The most important role of carrying a shell is to reduce evaporative water loss [16], as megalopae and adult land hermit crabs desiccate rapidly without a shell [17][18][19]. Desiccation is more rapid in smaller hermit crabs with higher surface-area-to-volume ratios as compared to larger hermit crabs [21]. In addition, gastropod shells also protect the hermit crabs from predation, and the shell-less condition is prone to cannibalism. Even though carrying shells are vital for the hermit crabs, they are energetically costly [22].
A hermit crab is most concern about the size of the opening of the shell [23]. When the crab is interested in the particular shell, it will stick its large claw into the new shell to check if the opening is the correct size. If the size is right, it will roll the shell over to empty any debris. When the hermit crab is happy, it will position its body in such a way to minimise the exposure of its abdomen when changing its shell. When other hermit crabs are around, it will attempt to hold on to its old shell and stick one of its foot into the new shell until it is happy with the new one to avoid 'buyers remorse.
The video below shows the intense competition of shells between the hermit crabs!
Shells of the hermit crabs are just like their homes. Shells of the hermit crabs are supposed to make the hermit crabs feel comfortable and safe. You can also do your part by designing the house of the hermit crabs for the hermit crab community! 3D printing is commonly used for human uses, but it can also be used to help the animal kingdom, such as the hermit crabs. You can start by designing 3D printed shells for the hermit crabs, where they can call 'home' and upload your artwork to Thingverse. Furthermore, shells of hermit crabs can also be designed for other purposes such as for cultural values by Japanese creator Aki Inomata:
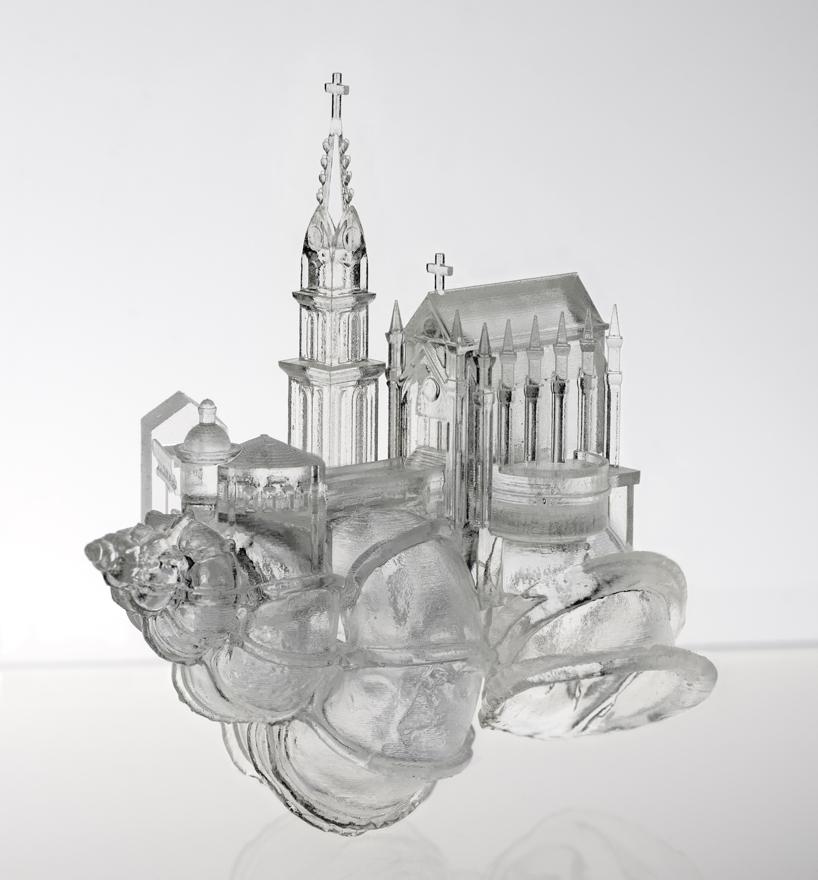 |
| Figure 16: Hermit crab shell creation using 3D printing. (C) AKI INOMATA in the courtesy of MAHO KUBOTA GALLERY (Permission granted). |
 |
| Figure 17: A hermit crab carrying a transparent plastic shell. (C) AKI INOMATA in the courtesy of MAHO KUBOTA GALLERY (permission granted). |
8. Taxonavigation
8.1 Synonyms [3]:
Cenobita cavipes Stimpson, 1858 (wrong spelling)Coenobita baltzeri Neumann, 1878 (junior synonym)
8.2 Type Specimen
Applied name: Coenobita baltzeri Neumann, 1878Accpeted name: Coenobita cavipes Stimpson, 1858
Collection data as follows :
- Basis of record: PRESERVED_SPECIMEN
- Collection code: Collection Crustacea
- Institution code: SMF
- Type status: Holotype
- Catalogue number: 19223
- Locality: Ostindien
8.3 Original description & History
The common land hermit crab C. cavipes was described from Loo Cho (Ryukyu Islands, Japan) [24]. This species was first confused with C. violascens Heller, 1862, probably because both species have purple or violet chelae, and lack laminar teeth on the upper part of the outer surface of their left palm (i.e., a stridulatory ridge) [28].8.4 Classification
Obtained from WoRMS| Kingdom |
Animalia |
| Phylum |
Arthropoda |
| Subphylum |
Crustacea |
| Superclass |
Multicrustacea |
| Class |
Malacostraca |
| Subclass |
Eumalacostraca |
| Superorder |
Eucarida |
| Order |
Decapoda |
| Suborder |
Pleocyemata |
| Infraorder |
Anomura |
| Superfamily |
Paguroidea |
| Family |
Coenobitidae |
| Genus |
Coenobita |
| Species |
Coenobita cavipes |
8.5 Phylogeny
The use of protein-coding genes:
The study done by Tsang et. al (2008) has applied two nuclear protein-coding genes, phosphoenolpyruvate carboxykinase (PEPCK) and sodium-potassium ATPase alpha-subunit (NaK) for decapod phylogenetics. It was hypothesized that the use of protein-coding genes to construct the phylogenetic tree of Decapoda has less controversy. The best-fit model was selected using Modeltest was GTR+I+G for Maximum likelihood (ML) analysis; the same model was used using Bayesian interference (BI). Only the support values (the thicker the line, the higher the support value and grey lines have low support values) of ML and BI are shown in the ML tree below. Results have shown that Anomura and Brachyura from Meiura with intermediate support. It is beyond the scope of the present study to reconstruct a detailed phylogeny within Brachyura or Anomura, but their sister relationship corroborates the Meiura concept [25].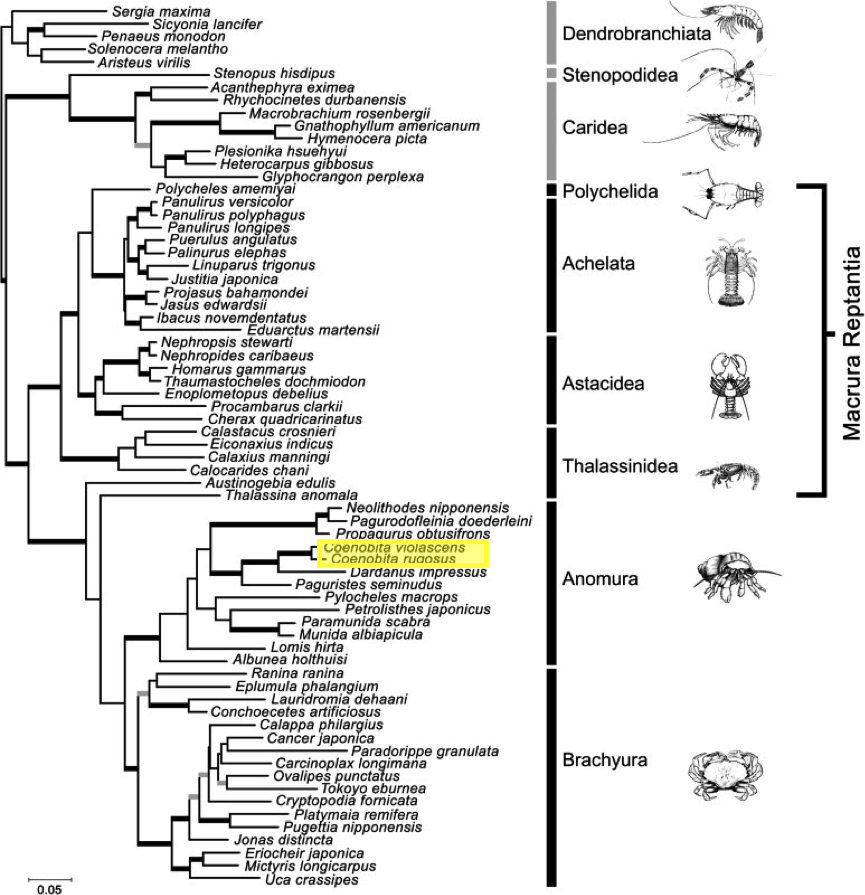 |
| Figure 18: Maximum likelihood tree from combined PEPCK and NaK analysis under the best-fitting model GTR+I+G. The branches strongly supported by both ML (BP ⩾ 70) and BI (PP ⩾ 0.95) are indicated by thick black lines; branches only strongly supported by one of the analyses are indicated with thick grey line. Adapted from: Tsang et al., 2008. |
The use of protein-coding genes is more reliable because the use of mitochondrial DNA markers has led to rapid substitution saturation which limits their utility in resolving deep nodes,
whereas the use of nuclear DNA markers has suffered from alignment ambiguities. These disadvantages can complicate analysis and hamper accurate recovery of phylogenetic signal. Consequently, nuclear protein-coding genes could serve as an excellent new source of information. These genes are easy to align, with many potential candidates in the genome [26]. Hence, this phylogenetic tree has shown that nuclear protein-coding genes, like the two genes we have used here, should become core markers for future phylogenetic studies of decapods, especially for reconstructing deep nodes. More markers should also be explored for their phylogenetic potential. In combination with the available mtDNA and nuclear ribosomal DNA markers, the use of the protein-coding genes could lead us closer to the goal of reconstructing the tree of life of Decapoda.
The above phylogenetic tree on Decapoda is supposed to be the most accurate and hence should be used, because of several controversies on the phylogenetics of Decapoda.
The extraordinary morphological diversity of Decapods has posed challenges to their phylogenetic study, and many taxonomic schemes and phylogenetic hypotheses have been proposed for Decapoda [24]. This is probably because morphological cladistic analyses are impeded by the sheer diversity of forms and limited available character sets, yet comprehensive molecular phylogenetic studies of high-level relationships were not available until recently. In several cases, even though the same data sets were used, different results were obtained. It can be seen in the figure below, where the clade 'Anomala' were placed at different positions despite the same data set where mitochondrial 16S, nuclear 18S and 28S were used.
.
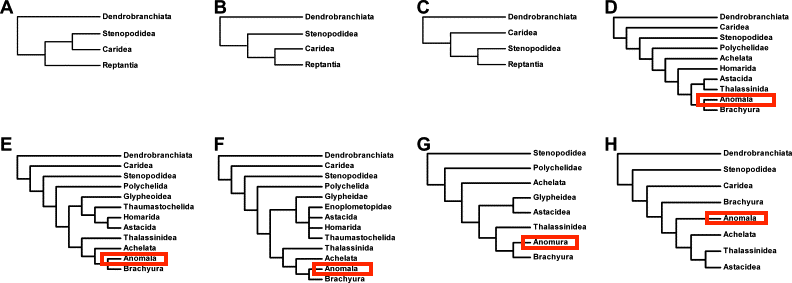 |
| Figure 19: Hypotheses of phylogenetic relationships among Decapoda lineages. (A–C) The relationships of Reptantia relative to natant lineages based on morphology; (D–F) relationships among reptant lineages based on morphological cladistic analyses; (G–H) phylogenetic hypotheses of decapod infraorders based on molecular data. Adapted from: Hamasaki et al., 2017 |
Yet, because of its maternal inheritance, the absence of recombination and fast evolution, mitochondrial DNA (mtDNA) has been intensively used to study population structure, phylogeography and phylogenetic relationships at various taxonomic levels [25].
In the paper by Hamasaki et al. (2017), the phylogenetic relationships amongst various coenobitid species were analysed based on cytochrome c oxidase I (COI) and 16S sequence data. Their findings could provide information to determine evolutionary significant unit. For phylogenetic analysis, the COI (573 bp) and 16S (311–312 bp) gene sequences of each species were aligned in CLUSTALW [26]. The intra-specific and inter-specific pairwise nucleotide divergences of the COI and 16S sequences were calculated using the Kimura two-parameter model (K2P) [27] in MEGA.
COI enzyme catalyzes oxygen to water and therefore is an essential enzyme for aerobic metabolism. However, 16S rDNA gene is less variable than COI gene and therefore valuable for reconstructing phylogenetic relationship. The K2P genetic distances were smallest amongst Coenobita species at 2.9–7.3% (Fig. 20) and the neighbour-joining (NJ) and maximum likelihood (ML) trees based on the COI and16S replicates under the best-fit substitution model.
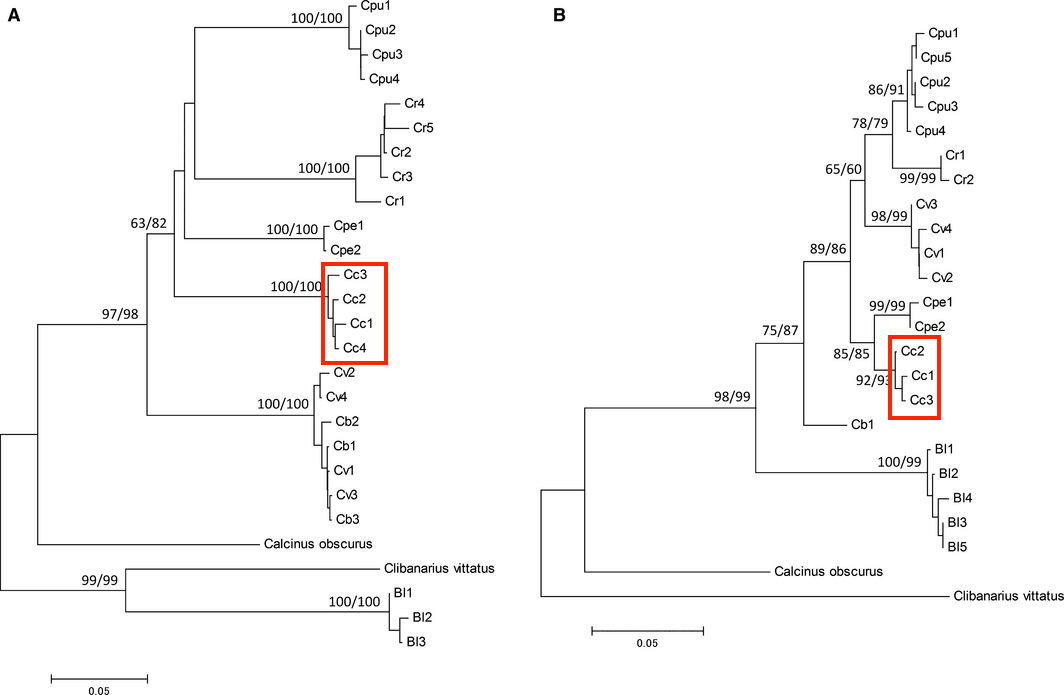 |
| Figure 20: Neighbour-joining tree of phylogenetic relationships among coenotibid crabs based on mitochondrial DNA cytochrome c oxidase I (A) and 16S (B) halotypes: Coenobita brevimanus (Cb), Coenobita cavipes (Cc), Coenobita perlatus (Cpe), Coenobita purpureus(Cpu), Coenobita rugosus (Cr), Coenobita violascens (Cv) and Birgus latro (Bl). Bootstrap supports (>50%) of neighbour-joining and maximum likelihood trees are shown on main nodes. Adapted from: Hamasaki et al., 2017. |
The use of two different genes has yielded different results, and the use of COI has a lower support as compared to the use of 16S rDNA gene. This is because the 16S rDNA gene, which is a structural, non-coding gene, and is less variable than the COI gene and hence appears to be more valuable for reconstructing the phylogenetic relationships amongst coenobitid crabs. Even though 16S rDNA gene appears to be a more reliable marker, COI can still be used as it catalyses the reduction of oxygen to water and therefore is an essential enzyme for aerobic metabolism, and hence can also be used.
9. References
[1] Davison G.W.H., Ng P.K.L. & Ho H.C. (2008). The Singapore Red Data Book (2nd Edition). Singapore: Nature Society (Singapore). pp. 285.
[2] Lim K.K.P, Murphy D.H, Morgany T., Sivasothi N., Ng P.K.L., Soong B.C., Tan H.T.W., Tan K.S. & Tan T.K. (2001). A Guide to Mangroves of Singapore. Raffles Museum of Biodiversity Research, Volumes 1&2.
[3] McLaughlin P.A., Komai T., Lemaitre R. & Rahayu D.L. (2010). Annotated checklist of anomuran decapod crustaceans of the world (exclusive of the Kiwoaidea and families Chirostylidae and Galatheidae of the Galatheoidea. Zootaxa, 23: 5-107.
[4] Anonymous (no date). Land Hermit Crab. Smithsonian's National Zoo & Conservation Biology Institute.
[5] Rahayu D.L., Shih H.-T. & NgP. K. L. (2016). A new species of land hermit crab in the genus Coenobita Latreille, 1829 from Singapore, Malaysia and Indonesia, previously confused with C. cavipes Stimpson, 1858 (Crustacea: Decapoda: Anomura: Coenobitidae). The Raffles Bulletin of Zoology, 34: 470-488.
[6] Hsu C.-H & Soong K. (2017). Mechanisms causing size differences of the land hermit crab Coenobita rugosus among eco-islands in Southern Taiwan.
PLoS ONE12(4): e0174319. https://doi.org/10.1371/journal.pone.0174319.
[7] KUNZE, J., and ANDERSON, D. T., 1979, Functional morphology of the mouthparts and gastric mill in the hermit crabs Clibanarius taeniatus (Milne Edwards), Clibanarius virescens (Krauss), Paguristes squamosus McCulloch and Dardanus setifer (Milne Edwards) (Anonmra: Paguridae). Australian Journal of Marine and Freshwater Research, 30, 683-722.
[8] Hartnoll R.G. (1988). Evolution, systematic, and geographical distribution. Biology of the Land Crabs, pp 6–54. Cambridge University Press, New York.
[9] Brodie R. & Harvey A.W. (2001). Larval development of the land hermit crab Coenobita compressus H. Milne Edwards reared in the laboratory. Journal of Crustacean Biology, 21(3): 715-732.
[10] Schiller C.D., Fielder R., Brown I.W., & Obed A. (1991). Reproduction, early life-history and recruitment. In: ACIAR Monograph 8. The Coconut Crab: Aspects of Birgus latro Biology and Ecology in Vanuatu. pp 13–35. Australian Centre for International Agricultural Research, Canberra, ACT.
[11] Nakasone Y. (2001). Reproductive biology of three land hermit crabs (Decapoda: Anomura: Coenobitidae) in Okinawa, Japan. Pacific Scientific, 55: 157–169.
[12] Hamasaki K., Kato S., Murakami Y., Dan S. & Kitada S. (2015b). Larval growth, development and duration in terrestrial hermit crabs. Sex. Early Development Aquatic Orginal, 1: 93–107.
[13] Rutherford J.D. (1997). Removal of living snails from their shells by a hermit crab. The Veliger 19: 438-439.
[14] Kellogg C.W. (1976). Gastropod shells: a potentially limiting resource for hermit crabs. Journal of Experimental Marine Biology and Ecology, 22: 101-111.
[15] Bach, C., Hazlett B. & Rittschof D. (1976). Effects of interspecific competition on fitness of the hermit crab Clibanarius tricolor. Ecology, 57: 579-586.
[16] Bertness M.D. (1980). Shell preference and utilization patterns in littoral hermit crabs of the Bay of Panama. Journal of Experimental Marine Biology and Ecology, 48: 1-16.
[17] Turra A. & Leite F.P.P. (2000). Clustering behavior of hermit crabs (Decapoda, Anomura) in an intertidal rocky shore at São Sebastião, Southeastern Brazil. Brazilian Journal of Biology, 60: 39- 44.
[18] Williams J.D. & McDermott J.J. (2004). Hermit crab biocoenoses: a worldwide review of the biodiversity and natural history of hermit crab associates. Journal of Experimental Marine Biology and Ecology, 305: 1-128.
[19] Greenaway P. (2003). Terrestrial adaptations in the Anomura (Crustacea: Decapoda). Memoirs Museum of Victoria, 60: 13–26.
[20] Herreid C.F. (1969). Water loss of crabs from different habitats. Comparative Biochemistry and Physiology, 28: 829–839.
[21] de Wilde P.A.W.J. (1973). On the ecology of Coenobita clypeatus in Curacao with reference to reproduction, water economy and osmoregulation in terrestrial hermit crabs. Studies on Fauna of Curacao and other Caribbean Island, 144: 1–138.
[22] Brodie R.J. (2005). Desiccation resistance in megalopae of the terrestrial hermit crab Coenobita compressus: water loss and the role of the shell. Invertebrate Biology, 124: 265–272.
[23] Osorno J.L., Contreras-Garduño J., & Macías-Garcia C. (2005). Long-term costs of using heavy shells in terrestrial hermit crabs (Coenobita compressus) and the limits of shell preference: an experimental study. Journal of Zoology, 266: 377–383.
[25] Anonymous (no date). Shell Selection. The Hermit Crab Patch. URL: http://www.hermitcrabpatch.com/Hermit-Crab-Shell-Selection-a/149.htm.
[26] Scholtz G. & Richter S. (1995). Phylogenetic systematics of the reptantian Decapoda (Crustacea, Malacostraca). Zoological Journal of the Linnean Society, 113: 289-328.
[27] Porter M.L. & Perez-Losada M. & Crandall K.A. (2005). Model-based multi-locus estimation of decapod phylogeny and divergence times. Molecular Genetics and Evolution, 37: 355-369.
[28] Avise J.C. (2000) Phylogeography, pp. 447. Harvard University Press, London.
[29] Thompson J.D., Higgins D.G., Gibson T.J. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research, 22: 4673–4680.
[30] Kimura M. (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution, 16:111–120.
[31] Nakasone Y. (1988) Land hermit crabs from the Ryukyus, Japan, with a description of a new species from the Philippines (Crustacea, Decapoda, Coenobitidae). Zoological Science, 5: 165–178.
[32] Senckenberg (2010). Collection Crustacea SMF. Occurrence Dataset https://doi.org/10.15468/mc7ysi accessed via GBIF.org on 2017-11-11. https://www.gbif.org/occurrence/207795822.