Draco melanopogan (Boulenger, 1887)
 |
| Photograph taken at Lower Peirce Forest, Singapore. (Image courtesy www.wildsingapore.per.sg) |

Introduction | Interesting Facts! | Biology | Distribution | Status & Threats | More on the Patagia | Evolution of Sexual Dimorphism | Phylogeny | Taxonavigation | Useful Links! | References and Literature
Introduction
 |
| A female D. melanopogon. (From http://www.flickr.com/photos/steven_wong/5943424585/in/photostream/) |
Draco is a genus of agamid lizards, commonly known as the Flying Dragons, owned to their famous gliding locomotor strategy. Linnaeus derived the name of this genus from the Latin term for mythological dragons in 1758. Boulenger then named this specific species D. melanopogon in 1887 (1). The name is derived from Greek for "black-bearded", after the black gular flaps in males. ("melanin" for black, and "-pogon" for beard-like).
They are widespread in Southeast Asia and southwest India. The genus is composed of approximately 45 species, with currently 39 currently recognized and several new species awaiting description (18).
In Singapore, these lizards can be spotted in the nature reserves, the Botanical Gardens, Kent Ridge, and even housing estates (5).
Interesting Facts!
- The remarkable talent of Draco lizards (2):
(Video from Animal Planet's official Youtube Channel: http://www.youtube.com/user/AnimalPlanetTV/videos?query=draco+lizard)
- Draco lizards are not, strictly speaking, flyers. A more appropriate term for them is gliders. Their wings do not provide any power, instead they increase the lizards’ lift, enabling them control over their direction. Also, the lizards employ their long tails to maneuver (3).
- They can glide for distances of up to 30 feet (3)!
- They are not seen on the ground much, as they spend most of their time in the trees where they are safer from predators (3).
- The 40 over species of Draco lizards each has its own distinctive body pattern, wing color, and gliding capabilities (3).
- Draco lizards are safe from human predation in the jungles of the Philippines due to a common but erroneous belief there that they are poisonous (4).
Biology

© 2002 Arie van der Meijden (From http://eol.org/pages/794987/overview )

 |
| (From http://www.flickr.com/photos/steven_wong/5943995898/in/photostream/) |
Total length: 22-27 cm (5)
Dorsal surface is generally greenish. Body is long and slender. Tail comprises around 60% of total length (6).
Morphology (7)
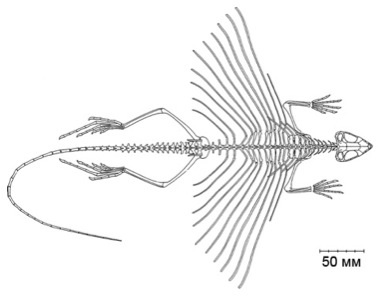 |
| (From http://westlakesscientific.org/particle/761) |
 |
| (From http://westlakesscientific.org/particle/761) |
In Draco, males have long, black, and extendable throat flap (also known as the gular flap or dewlap), which is used for territorial and courtship displays (5). Females lack the gular flap and lappets (6). To find out more about the differences between males and females D. melanopogon: Click here!
A male flapping its dewlap (8):
The ribs extend way out of the body to support a flap of skin on each side, known as the Patagium. It is black, sometimes with small yellow spots. By launching themselves from great heights into the air and extending the ribs forward and outward, the patagium spreads out and enable the lizards to fall at a controlled pace, gliding to another vertical surface nearby (9). They can travel up to 5m for every metre of height loss (10). To learn more about the Patagia: Click here!
 |
| (Picture taken as a snapshot from Animal Planet's official Youtube Channel: http://www.youtube.com/user/AnimalPlanetTV/videos?query=draco+lizard) |
Behaviour
 |
| Effective camouflage (Image courtesy Nick Baker, www.ecologyasia.com) |
Arboreal (live on elevated substrates, such as trees) (9): The lizards glide from tree to tree in search of food, mates, to chase territorial males, and to avoid predators.
Diurnal (active in the day, sleep at night) (9)
Camouflage: Their greenish and brown dorsal surface enables effective camouflage with lichen-covered tree barks, allowing them to evade predators such as tree snakes.
Diet
Feeds on small invertebrates, mainly ants (9).Also feeds on small beetles, millipedes, isopods, and termites (11).
Reproduction
Oviparous (lay eggs) (9).The only time Draco ventures to the ground is when a female is ready to lay her eggs. Upon descending from the tree she is on, the female makes a nest hole by forcing her head into the soil. A typical female lays about 2–5 eggs before filling the hole. She remains on the ground for approximately 24 hours, fiercely guarding the nest, then returns to the trees and leaves the eggs to their fate. (4)
Female perched on a tree branch:
 |
| (From http://www.flickr.com/photos/steven_wong/5943465491/in/photostream/) |
Habitat
There are 3 species extant in Singapore: D. melanopogan and D. quinquefasciatus are confined to Bukit Timah Nature Reserve and Central Catchment Nature Reserve. D. sumatranus occurs in exposed habitats, and is often seen on trees in housing estates (5). |
| D. quinquefasciatus (From: http://lazy-lizard-tales.blogspot.sg/2012/01/flight-of-dragons.html) |
 |
| D. sumatranus (Image courtesy of <dansengohsp>. http://lazy-lizard-tales.blogspot.sg/2012/01/flight-of-dragons.html) |
 |
| (Image Courtesy of NParks: http://www.nparks.gov.sg/cms/index.php?option=com_visitorsguide&task=naturereserves&id=46&Itemid=75) |
They inhabit lowland dipterocarp-dominated forests, often seen at tree trunks on eye level, but can also inhabit the canopy.
Smaller species (such as D. melanopogon, D. quinquefasciatus of Borneo) tend to occupy lower levels of the trees because their large wing surface area: body mass ratio requires a shorter initial 'ballistic dive' before achieving enough lift for a level glide, whereas larger species (such as D. fimbriatus) are high canopy specialists, requiring an initial launch from higher up to generate enough lift to carry their greater body mass. (18)
Back to Table of Contents
Distribution
Genus Draco: (12)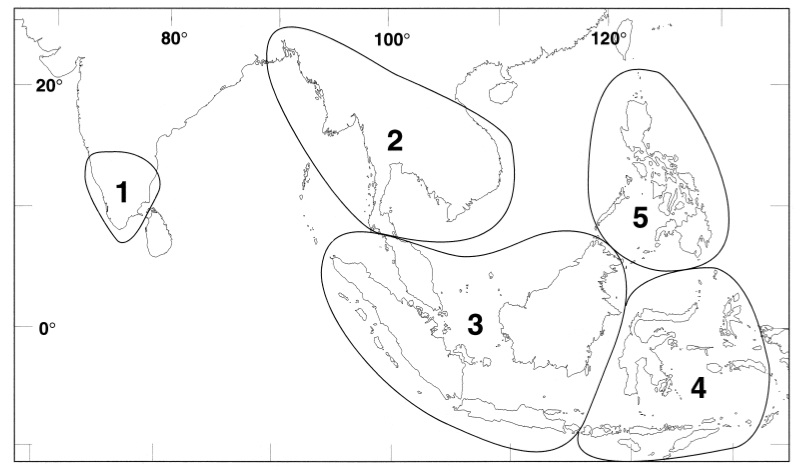 |
| A map of Southeast and South Asia showing distribution of Draco. Following five areas are recognized on the basis of distributional patterns in Draco and other taxonomic groups: 1, Southern India; 2, Indo-China Peninsula; 3, Malay Peninsula and Greater Sunda Islands exclusive of Sulawesi; 4, Lesser Sunda Islands, Sulwaesi and Maluk Islands; 5, Philippines. (From Honda et.al, 1999) |
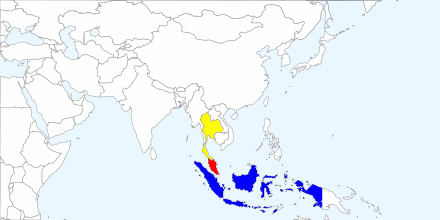
Species D. melanopogon:
South Thailand (south of Isthmus of Kra)
Malaysia (Pulau Tioman, Johor, Pulau Sibu)
Indonesia (Sumatra, Borneo, Natuna Island)
Singapore
(13)
Back to Table of Contents
Status & Threats
Status in Singapore:Native (indigenous species which have survived prior to urbanization) (9)
Uncommon (infrequently seen but widespread, recorded on the average less than twice a year) (9)
IUCN status - Not Evaluated (species which have not been accessed yet) (14)
Main Threat in Singapore:
Habitat destruction (10)
More on the Patagia
(15)The agamid lizard genus Draco and four lineages of extinct reptiles have each independently evolved musculo-skeletal adaptations for gliding on a pair of flight membranes known as the patagia, which expand laterally from the dorsal trunk region. They are supported by rod-like structures made of bone or dermal deposits, and unfolded through the action of specialized muscles and ligaments. In Draco, elongated dorsal thoracic ribs support a broad patagium that flares outwards between the fore and hind limbs. During flight, the membrane extends and moves under voluntary control via rib-associated muscles and ligaments.
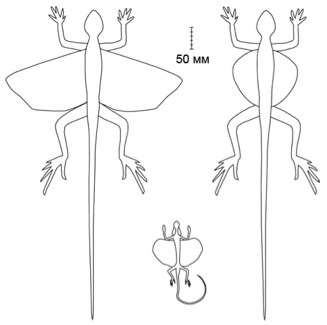
Dorsal and ventral views of a male D. melanopogon showing the outstretched patagia.
This gliding membrane folds back against the side of the body when not in use (Shine et. al, 1998):
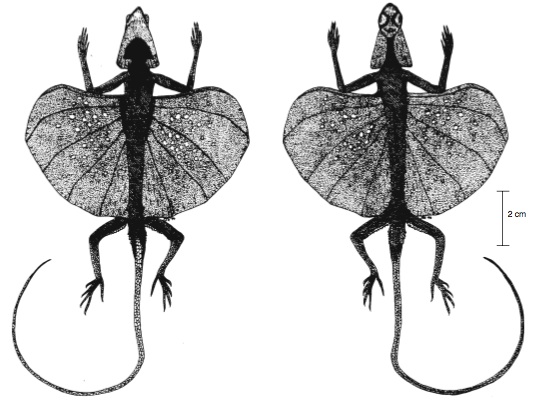 |
| (Image Courtesy of Wiley. From Shine et. al. "Costs of reproduction and the evolution of sexual dimorphism in a 'flying lizard' Draco melanopogon (Agamidae).") |
Such dorsal ribs have been found to support gliding membranes in several extinct reptiles, including the basal squamate Xianglong zhaoi (from Early Cretaceous), the lepidosaurs Kuehneosaurus and Icarosaurus siefkeri (from Late Triassic), and the very distantly related Archosaur Mecistotrachelos apeoros (Mid-Triassic). A late Permian lepidosaur, Coelurosauravus jaekeli, has been identified as gliding on a rod-supported patagium, but in this species, the support structure is not rib-derived; instead, it is made of bony material deposited within the skin. It has been hypothesized that these reptiles all evolved rod-supported patagia independently, a conclusion supported by observed variation in patagium outline, aerodynamic properties and precise musculo-skeletal arrangements between species. Indeed, the Xianglong exhibits possibly the most aerodynamic and maneuverable rib-supported patagium, comprising a typical aerofoil cross-section (thickened at the leading edge, tapered to the trailing edge) and wings that were much longer laterally (in contract, Draco has pantagia longer in the anterior-posterior dimension than transversely). Also, Mecistotrachelos was probably more maneuverable than Draco, due to thickened elongated ribs near the base of its unusually long neck, which suggests strong muscle attachment for excellent control over its membranous 'wings'. (15)
 |
| Xianglong zhaoi (From http://www.mapoflife.org/topics/topic_342_Gliding-reptiles/) |
Beyond general comparisons of gliding patagia, Xianglong points to a more subtle instance of convergent evolution – it strengthens its patagium with parallel collagen fibres in a fashion only seen elsewhere in very distantly related taxa, such as the pterosaurs (capable of true flight) and the Triassic delta-wing glider Sharovipteryx. It is proposed that these three reptile groups independently converged on the use of collagen fibres to critically reinforce their flight membranes. (15)
Extra Info-bites!
A flying snake hunts the Draco (19):(Video from National Geographic's official Youtube Channel: http://www.youtube.com/watch?v=kPGNbb5Y2a0)
 |
| Gliding tree snake (From http://www.mapoflife.org/topics/topic_342_Gliding-reptiles/) |
Back to Table of Contents
Evolution of Sexual Dimorphism
Male D. melanopogon: |
| (Image Courtesy of Ulrich Manthey: http://reptile-database.reptarium.cz) |
A study conducted by the University of Sydney (published, The Zoological Society of London) examined the evolution of sexual dimorphism in D. melanopogon. The specimens were collected from the Bukit Lagong Forest Reserve, Selangor, Malaysia (Shine et. al). (16)
It proposed that patterns of sexual dimorphism in D.melanopogon might be linked more directly to locomotor functions. Females grow larger than males, having larger heads and longer tails relative to body length. Not only are these traits unusual among agamid lizards, the two latter conditions seemed to be unique to Draco, and possibly unique among all lizards. This is surprising because, even though head sizes relative to size are frequently dimorphic, it is very often in the direction of larger head sizes in males, than in same-sized females. Similarly, in all other agamid species for which the study could find information on, males have tails at least as long as those of same-sized females. (16)
Female D. melanopogon:
The study proposes several hypotheses for such a phenomenon, for which two of the strongest arguments are: (16)
1. Females have bigger bodies to better carry eggs
Larger body sizes, relative head sizes and relative tail lengths of female Draco may be adaptations to improve a female's ability to glide while she is carrying eggs. Lowered mobility might reduce a female's ability to evade predators, capture prey, and move to egg-laying sites. By growing to a larger body size, a lower relative clutch mass is achieved, and hence the extent of wing-loading is reduced (ie. lower payload relative to surface area of the wing). By having large heads and long tails relative to body size, females could have stabilizing mass at either extreme of the body that could possibly enhance aerial mobility and gliding stability
The paper put forth that previous studies have similarly interpreted the larger body size of females in flying organisms as adaptations to reduce the relative mass of the clutch and hence, the degree of locomotor impairment of gravid females (eg. in most raptorial birds). In bats, females not only grow larger in many species but tend to have larger wings relative to body size.
2. Males have smaller bodies to increase maneuverability for mating
Smaller body size of males is an adaptation to increase maneuverability while gliding. If Draco males glide more often than females, in terms of social interactions among competing males, maneuverability could be more important for this sex. There appear to be sexual selection for small male size to enhance agility in three-dimensional contests. A similar hypothesis has been advocated to explain the ‘reversed' (males are smaller)dimorphism of some species of birds, seals, as well as aquatic versus terrestrial turtles.
Back to Table of Contents
Phylogeny
We will be looking at three different phylogenetic studies of the genus Draco - the first study looked at phylogenetic relationships among 12 species based on mitochondrial rRNA genes and allozymes (Honda et. al, 1999); the second study which was done much earlier proposed the primary dichotomy in Draco based on morphological characters (Musters 1983); the last study was based on mitochondria DNA sequence obtained from 53 Draco species/populations and 4 outgroup taxa (McGuire and Heang, 2000).
1. A study of phylogenetic relationships among 12 species of the genus Draco, inferred from mitochondrial 12S and 16S rRNA genes and allozymes, revealed the presence of at least four lineages within the genus (Honda et. al) (12).
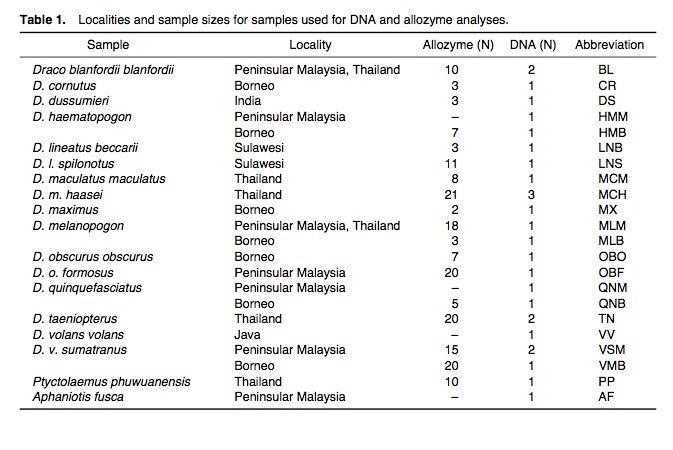
Neighbour Joining trees derived from distance matrix of allozyme and rRNA sequence data of Draco (Honda et. al):
At least four distinct lineages within the genus were observed. The first lineage consists of D. volans and D. cornutus; the second only of D. lineatus, which exhibits a great genetic divergence between two subspecies; the third is monotypic with D. dussumieri, the only species distributed in southern India; the fourth included all the remaining species. The third and fourth lineages are expected to exclusively share a common ancestor. It is suggested that the common ancestor of whole Draco originally diverged into three groups –the ancestors of the first, second, and other two lineages, by vicariance. In the fourth lineage, D.blanfordii, D. haematopogon, D. melanopogon, D. obscurus and D. taeniopterus are proposed to be exclusively close to each other.
The two dendrograms indicated three conflicts in terms of branching topology (at BPs≥50%):
(1) In the allozyme dendrogram, D. blanfordii was found in node V, together with D. haematopogon, D. melanopogon, D. obscurus and D. taeniopterus.
In the DNA dendrogram, D. blanfordii was exclusively clustered with D. maculatus.
(2) In the allozyme dendrogram, D. maximus was exclusively closest to D. quinquefasciatus.
In the DNA dendrogram, D. maximus formed a cluster with node IV (D. obscurus and D. taeniopterus).
(3) In the allozyme dendrogram, D. maximus–D. quinquefasciatus cluster was distantly located from the cluster consisting of the other species.
In the DNA dendrogram, D. haematopogon, D. maximus, D. melanopogon, D. obscurus, D. quinquefasciatus and D. taeniopterus formed an exclusive cluster.
However, these dendrograms were not in substantial conflict with each other in nodes II–V that were defined in at least one of the dendrograms with significant BP values (BPs≥70%).
Phylogenetic tree combining the two NJ trees of Draco above (Honda et. al):
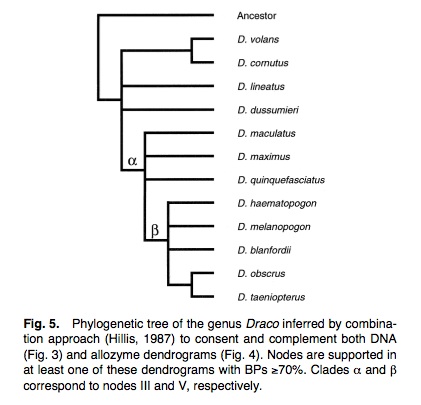
To maximize phylogenetic resolution, three clades were identified (clade α, clade β, and the D. obscurus–D. taeniopterus clade (corresponding to nodes III, V and IV of the previous dendrograms respectively).
The dendrograms are based on the Neighbor-Joining method (NJ) and it is key to note the advantages and drawbacks of such a method. The main benefit of NJ is that it is fast, making it suitable for analyzing large data sets and for bootstrapping (other methods such as maximum parsimony and maximum likelihood may be computationally prohibitive). Also, as long as the input distance matrix is correct, the output tree will be correct. Moreover, NJ allows correction for multiple substitutions. However, one drawback of NJ is that information is reduced (since it is distance matrix based). It has problems with taxa that have the same distances, this is because NJ randomly chooses which ones to connect, thereby giving only one tree out of several possible/optimal trees (known as "tie trees").
2. Let us now take a look at a previous study. Before genes and allozymes were analyzed, Musters (1983) had proposed the primary dichotomy in Draco based on morphological characters.
Phylogenetic tree based on morphological characters and geographical range of Draco (Honda et. al):
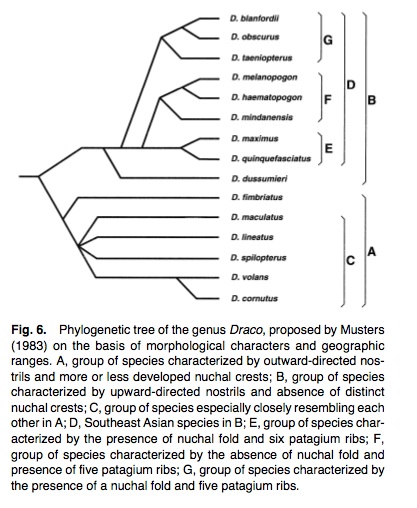
There are two major clades in his phylogenetic tree; one was characterized by outward-directed nostrils and more or less developed nuchal crests (ie. Group A), the other major clade (Group B) was characterized by upward-directed nostrils and the absence of distinct nuchal crests.
Group D consists three further clades; one with species exhibiting a nuchal fold and six ribs in the patagium (Group E), one with species without a nuchal fold and with five patagium ribs (Group F), and its sister group (Group G) characterized by the presence of a nuchal fold and five patagium ribs.
As compared to the previous study by Honda et. al, phylogenetic relationships of Draco hypothesized based on DNA and allozyme analysis do not support the monophyly of the Group A in Musters at all. The characters of Musters' Group A (ie. outward-directed nostrils and more or less developed nuchal crests) are considered to be primitive, as they are common to the outgroups (Aphaniotis and Ptyctolaemus) as well.
3. Other studies have employed analysis of mitochondrial DNA sequence data using minimum evolution (of log-determinant distances) and maximum-likelihood optimality criteria to provide a estimate of Southeast Asia Draco phylogenetic relationships (McGuire and Heang). Mitochondria DNA sequence were obtained for 53 Draco species/populations and 4 outgroup taxa. (17)
Maximum Likelihood analysis (McGuire and Heang):
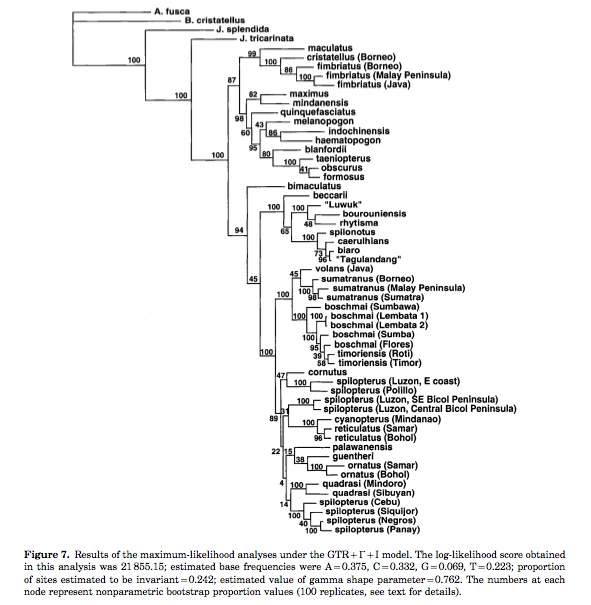 |
| Image Courtesy of Wiley. |
The Maximum Likelihood (ML) analysis attempts to find a model consisting of tree, branch lengths, nucleotide frequencies and substitution rates that explains the data with the highest likelihood. In another words, given a probable model for a character change, the analysis picks the tree that has the highest probability of generating the observed data. According to the study, the ML analysis under the specific model (refer to above tree) explains the data significantly better than other analyses based on less parameterized models. Yet, the ML analysis has its drawbacks as well. First, it will find the correct tree only if the correct evolutionary model is used. Thus, if a model is chosen incorrectly, long branch attraction and repulsion problems will arise. Also, there is often computational problems with optimizing so many parameters simultaneously, therefore most solutions are far from being optimal.
Minimum Evolution analysis (McGuire and Heang):
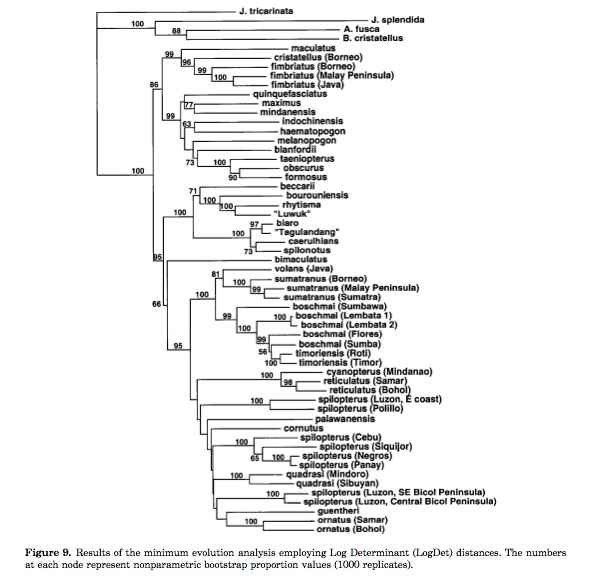 |
| Image Courtesy of Wiley. |
The Minimum Evolution (ME) criterion differs from ML in that observations are not used directly to calculate the tree score. Instead the data are transformed to pair-wise distances, and the score is calculated from there. There is an advantage is using pair-wise distances for data such as DNA-DNA hybridization, where the data from the experiments are pair-wise differences. However, using pair-wise distances compared to using the full data set carries with it certain drawbacks. Some information is lost when characters are transformed to pair-wise distances. Moreover, the branch lengths may sometimes be uninterpretable (e.g. there could be 1/2 a change for the length of a branch) or nonsensical (e.g. the number of changes needed to explain the differences in characters could exceed the total length of a ME tree).
Back to Table of Contents
Taxonavigation
Classification: (1)| Kingdom |
Animalia |
| Phylum |
Chordata |
| Class |
Reptilia |
| Order |
Squamata |
| Suborder |
Iguania |
| Family |
Agamidae |
| Genus |
Draco |
| Species |
D. melanopogon |
Subspecies: (13)
D. melanopogon melanopogon (Boulenger 1887)
D. melanopogon nigriappendiculatus (Bartlett 1894)
Synonyms: (13)
Draco melanopogon - BOULENGER 1887: 492
Draco nigriappendiculatus - BARTLETT 1894 (fide HENNIG 1936)
Draco melanopogon - DE ROOIJ 1915: 84
Draco melanopogon - TAYLOR 1963: 848
Draco melanopogon - HENDRICKSON 1966: 58
Draco melanopogon - GRANDISON 1972: 78
Draco melanopogon - MUSTERS 1983
Draco melanopogon - MANTHEY & GROSSMANN 1997: 171
Draco melanopogon - COX et al. 1998: 101
Draco melanopogon - MANTHEY & SCHUSTER 1999: 56
Draco melanopogon - GRISMER 2011
There are currently 2 syntypes from Malacca, Malaysia - BMNH RR 1946.8.26.87-90 (formerly BMNH 86.12.28.8-11) from the British Museum of Natural History. (20)
A syntype refers to any one of two or more specimens listed in a species description where no holotype was designated. (21) Attempts to publish species description based on syntypes are generally frowned upon by practicing taxonomists, and most are gradually being replaced by lectotypes - whereby a specimen is later selected to serve as the single type specimen for species originally described from a set of syntypes.
When one single specimen is clearly designated in the original description, this specimen is called the holotype of that species. The holotype is typically placed in a major museum or similar well-known public collection, so that it is freely available for later examination by other biologists. This is important as it allows biologists from all over the world to compare their specimens with a common single specimen, thereby reducing taxomomic problems such as incorrect species identification and confused use of names (eg. the case where a same species is named several times).
Back to Table of Contents
Useful Links!
1. Youtube Video: D. sumatranus spotted in the Botanical Gardens, Singapore.
[http://www.youtube.com/watch?v=OqpjK94Axy4]
2. Youtube Video: D. dussumieri spotted in the Anshi National Park, India.
[http://www.youtube.com/watch?v=mGKJJm4YIQg]
3. Youtube Video: Draco lizard spotted in Komodo Island.
[http://www.youtube.com/watch?v=m1mAdq1-X-Y]
4. High Quality Images from Flickr: http://www.flickr.com/search/?q=draco%20melanopogon
5. GenBank:
Taxonomy (http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=89028)
Nucleotide sequences (http://www.ncbi.nlm.nih.gov/nuccore/?term=txid89028[Organism:noexp]);
Protein sequence (http://www.ncbi.nlm.nih.gov/protein/18138159);
Popset, FASTA sequences (http://www.ncbi.nlm.nih.gov/popset/18138098?report=fasta)
6. Journal article on Sexual Dimorphism (Shine et. al): http://onlinelibrary.wiley.com/doi/10.1111/j.1469-7998.1998.tb00149.x/abstract (PDF available)
7. Journal article on Phylogenetic Trees (Honda et. al): www.bioone.org/doi/pdf/10.2108/zsj.16.535 (PDF available)
8. Journal article on Phylogenetic Trees (McGuire and Heang): ib.berkeley.edu/labs/mcguire/McGuire.Kiew.BJLS.pdf (PDF available)
References and Literature
1. Ross, Piper. (2007). Extraordinary Animals: An Encyclopedia of Curious and Unusual Animals, Greenwood Press.
2. AnimalPlanetTV, Youtube Channel. (2008). The Draco Lizard, "Fooled by Nature" series, Animal Planet.
[http://www.youtube.com/watch?v=FxSGpCOtkSc&list=PL89DA141A85CA6DDA&index=3&feature=plpp_video]
(Last viewed: 16th November 2012)
3.McArthur, Yvonne. (2012). The Flying Dragon Lizards of Southeast Asia. Environmental Graffiti.
[http://www.environmentalgraffiti.com/animals/news-flying-dragon-lizards-southeast-asia]
(Last viewed: 8th November 2012)
4. National Geographic, Draco Lizard.
[http://animals.nationalgeographic.com/animals/reptiles/draco-lizard/]
(Last viewed: 8th November 2012)
5. Ng, K.L.P., Corlett, R.T., and Tan, T.W.H. (2011). Singapore Biodiversity: An Encyclopedia of the Natural Environment and Sustainable Development. Raffles Museum of Biodiversity Research, Singapore. 316 pp.
6. Ecology Asia, Black-bearded Gliding Lizard.
[http://www.ecologyasia.com/verts/lizards/black-bearded_gliding_lizard.htm]
(Last viewed: 16th November 2012)
7. Benton, Michael. (2011). Aerodynamics of flying lizards shared.
[http://westlakesscientific.org/particle/761]
(Last viewed: 15th November 2012)
8. Wong, Steven. (2012). Male Draco melanopogon flapping its dewlap.
[http://www.youtube.com/watch?v=MexbCDHUwP0]
(Last viewed: 15th November 2012)
9. Lim, K.P. and Lim, L.K., 1992 (revised 2002). A Guide to the Amphibians and Reptiles of Singapore. Singapore Science Centre. 110 pp.
10. Wildlife Singapore, Black-bearded Flying Lizard Draco melanopogon.
[http://www.wildsingapore.per.sg/discovery/factsheet/lizardblkbeard.htm]
(Last viewed: 15th November 2012)
11. The DNA of Singapore, Draco melanopogon.
[http://rmbr.nus.edu.sg/dna/organisms/details/724]
(Last viewed: 15th November 2012)
12. Honda, Masanao; Ota, Hidetoshi; Kobayashi, Mari; Nabhitabhata, Jarujin; Yong, Hoi-Sen and Hikida, Tsutomu. (1999). Phylogenetic Relationships of the Flying Lizards, Genus Draco (Reptilia, Agamidae). Zoological Society of Japan. Zoological Science 16: 535–549.
13. Uetz, Peter. Draco melanopogon BOULENGER, 1887. The Reptile Database.
[http://reptile-database.reptarium.cz/species?genus=Draco&species=melanopogon]
(Last viewed: 15th November 2012)
14. Uetz, Peter. Draco melanopogon Black-barbed Flying Dragon. Encyclopedia of Life.
[http://eol.org/pages/794987/overview]
(Last viewed: 15th November 2012)
15. Map of Life - Convergent Evolution Online, Gliding Reptiles.
[http://www.mapoflife.org/topics/topic_342_Gliding-reptiles/]
(Last viewed: 15th November 2012)
16. Shine, Richard; Keogh, Scott; Doughty, Paul and Giragossyan, Hamlet. (1998). Costs of reproduction and the evolution of sexual dimorphism in a 'flying lizard' Draco melanopogon (Agamidae). The Zoological Society of London. J. Zool., Lond. 246: 203-213.
17. McGuire, Jimmy A., and Heang, Kiew Bong. (2001). Phylogenetic systematics of Southeast Asian flying lizards (Iguania: Agamidae: Draco) as inferred from mitochondrial DNA sequence data. Biological Journal of the Linnean Society. 72: 203–229.
18. McGuire, Jimmy A., and Dudley, Robert. (2011). The Biology of Gliding in Flying Lizards (Genus Draco) and their Fossil and Extant Analogs. Integrative and Comparative Biology, 51(6): 983–990.
19. National Geographic, Youtube Channel. (2012). "Flying Snake Hunts Leaping Lizard", National Geographic.
[http://www.youtube.com/watch?v=kPGNbb5Y2a0]
(Last viewed: 16th November 2012)
20. A Guide to the Lizards of Borneo.
[http://www.arbec.com.my/lizards/agamidae/agamidae4g.php]
(Last viewed: 20th November 2012)
21. Wikipedia. Type (Biology).
[http://en.wikipedia.org/wiki/Syntype#Syntype]
(Last viewed: 20th November 2012)
Back to Table of Contents
(Or send comments/information to: tzeryeun@gmail.com )
