
Overview
Common names:
- Red cotton bug
- Cotton stainer bug
Dysdercus cingulatus crawling around
Dysdercus cingulatus is a species of bug from the family Pyrrhocoridae, more commonly referred to collectively as cotton stainer bugs.
The common name "Cotton Stainer" refers its tendency to leave stains on the cotton bolls as it feeds on the plants parts.
They are conspicuous due to their bright contrasting colours often attracting attention when they gather in large numbers under the leaves of its host plants.
Are all insects "Bugs"?
The answer is no! All bugs are insects, but not all insects are bugs! When an insect has the term "Bug" attached to the end of its common name, it is classified as an insects belonging to the order Hemiptera. This order of insects has the common name "True Bugs" a distinction made to clarify the blatant misuse of the term bug to subsume all insects. Here are some examples of True Bugs.
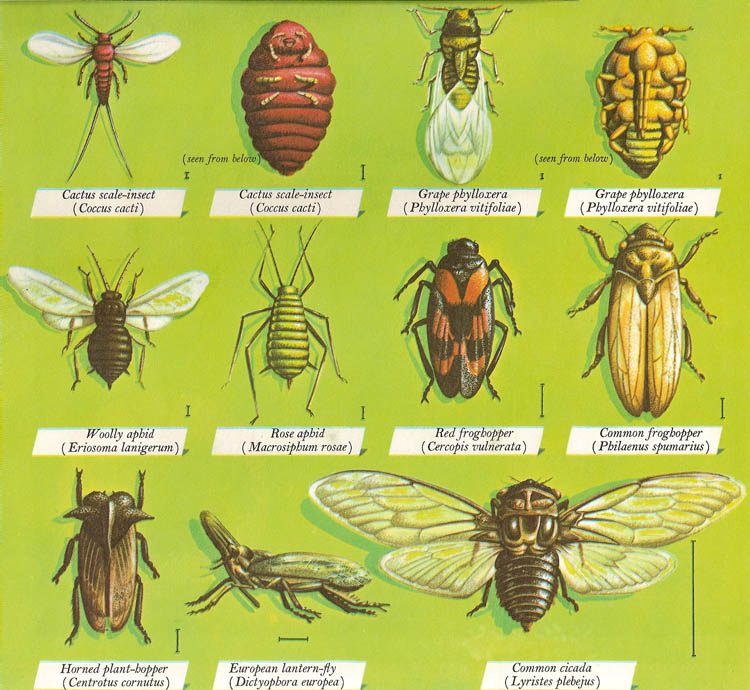
Photo credit: David Darling (Permission Pending)
The word Hemi- means half and ptera- means wing, which makes them "half wings'". The name refers to a distinctive feature in all bugs where half of their front wings are hardened, possibly for protection. The other half is soft and membranous, like other insect wings.
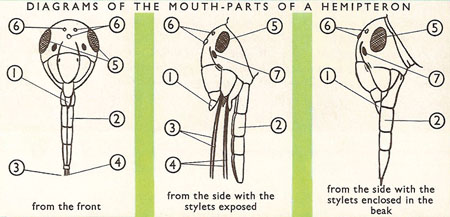
Another distinctive feature present in all bugs are their piercing and sucking mouth parts. This long hardened beak-like structure is known as the rostrum which it uses to pierce its food. It then uses
the stylets which are hidden within the beak to suck the fluids of its food similar to Mosquitos but not exactly identical (Mosquitoes are not actually bugs, they belong to another order Diptera, more info here!).
Most bugs feed on fluids from plants (e.g plant sap, nectar, oils), but some bugs are predatory and feed on the fluids of other animals, mostly small invertebrates, but sometimes blood from large mammals (e.g Kissing Bug).
For some general info on Hemipterans, and a few examples watch the video below!
For more information you can visit this website
http://www.daviddarling.info/encyclopedia/H/Hemiptera.html
Directory
Distribution | Biology | | Economic Impact | Morphology | Taxonomy | Comparison within Dysdercus | | Phylogeny | Literature Cited
Distribution
The genus Dysdercus of which Dysdercus cingulatus belongs to, is one of the few genera which is represented in all zoogeographical regions, it is found in both the new world and old world. (1). D.cingulatus is mainly found in the continents of Asia and Oceania.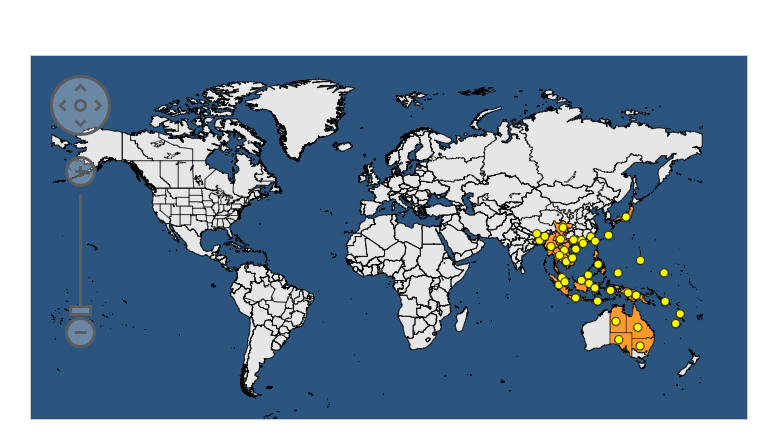 Global distribution of D.cingulatus. Information taken from EPPO Global Database (Fair Use) https://gd.eppo.int/taxon/DYSDCI/distribution |
D.cingulatus is found in:
- North-eastern India
- China
- Japan
- Taiwan
- Bangladesh
- Sri Lanka
- The Indo-Chinese peninsula (Including Malaysia and Singapore)
- Sumatra
- Borneo
- Philippines
- Papua New Guinea
- Australia.
Locally it is mostly distributed near coastal areas such as mangroves, their distribution is highly dependent on the distribution of their host plants (22).
Biology
Life Cycle
Dysdercus cingulatus like all other true bugs are hemimetabolous. This term refers to the general pattern of its life cycle. For example Butterflies (Order: Lepidoptera) are holometabolous, they undergo complete (holo- ) metamorphosis (metaboly-). All true bugs on the other hand undergo partial (hemi-) metamorphosis. The difference is in holometabolous insects, the immatures (Larvae) often look and behave completely differently than the adult. The immatures of hemimetabolous insects however are called nymphs which generally behave similarly and resemble the adult. Photo credits: H. Maxwell-Lefroy and F.M. Howlett (Fair Use) |
Within the species Dysdercus cingulatus, the complete life cycle from the beginning of development in the embryo to the mature adult takes 50-90 days (2). The eggs hatch after 7 days and the nymph emerges (2). The nymphs then go about foraging for food to gain enough energy to grow. When the outer shell (exoskeleton), gets too small. It sheds its exoskeleton and forms a new one from inside it, this is known as moulting (Ecdysis). Nymphs of D.cingulatus moult 5 times in their lifespan, the fifth and final moult gives rise to fully mature adult. After emerging from the final moult, the wings are fully developed allowing them to fly and migrate in search of food sources. |
Foraging Behaviour
Abelmoschus esculentus Photo credits: Delince J (Creative Commons) |
 Hibiscus tiliaceus Photo credits: Tau'olunga (Creative Commons) |
Host Plants Associated with Dysdercus cingulatus
Some cotton stainers like Dysdercus decussatus, another species that occurs in Singapore, have a more restricted selection of host plants. D.cingulatus however can be found in many different host plants. All the plants listed below can be found around South-east Asia, though not all are found in Singapore.From family -Malvaceae:
- Abelmoschus moschatus
- Abelmoschus esculentus (Okra)
- Hibiscus tiliaceus
- Hibiscus rosa-sinensis
- Hibiscus syriacus
- Hibiscus mutabilis
- Hibiscus makinoi
- Hibiscus cannabinus (the Kenaf plant)
- Thespesia populnea
- Abutilon indicum
- Malvastrum coromandelianum
- Gossypium arboreum (Cotton plant)
From family Bombacaceae:
- Chorisia speciosa
- Bombax ceiba
Foraging
Dysdercus cingulatus will feed on the most profitable host plant it can find, if that host plant deteriorates and becomes unsuitable, individuals will move to a less preferred host (4). It is studied that D. cingulatus can withstand several days of starvation while migrating and looking for suitable host plants.
Dysdercus cingulatus nymphs feeding on cotton bolls
D.cingulatus, like most other bugs from the genus Dysdercus, feed on sap found in the seed of cotton bolls. They pierce the outer covering of the seeds and suck the plant saps within the seed. Constant feeding of the seeds may weaken them, causing it to dry up eventually killing the cotton bolls. They can also leave stains on the cotton bolls preventing commercial use, this behaviour lends them their infamous title "cotton stainers" (1). When they use their beaks to puncture the bolls, they may also inadvertently introduce the fungus Atospora gossypii which results in internal boll disease (5). Both excrements from feeding D. cingulatus and internal boll disease damage the cotton bolls and prevents it from being used for cloth production. This results in major economic losses in old world. (6). In fact old world species are more harmful crop pest than the new world variety (6).
Reproductive Behaviour
 Photo credits: Kwan © www.NatureLoveYou.sg (Permission Pending) |
In most Dysdercus reproduction is linked with foraging behaviour. Fully mature adult females of D.cingulatus will migrate immediately after ecolosion and find a suitable food source. Their wings will allow them to fly to find a suitable host plant (4). Once a suitable food source is available, females histolyse (breaking down of tissue) their flight muscles making them flightless. Histolyzing of flight muscles in D.cingulatus provides a large source of nitrogen (7). This survival strategy redirects energy and nutrients from flight activity toward reproductive activity. The flightless females than begin producing unfertilized eggs (oogenesis) in preparation for mating. Once histolysis of wing muscles occur, regeneration of flight muscle is not possible (8). Males on the other hand retain flight the ability to fly (9). This helps them to get around and to mate with multiple females (10).
During mating, the fertile male will approach the female sensing their bodies with the antenna, the female may attempt to runaway (it cannot fly remember) if it rejects the advances of the male if not they will allow copulation. The male inserts its penis (adeagus) into the female to fertilize the eggs. During copulation they are positioned end to end, and it continues for several days. It is believed the extended duration of copulation is to prevent other males from attempting to mate with the female (11). Females will then lay the fertilized eggs using their ovipositor. Eggs are usually laid in moist soil or small crevices in the ground. Each female is able to lay 100-130 eggs (2).
A mating pair can still move around and feed while remaining in the mating position
Predator-prey Interaction
Aposematic Colouration
A common strategy for prey to avoid predation is to remain hidden through camouflage. All cotton stainer bugs are often colourful and attractive, this may seem at first counterproductive for survival. However this is actually the phenomenon known as aposematic colouration. It is an alternative strategy where the prey is harmful to the predator in some way and it present their bright colours openly, acting as warning signals to potential predators. The prey usually contains some kind of chemicals that make them distasteful or are actually toxic and can kill the predator. This is common in bugs, butterflies, frogs, snakes and several other groups of animals.In the case of D.cingulatus, they contain chemicals in their blood that make them distasteful to most vertebrate predators. (1). The similarity in colours and pattern of all Dysdercus maybe an example of Mullerian mimicry.
Natural Enemy
The is very little evidence of vertebrate predators of cotton stainers, however they have many invertebrate predators. The most commonly cited natural enemy of the cotton stainer are the Assassin bugs belonging to the family Reduviidae. Most of the predatory assassin bugs are generalist, feeding on many kinds of insects. They typically grab their prey using their front legs and pierce the body of the prey with their beaks and suck the fluids out. Most literature suggest an assassin bug Phonoctonus spp. as a common predator of Dysdercus cingulatus (12).The family of cotton stainer bugs (Pyrrhocoridae) are almost exclusively herbivorous, there is however an exception. The bug Antilochus conquebertii also belong to the cotton stainers, but they exhibit predatory behaviour. Its prey consists of other Pyrrhocorids including Dysdercus cingulatus (13).
A.conquebertii waits patiently still for its prey, using its eyes and antenna to sense its surrounding until a prey comes close (14). Once a prey is within reach, it pounces and captures its prey, subduing it by piercing the neck or head region using its long proboscis (14). Its method of prey capture is very similar to the assassin bug. It then inserts the stylet into its prey and begins feeding by sucking the bodily fluid of the prey like a straw (14). It has been known to capture prey larger than itself, even a snail! (15).
A.conquerbetii feeding on D.decussatus
Economic Impact
As stated above, Dysdercus cingulatus is a major crop pest in the Asian region. D.cingulatus is native to two of the major cotton producing countries: China and India. Understandingly a lot of researchers from these countries have studied these crop pest hoping to find solutions to mitigate the economic impact these pest cause.Pest Control
In general cotton stainers, including Dysdercus cingulatus, is not major economic pest in Singapore since we do not produce cotton locally. It is also not an ecological pest, as they only cause considerable damage when their populations get out of control which only happens when there is a high density and perennial flowering of cotton producing plants. This situation usually only happens in an agricultural context. The natural density of host plants of D.cingulatus in the natural habitats of Singapore is usually not high and they do not flower all year round, so the population of D.cingulatus is moderate.
However, if you are a cotton farmer, here are some helpful tips to root out infestations by these pesky bugs:
- Prevention
- Remove debris and ratoon cotton (underground stems that can give rise to succeeding growth)
- Avoid planting crops near other possible host plants
- Planting cotton annually, if crops are maintained all year round, it increases the chance for cotton stainer populations to persist
- Assessing the problem
- Despite taking precautionary measures, infestation may still happen
- Cotton stainer bugs appear late season when the cotton bolls are beginning to ripen
- Damaged cotton bolls and flowers are obvious indication of cotton stainers
- Cotton stainers, regradless of species, are very distinctly coloured so its not easy to miss them. If you spot these red and black insects crawling on your crops, just be aware and keep a look out. It may not be necessary to take action yet
- If there are 10 or more colonies on a single plant per acre, that would count as an infestation and action should be taken
- Direct control
- Fresh custard apple leaf extract can be sprayed on the crop plants as a repellent/insecticide
- Other stronger chemicals can be used but most of these require legal permits
- Pyrethrum powder
- Alpha cypermethrin-based products
- Lambda-cyhalothrin-based products
- Cypermethrin-based products
- Alternatively, if numbers are small, brushing them into a pale of water works as a temporary measure
For more detailed information, please refer to these guides:
https://www.plantwise.org/FullTextPDF/2016/20167801478.pdf
https://www.plantwise.org/KnowledgeBank/FactsheetAdmin/Images/Uploads/PDFs/20167800155.pdf
Morphology
 Dorsal view Photo credits: Khono Katsuyuki (Permission Pending) |
 Ventral view Photo credits: Khono Katsuyuki (Permission Pending) |
Adults of Dysdercus cingulatus are 12-18mm long (1). Transverse white bands can be seen in the collar, just behind the head and on the underside along the margin demarcating each thoracic and abdominal segment. Legs are dark red. Forewings like all other Hemiptera are half membranous and half sclerotised (6). The sclerotised region of the forewings are red in colour with black spots, the membranous region of the forewing is black. D. cingulatus also has an elongated sucking mouth part similar to other true bugs.
Photo credits: Clarice Dorocinski (Permission Pending)
Photo credits: Clarice Dorocinski (Permission Pending)
Taxonomy
Dysdercus cingulatus was initially described as Cimex cingulatus by Fabricius in 1775. At this point the genus Dysdercus was not yet described. The genus Dysdercus was first described by Amyot and Serville in 1843. The first revision of Dysdercus in the old world, sought to rectify the authorship of the genus. Freeman argued that authorship of Dysdercus belonged to Boisduval, who designated Dysdercus decussatus (Boisduval 1835) as the holotype for Dysdercus (16). In reply, a revision of the new world Dysdercus was published, which debated that authorship belonged to Meneville who described Dysdercus peruvianus in an earlier publication during 1831 (17). The original genus Cimex, in the 1775 description, belongs to the Cimicidae a family of bed bugs. In 1794, it was revised to genus Lygaeus, belonging to Lygaeidae a family that closely resembles the current Dysdercus genus. According to Freeman, the different species within the genus Dysdercus are largely differentiated using genitalia.
Taken from Fabricius 1775 (Fair Use) (20)
Synonyms
Dysdercus megalopygus Breddin, 1909Cimex cingulata Fabricius, 1775
Lygaeus cingulatus (Fabricius) Fabricius, 1794
Type
Type data: UnknownType locality: Australia
Who was Fabricius?

Johan Christian Fabricius is considered one of the greatest entomologists of the 18th century. He was born in Denmark in 1745 and studied in the University of Copenhagen in 1762. Johan then studied in Uppsala University in Sweden under the tutelage of Carl Linnaeus for two years (18). Johan was a great observer of insects and was responsible for describing several insect species.
Linnaeus described insect species as well, but tended to be more botanically-minded (19). Fabricius distinguished insect species using there mouth parts instead of general form or number of wings (which Linnaeus usually used as a distinguishing features). The classification of insect orders (e.g Beetles, Butterflies) are still in use today, though the names have changed. Fabricius named 9,776 species of insects, compared to Linnaeus' tally of around 3,000 (19).
Comparison within Dysdercus

Comparison of three species of Cotton Stainer (Left to Right): Dysdercis cingulatus, Dysdercus poelicus and Dysdercus decussatus
Photo credits: Khono Katsuyuki (Permission Pending)
There are two species of Dysdercus in Singapore. The mentioned Dysdercus cingulatus and Dysdercus decussatus. The main difference is the pattern on their wings, D.cingulatus wings are reddish with a black dot and black tips. D.decussatus on the other hand has blackish wings with a yellowish band which forms a cross when the wings are folded above its abdomen. Their behaviour is largely the same, however D.decussatus are more selective and mostly found feeding on two species of plants in Singapore:Thespesia populnea and Hibiscus tiliaceus.
.
 Nymph of Dysdercus cingulatusPhoto credits: Kwan © www.NatureLoveYou.sg (Permission Pending) |
 Adult of Dysdercus cingulatusPhoto credits: Kwan © www.NatureLoveYou.sg (Permission Pending) |
 Nymph of Dysdercus decussatus Photo credits: Kwan © www.NatureLoveYou.sg (Permission Pending) |

If you look around the nature areas in Singapore you may find what looks like a third species of Dysdercus. This other species is designated Dysdercus simon (Tauber, 1927). Comparing both Dysdercus decussatus and Dysdercus simon, the only visible difference is the colour of its head, D.simon being black and D.decussatus red. According to scientific literature, D.simon is not actually an authentic description, therefore it is not a separate species but regarded as a synonym of D.decussatus (16). However, this taxonomic revision was done using exclusively morphological and anatomical data, in 1947. This was of course before the paradigm shift in taxonomy brought about by the advent of genetics and other more sophisticated molecular techniques. If another taxonomic revision was to be conducted using DNA and molecular data, it may be possible to differentiate them into separate species.

Male and female of Dysdercus decussatus (Top row), Male and female of Dysdercus simon (Bottom row)
Photo credits: Khono Katsuyuki (Permission pending)
Phylogeny
The tree was taken from a study which compared the phylogeny of phytophagous insects and their bacterial symbionts (21). Protein-coding sequences (COI and COII) were aligned based on their amino-acid translation in Geneious Pro 5.4 and partial 18S rRNA sequences were aligned using the SINA aligner. The individual alignments were concatenated and used for phylogenetic reconstruction with maximum likelihood algorithms (ML) and Bayesian Inference (BI), respectively. An ML tree was computed with FastTree 2.1 using the GTR model, and local support values were estimated with the Shimodaira–Hasegawa test based on 1000 resamplings without reoptimizing the branch lengths for the resampled alignments. For BI (computed using MrBayes 3.1.2), the data set was partitioned into the three genes, with six substitution types for the CO genes (GTR model), and one for the ribosomal gene (F81 model). Trees were sampled every 1000 generations, and a ‘burn-in’ of 1000 was used ( = 10%). They computed a 50% majority rule consensus tree with posterior probabilities for every node.
Literature Cited
1. Schaefer, C. & Panizzi, A. (2000). Heteroptera Of Economic Importance. Boca Raton, Fl: Crc Press.2. Central Institute for Cotton Research (2015). Cicr Technical Bulletins, Indian Council Of Agricultural Research, India, English Language Http://Www.Cicr.Org.In/Pdf/Kcyp_Stainer.Pdf
3. Kohno, K. (2001). Host Plant of Dysdercus Poecilus (Heteroptera: Pyrrhocoridae) And Its Relative Species in Ishigaki-Jima Island, The Ryukyus, Japan. Rostria 50:31–34 (In Japanese With English Summary).
4. Dingle, H. & Arora, G. (1973). Experimental Studies of Migration in Bugs of The Genus Dysdercus Oecologia 12:119–140.
5. Frazer, H. L. (1944). Observations on The Method of Tranmission Of Internal Boll Disease Of Cotton By The Cotton Stainer Bug Ann Appl Biol 31:271-290
6. Grazia, J., Panizzi, A. & Schaefer, C. (2015). True Bugs (Heteroptera) of the Neotropics. Springer.
7. Davis, N. (1975). Hormonal Control of Flight Muscle Histolysis In Dysdercus Fulvoniger . Ann Entomol Soc Am 68:710–714
8. Nair, C. & Prabhu, V. (1985). The role of feeding, mating and ovariectomy on degeneration of indirect flight muscles of Dysdercus cingulatus (Heteroptera: Pyrrhocoridae). Journal Of Insect Physiology, 31(1), 35-39. http://dx.doi.org/10.1016/0022-1910(85)90039-3
9. Edwards, F. J. (1969a). Development and Histolysis Of The Indirect Flight Muscles In Dysdercus Intermedius. J. Insect Physiol. 15, 1591-1599
10. Dingle, H. (1966a). The Effect of Population Density On Mortality And Sex Ratio In The Milkweed Bug, Oncopeltus, And The Cotton Stainer, Dysdercus (Heteroptera). Amer. Natur. 100, 465470
11. Alcock, J. (1994) Post Insemination Associations Between Males and Females - The Mate-Guarding Hypothesis. Annu Rev Entomol 39: 1-21.
12. Schaefer, C. W. And I. Ahmad (1987). "Parasites and Predators of Pyrrhocoroidea Hemiptera, And Possible Control of Cotton Stainers By Phonoctonus Spp Hemiptera, Reduviidae." Entomophaga 32(3): 269-275.
13. Muthupandi, M., Bertrand H, Evangelin G, William Sj. (2014). Biology of Pyrrhocorid Predator, Antilochus Conquebertii Fabr. (Hemiptera: Pyrrhocoridae) And Its Predatory Potential on Dysdercus Cingulatus Fabr. (Hemiptera: Pyrrhocoridae). Journal of Entomology and Zoology Studies; 2(2):91-96.
14. Evangelin, G., Bertrand, H., Jino M., William Sj. (2015). Feeding Behaviour of Antilochus conquebertii (Hemiptera: Pyrrhocoridae) and its systematic positioning. Journal of Entomology and Zoology Studies; 3(1):199-203.
15. Barker, Gm.(2004). Natural Enemies of Terrestrial Molluscs, 485-495.
16. Freeman, P. (1947). A Revision of Genus Dysdercus. Entomol Soc 98:372-424
17. Van Doesburg, Ph. Jr (1968). A Revision of The New World Species Of Dysdercus Guérin Méneville (Heteroptera, Pyrrhocoridae). Zool Med 97:1–215
18. "Johan Christian Fabricius" (1891). //Dansk biografisk leksikon//(in Danish). 5 (1st ed.). Projekt Runeberg. pp. 24–30.
19. S. L. Tuxen (1967). "The Entomologist J. C. Fabricius". Annual Review Of Entomology. 12: 1–15. Doi:10.1146/Annurev.En.12.010167.000245.
20. Fabricius J C (1775) Systema Entomologiae, Sistens Insectorvm Classes, Ordines, Genera, Species, Adiectis Synonymis, Locis, Descriptionibvs, Observationibvs. - Pp. [1-31], 1-832. Flensbvrgi, Lipsiae. (Kort). (P. 719) [Link] [Link To P. 719]
21. Sudakaran, S., Retz, F., Kikuchi, Y., Kost, C., & Kaltenpoth, M. (2015). Evolutionary transition in symbiotic syndromes enabled diversification of phytophagous insects on an imbalanced diet. The ISME Journal, 9(12), 2587-2604. http://dx.doi.org/10.1038/ismej.2015.75
22. Murphy, D, H. (1990) The Natural History of Insect Herbivory on Mangrove Trees in and Near Singapore. Raffles Bulletin of Zoology. 38(2):119-203

