Overview

Scientific name: Echinophyllia aspera
Common name: Golden eye chalice coral [1] , Australian watermelon chalice coral [2] , Flat lettuce coral [3]
Echinophyllia aspera was described by Ellis and Solander in 1788 and is more commonly known as the chalice coral. This species of colonial hard coraloccupies a huge range of environment parameters [4] and is widely distributed across the Indo Pacific[5] including Singapore. Its resilience to disease, ability to tolerate low light and reject sediments suggest that they are well-adapted to Singapore’s turbid waters. The Echinophyllia genus have been successfully propagated in captivity and E. aspera obtained its aquaculture name of Golden eye chalice and Australian watermelon chalice.
Table of Contents
Singapore's reefs
Coral reefs are one of the world’s richest and most biologically diverse ecosystems. They provide food, shelter and nursery grounds for a wide range of marine organisms. They also supply various resources for seafood, pharmaceuticals, aquarium trade, tourism and provide ecological services like coastal protection and carbon sequestration [6] .Singapore's reefs are found skirting many of the islands south of mainland Singapore. These comprise of mainly fringing and patch reefs. Approximately 60% of Singapore's reefs are lost due to coastal reclamation and dredging along almost the entire shoreline of Singapore. The remaining 40% of the reefs are subjected to sediment impacts. The reef community has undergone major shifts.Reefs are more compact and shallow[7] and their growth zone is reduced to the upper 6m of reef slopes due to the turbid waters[8] . Recruitment rates are low as it is difficult for larvae to settle on loose rubble while coral species dominance shifted to favour those more tolerant of reduced light condition. Reefs are also concentrated on sloping hard surfaces to disallow sediment accumulation which is more suitable for settling coral larvae . Despite the turbid waters, Singapore reefs host 255 hard coral species, that is approximately one-third of the world scleractinian diversity and more species are still being found.
Echinophyllia aspera is known to be comparatively more resilient to bleaching and tolerant to low light conditions. This explain their occupation of a wide range of habitats over a huge geographical range. This also explains why they are well adapted to Singapore's turbid waters.
Habitats
Echinophyllia aspera is widespread across the Indo-Pacific and are commonly found in most reef environments. They are mostly found on lower reef slopes, lagoons and fringing reefs [9] which protects E. aspera from wave action. They are also found to have a preference for slightly shaded spots on the reef slopes [10] .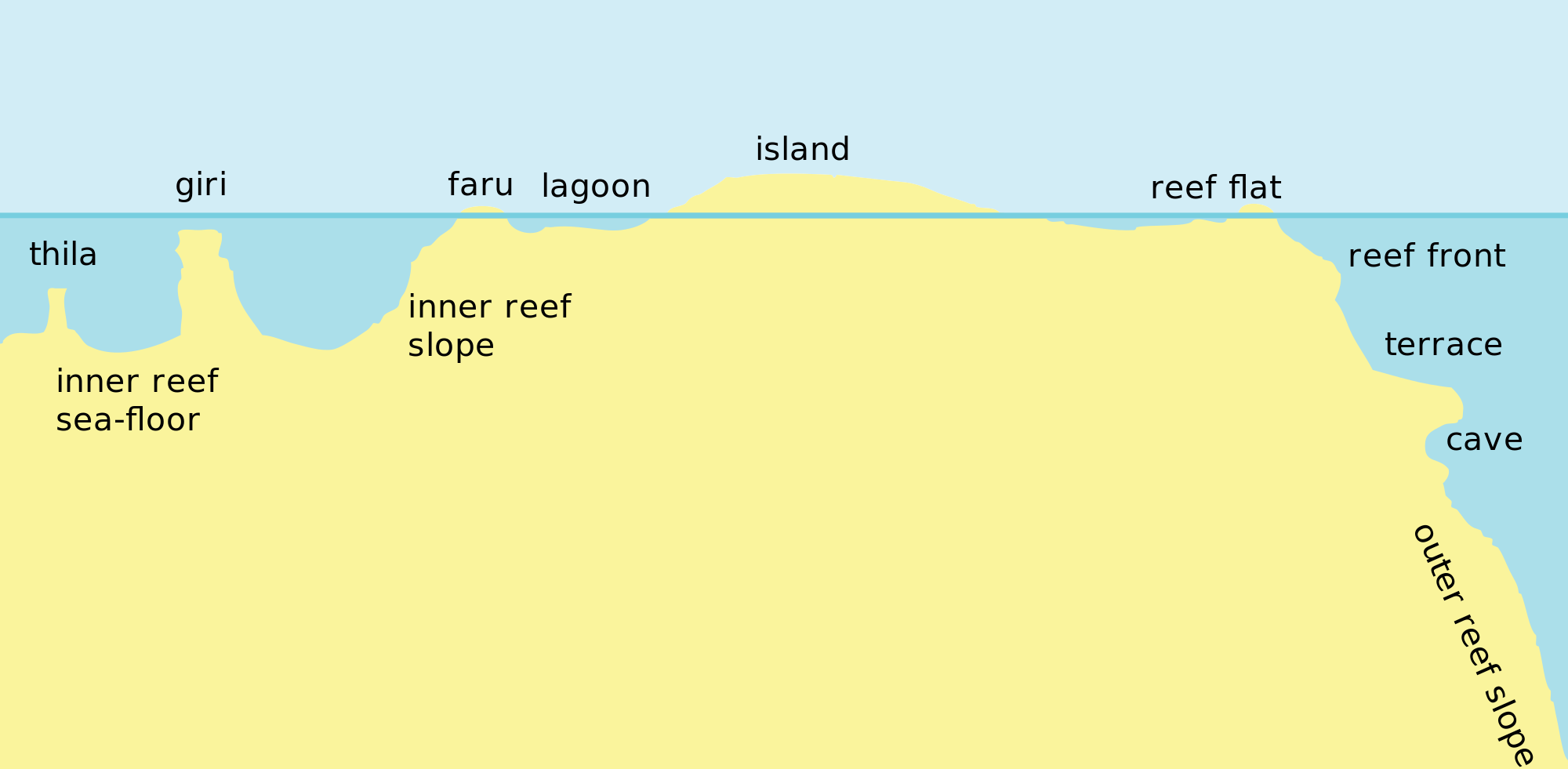
Cross section of a reef profile. Image obtained from Wikimedia (Creative Commons)
Environmental parameters occupied by E. aspera [11]
Depth(m) 1-60
Temperature range (°C): 22.219 - 28.122
Nitrate (umol/L): 0.046 - 4.021
Salinity (PPS): 34.337 - 35.506
Oxygen (ml/l): 4.452 - 4.969
Phosphate (umol/l): 0.081 - 0.566
Silicate (umol/l): 0.900 - 4.087
In a study done in mesophytic reefs in the Great Barrier Reef, the depth of E. aspera recorded ranges from 55m to 90m. They are common over a wide variety of reef habitats and depth ranges, and as such represent "depth generalist" species.
Distribution
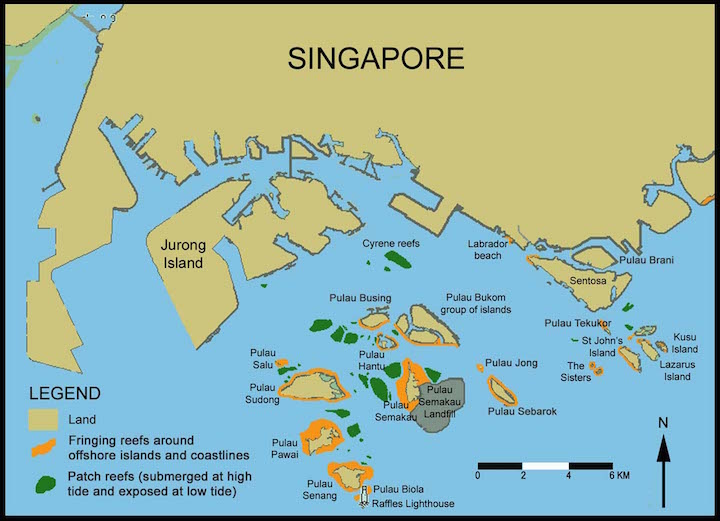

Local
E. aspera is recorded on seven of the eight reefs surveyed pre-2005 and during the 2006-2007 field surveys (there were no new records) [13] . The localities of E. aspera found on Singapore's reefs are Raffles Lighthouse (RL), Pulau Semakau (SE), Sisters Island (SI), Pulau Hantu (HA), Lazarus Island (LA), St. John’s Island (SJ), Kusu Island (KU) and Cyrene Reefs (CY).Global
RangeE. aspera is widely distributed through the Indo-West Pacific, from the Red Sea to French Polynesia. This species is found in the Red Sea and the Gulf of Aden, the southwest and northwest Indian Ocean, the Persian Gulf, the central Indian Ocean, the central Indo-Pacific, west, north and east Australia, South-east Asia, Japan and the South China Sea, the oceanic West Pacific, and the Central Pacific [14] .
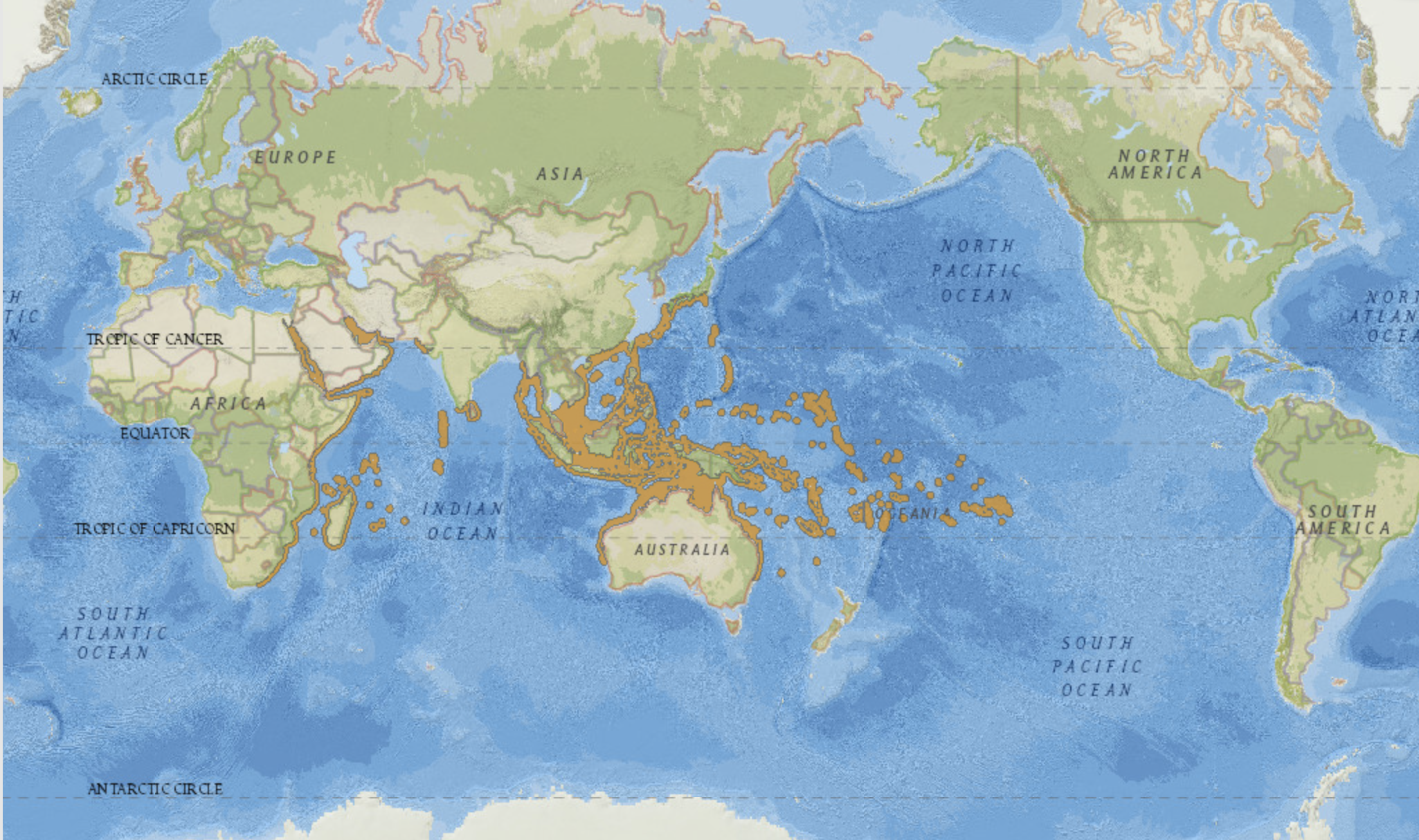
Abundance
It was listed as "rare but distinctive" on Coral AIMS[15] . However, other studies have stated that it is common and widely distributed[16] [17] . The population size is not clearly known [18] .
Biology
Feeding
Symbiotic relationship with zooxanthellaeLike most scleractinian corals, Echinophyllia aspera have a symbiotic relationship with zooxanthellae (Symbiodinium sp.), a type of unicellular algae called dinoflagellates. Symbiosis is a close and long term relationship between two organisms, in this case, the zooxanthellae and their host coral. Corals may obtain up to 90% of their energy from the sugars provided by these photosynthetic zooxanthellae. This is used by the polyps to produce proteins and carbohydrates to build their calcium skeleton. In return, the zooxanthellae obtain nutrients from the coral and also gain housing and protection. These zooxanthellae reside in the living tissue of the corals and are often concentrated in their tentacles.
Stinging tentacles for feeding
E. aspera is a stationary epifaunal photosymbiotic-suspension feeder. Similar to most corals, they obtain food from symbiotic zooxanthellae. Their polyps are expanded only at night, and they have translucent, long and thin sweeper tentacles armed with cnidocytes. These cnidocytes are used to capture food for feeding. Corals feed on zooplankton to take in alternative sources of energy and the synthesised proteins are used to make ammonia that their symbiotic zooxanthellae needs.
Youtube video of E. aspera with their stinging cells extended at night
Colour
Symbiotic relationship with zooxanthellaeZooxanthellae cells reside in the coral tissue, give them their brown colour. The species of zooxanthellae known to reside in E. aspera in Australia's Great Barrier Reef is Symbiodinium thermophilum variant C3h. This variant is a generalist clade that is also found to inhabit at least 24 coral genera over a broad range of depths [19] .
Type C3h also contains intragenomic variants C3 and C21.

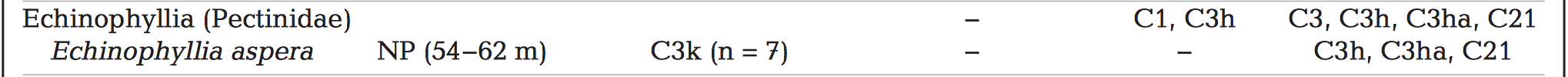
Interestingly, host generalist Symbiodinium type C3 and C3h are found in E. aspera at shallow depths but are not found at mesophytic depths. Meanwhile, Symbiodium type C3k is were found in E. aspera at mesophotic depths but have not been reported in E. aspera at shallow depths. C3k is also found in other corals that reproduce by broadcast spawning and transfer symbionts horizontally . This means that their zooxanthellae (Symbiodinium sp.) are acquired from the environment and not passed down from their parent.
Biofluoresence
Other fluorescent pigment comes from the coral protein. They cause the polyps to appear brightly coloured and glow at night or in blue light.


It was suggested that orange-red light fluorescence has some as of yet known biological function in deeper waters. Possible functions for fluorescent proteins in low light environments are enhancements for photosynthesis, visual cues for symbiotic fish and camouflage against certain fishes. Under low light conditions, these zooxanthellae are localised in the coral's endoderms (lower tissue layer), allowing these proteins to reflect and fluoresce light back to the zooxanthellae for photosynthesis. Conversely, under high light conditions, fluorescent proteins are located in the ectoderm (upper tissue layer) above the zooxanthellae, possibly to reflect excess visible light and prevent their tissues and symbiotic algae from light damage [21] .
Competition and defence
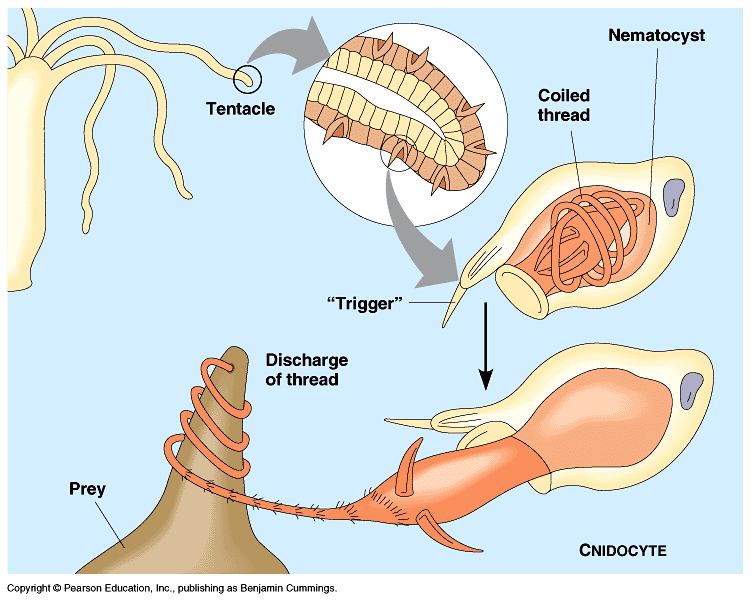
E. aspera is known to be aggressive and are advised to be kept away from other corals in aquariums [23] . These stinging tentacles can extend up to a feet long and are used to defend and compete for space.
Youtube video of E. aspera competing for space.
Their nematocysts are not venomous to humans but can cause stings and swells. They also retract their polyps to defend themselves when they sense danger.
Sediment removal
Youtube video of E. aspera rejecting sediments
Different corals have different mechanisms of removing sediments. They include localised tissue expansion, tentacular action and mucus secretion. The mechanism employed by E. aspera (as deduced from the video above) is ciliary beating whereby sediments are moved across the colony's surface by ciliary action.
Reproduction
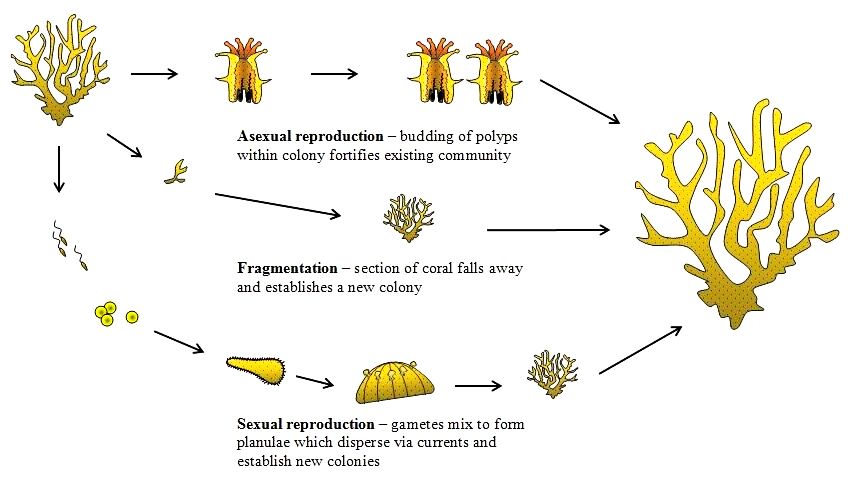
Corals can reproduce sexually or asexually. E. aspera are hermaphrodites [24] , meaning that they contain both male and female organs. They are also broadcast spawners and larvae brooders simultaneously.E. aspera has also been observed to spawn during mass-spawning events[25] .
Sexual
E. aspera has been documented as broadcast spawner. This mode of reproduction involves the shedding of gametes into water column where external fertilisation and development of the embryo can occur. They are hermaphroditic, thus they release both eggs and sperm packaged together in buoyant egg- sperm bundles during a spawning event. These egg-sperm bundles aggregate on the surface and concentrate the gametes to ensure that fertilisation can occur in a small space [26] .
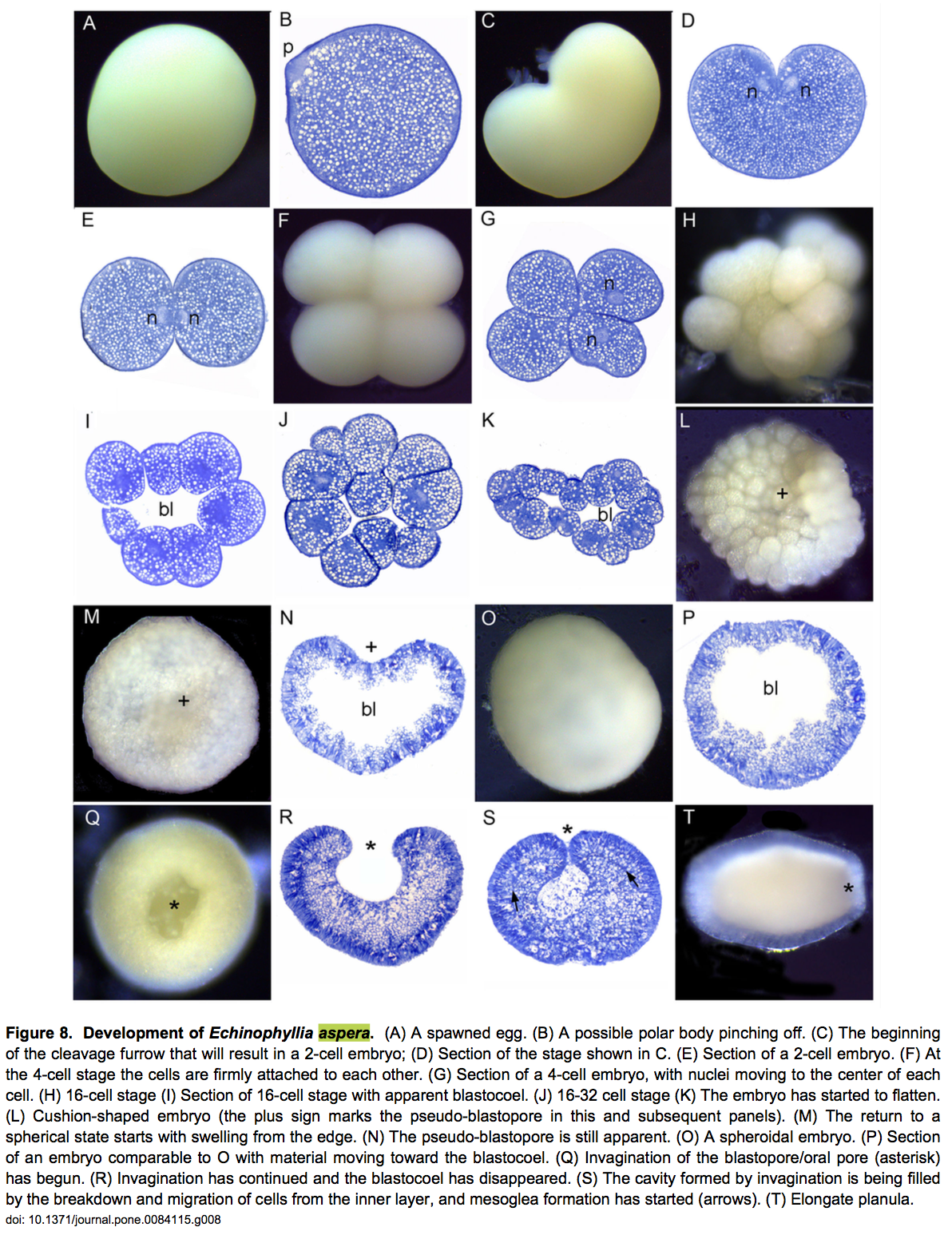
Development of the embryo [28]
The egg is almost rounded with fine yolk granules evenly distributed (Figure 8A-B). The first cleavage forms a heart-shaped embryo (Figure 8C,D) which then divide to form 2 equal blastomeres (Figure 8E). This division continues and after 3 hours the embryo consisted of 8-32 cells (Figure 8F-J). When they are at the 16th cell, the cells start to arrange themselves in a hollow sphere (Figure 8J), which became flattened after 7 hours as the pseudo-blastopore developed and then started to disappear again (Figure 8K-N). The embryo then swelled and appears rounded (Figure 8O). The embryo continued as a hollow spheroid for roughly 5 hours starting approximately 8 hours after the first cleavage (Figure 8P) and gradually became spherical after 14 hours. Blastopore formation finished after 15 hours (Figure 8Q,R) and at this stage, the embryos started swimming. The blastopore then began to close and eventually became the mouth. Mesoglea developed, and lipid was released into the interior (Figure 8R,S). The embryo then elongated into a typical planula shape, and was swimming strongly by the stage shown in Figure 8T.
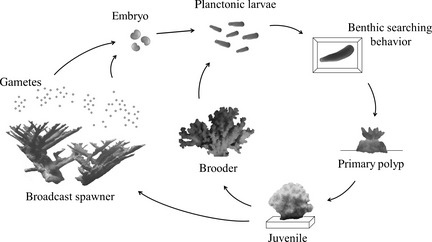
By this stage, planula larvae is free-swimming and will eventually settle on the substrate, forming plankters[30] . Later, this forms a tiny polyp which begins to excrete calcium carbonate and develops into a coral. Planula larvae are extremely vulnerable to predation and few survive.
Asexual
Colonies can reproduce asexually by budding when conditions are relatively stable.
Fragmentation/ Fragging:__
They can also reproduce by fragmentation. Fragmentation happens when fragments of corals break off naturally (sometimes due to storms) from their parent colony and establish themselves elsewhere. This is also known as fragging and it has been a popular tool for hobbyists to cultivate corals.
Online tutorial for fragging for Echinophyllia corals.
Propagation is easy for Echinophyllia corals. Asexual reproducing through "fragging" is a simple process that does not require elaborate equipment or specialised skills. The frag is usually taken from a healthy and settled colony and done via clean cuts in the skeleton with a blade. The frag is then mounted horizontally and put in a clean tank to recover.
This coral propagation methods have numerous environmental benefits as compared to wild collection, and perhaps should be practiced by aquarists seeking to propagate ornamental species sustainably. Researchers have found that growth rates of propagated corals are much faster than parent colonies, and fragmented corals can even fuse together very quickly. This is important for sustainable coral trade, coral conservation and restoration.
Conservation and threats
Status

Red List Category & Criteria: Least Concern v 3.1
Date assessed: 2008-01-03
Justification: It is widespread, common throughout its range, and found to 40m depth, and therefore is likely to be more resilient to habitat loss and reef degradation because of an assumed large effective population size that is highly connected and/or stable with enhanced genetic variability. However, the population trend is unknown.
Threats in Singapore
Habitat loss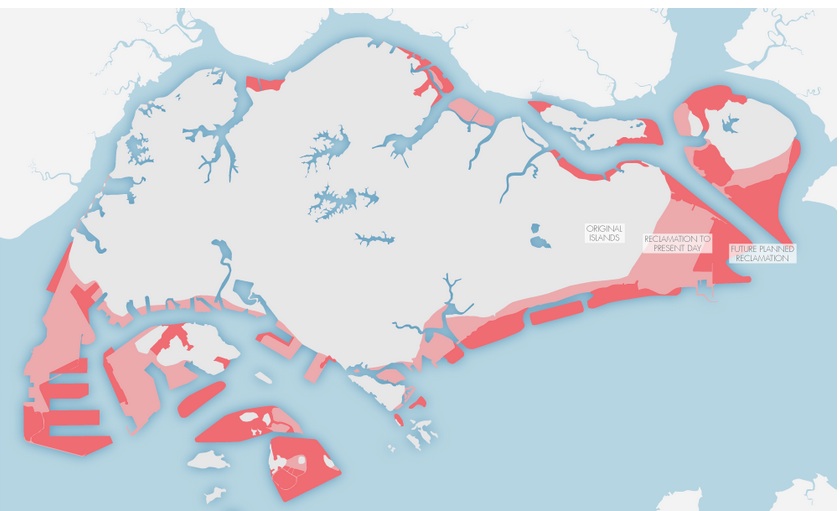
Map of Singapore showing original land, reclaimed land and future land reclamation plans (Permission pending)
Before 1970s, there were over 60 offshore islands and patch reefs around Singapore, most of them situated in the south of mainland Singapore. However, many of the south islands and coasts of the mainland have been reclaimed, increasing the total land area of Singapore by over 20% since 1970s. Many reefs were smothered by the reclamation whilst the remaining reefs are severely affected by turbid waters. Since 1986, most reefs have lost up to 65 % of their live coral cover. Future land reclamation plans will lead to further destruction of habitats.
Sedimentation
The general morphology of the corallum makes Echinophyllia sp. highly efficient passive sediment rejectors. Their abundance in turbid coastal areas, where sedimentation may be a factor, is suggested to be related to their sediment-rejection ability [31] .
While mature forms of E. aspera colonies are able to reject sediments, their larvae settlement is still adversely affected by sedimentation. Sediment accumulation hinders coral larvae settlement and effectively reduces the surface area that can be colonised. Meanwhile, successfully settled larvae are subjected to high post-settlement mortality due to sediment smothering and light attenuation.
Bleaching
Recent events of coral bleaching due to sustained elevated temperatures in June 1998 and June 2010 has affected Singapore’s reefs. Sea temperatures around Pulau Hantu and St John's Islands were elevated by 1-2 degree Celsius from March to June 1998. This leads to coral bleaching that extended to 6m, the lower growth depth limit for coral growth locally and 50-90% of reef organisms were affected. Sea temperatures only returned to normal in August 1998 and a study assessed that 10 out of 35 coral colonies died from stress. Meanwhile, surviving colonies showed various signs of stress, such as growth of turf algae and silt accumulation, leading to partial mortality [32] .
Although Echinophyllia sp. occurs in the deeper regions of the reef and might be less affected by bleaching, Reef surveys has reveal that coral cover of E. aspera were reduced from 1986 till 2012 [33] .
Climate change
Due to global warming, prolonged elevation of sea surface temperatures will further stress corals, especially scleractinian ones. We are likely to expect more frequent and intense bleaching events. Other stressors includes increased storms and rainfall due to climate change.
Global threats to E. aspera
Aquarium trade
IUCN Red list stated that the genus does not appear in trade statistics, suggesting that E. aspera is not collected for the aquarium trade[34] . However, there are strong evidence to suggest that this is not true. "Chalice corals" are extremely popular in aquarium trade.
A chalice coral comprises of genera Echinophyllia, Mycedium, Echinophora, Oxypora. E. aspera is a chalice coral with multiple variations- Australian Watermelon Chalice, Golden Eye Chalice, Red Chalice (limited edition). Golden Eye Chalice, locally called Cleveland Cavaliers Chalice due to its wine-coloured base and bright yellow eyes. These chalices are obtains from islands all over Indo-Pacific including Fiji, Tonga, Solomon Islands and the Great Barrier Reef.
There are detailed information available online on how to take care of these corals. For E. aspera kept in aquariums, they require moderate water movement. Too strong currents might overturn the chalice while low flow can allow detritus to settle on them leading to dead spots. Although Chalice corals relies on zooxanthellae to provide food, they are surprisingly aggressive and able to feed on frozen food to pellet foods. They will also need proper lightning to induce their attractive colours, if not they may fade to brown [35] . To bring out the its fluorescence ability, the corals are kept without lighting for a few days, before they were slowly exposed to very mild blue LED light (20-30 PAR). Without proposer acclimation, these corals will get stressed easily.

E. aspera can be bought from local reef shop. (Permission granted by Seasonal Aquarium Pte Ltd)
Bleaching
In general, the major threat to corals is global climate change, in particular, temperature extremes leading to bleaching and increased susceptibility to disease, increased severity of ENSO events and storms, and ocean acidification [36] .
The outlook for this coral is more positive than other. As explained above, they are depth generalist species and can be found in mesophytic reefs in depths of 55-90m. This makes them suitable candidates to recolonise reefs that have suffered coral mortality from disturbances such as bleaching and cyclones. Mesophytic reefs extend the range of species that also occur on shallower reefs and may therefore provide refuge from environmental disturbances that affect shallow reefs. These include light-enchanted warm water bleaching and severe tropical cyclones [37] .
Conservation in Singapore
While reef restoration is deemed a poor substitute to habitat conservation, the former is increasingly employed as an active intervention method to assist in the rehabilitation of damaged reefs, because leaving the reefs to recover by themselves may be too slow and ineffective. Various techniques have since been attempted, these includes establishing nurseries to rear sexual and asexual coral recruits, and transplanting coral material to seawalls[38] .Coral nurseries
Marine biologists from the National University of Singapore's Tropical Marine Science Institute (TMSI) are raising tiny fragments of corals to larger colonies so that they can be transplanted to seawalls and used for reef rehabilitation.Singapore's sea bed are filled with soft sediments, making it hard for the larvae to establish themselves. Loose coral fragments can be shifted about by currents and puts them at risk of abrasion by sand.
Thus, after about six months when the corals have grown to a reasonably large size able to withstand stress, they are moved out of nursery and attached to sites on Kusu and Lazarus island using marine epoxy, a type of glue. Since the project started in 2013, over 800 coral fragments have been transplanted to reefs off Kusu Island and seawalls of Lazarus Island and about 80 per cent have survived [39] .
Seawall
In highly urbanised Singapore where coastal defence infrastructures such as seawalls are ubiquitous, seawall comprise of approximately 63% of the coastline [40] .
213 corals are growing nurseries and transplanted onto sea walls on Lazarus island in 2013 and scientists recorded a survival rate of over 90% in 2017. Coral cover where one of the sites were also saw an increase in hard coral cover from 3 to 20 percent. This showed that man-made structures, such as sea walls, could be conducive for marine life [41] .
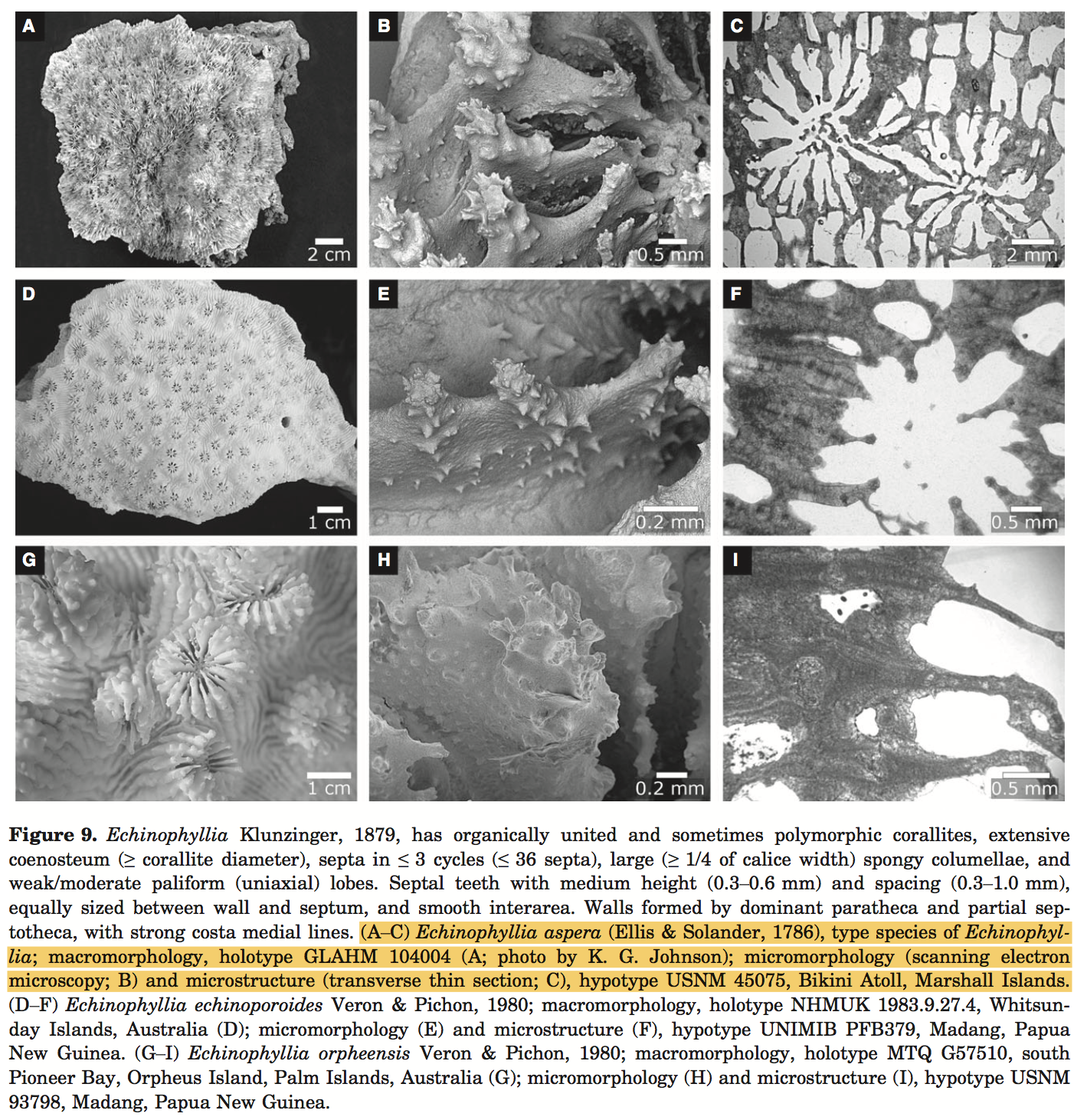
Morphology
Anatomy of corals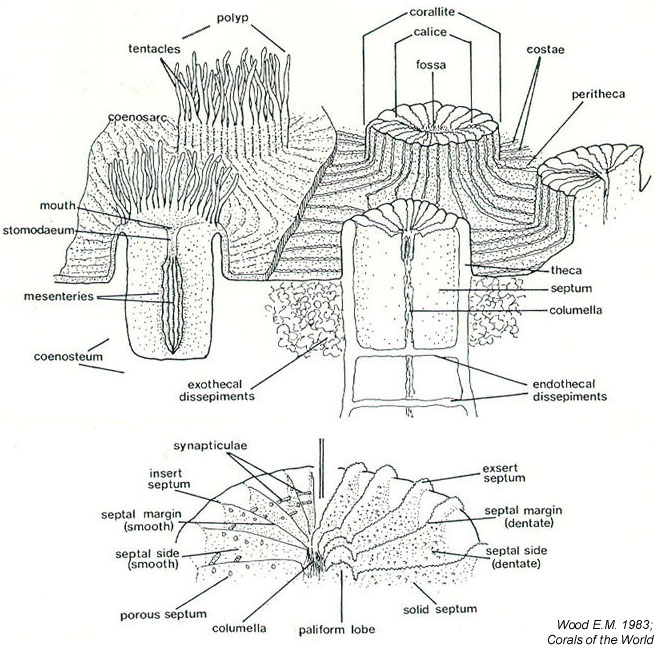
Colour
can be cream, grey, green on brown [43]
Growth forms
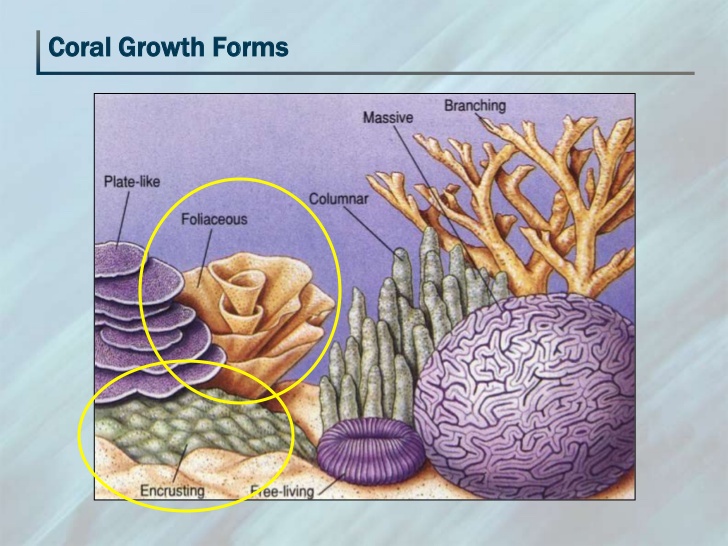
E. aspera is usually unifacial foliaceous or encrusting, occasionally semi-massive. Its central part is hillocky or sub-massive, while the peripheral parts become contorted, frequently forming overlapping whorls and tiers.
Corallite arrangement
Corallites are conical and protruding, usually inclined towards the periphery, and of varying sizes and shapes. A central corallite is usually distinguished in colonies of <20cm in diameter.
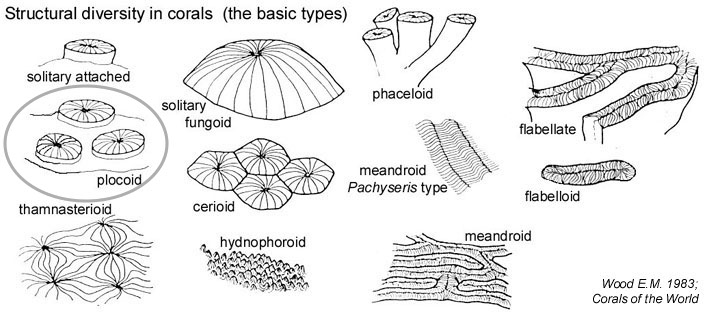
Calices: mostly plocoid, diameter ranging from 3-20mm and are loosely scattered and well spaced [46] .
Coenosteum: perforated next to chalices, costae toothed.
Costae: thick, with pointed, spins dentations.
Septa: The number of septa also varies greatly, and septa and not arranged in orders. Major septa radiate from the central corallite [47] , and are evenly exsert, up to 5mm, with 1-3 large dentations on their upper magins. Septa are often widely spaced and has margins with irregular sharp teeth.
Columella : well developed and conspicuous, consisting of twisted trabeculae.
Comparison with similar-looking general
Non-colonial: Genus Echinomorpha [48]
Colonial:
- Coenostial pits present: Genus Oxypora
- Coenostial pits absent + Corallites not inclined: Genus Echinophyllia*
- Coenostial pits absent + Corallites not inclined: Genus Mycedium
- Coenostial structures very conspicuous: Genus Pectinia
Taxonomy
Rank and taxa names
| Kingdom |
Animalia |
| Subkingdom |
Radiata |
| Phylum |
Cnidaria -- Hatschek, 1888 |
| Class |
Anthozoa -- Ehrenberg, 1834 |
| Subclass |
Hexacorallia |
| Order |
Scleractinia -- Bourne, 1900 |
| Suborder |
Faviina -- Vaughan and Wells, 1943 |
| Family |
Pectiniidae -- Vaughan and Wells, 1943 |
| Genus |
Echinophyllia -- Kluzinger, 1879 |
| Species |
Echinophyllia aspera -- (Ellis and Solander, 1786)[16] |
Echinopora aspera (previous combination)
Madrepoera aspera (original combination, basionym)
Oxyphyllia aspera (previous combination)
Oxypora aspera (previous combination)
Many synonyms exist for this species and the full inventory can be found here. The large number of snyonyms could be due to the variable appearance and wide geographical range of E. aspera, together with the nomenclatural reorganisations over the years.
Direct children
1. Echinophyllia aspera var. sugiyamai (Yabe & Eguchi, 1935) accept as Echinophyllia orpheensis (Veron & Pichon, 1980 -synonym)
2. Echinophyllia aspera var. tosaensis (Yabe & Eguchi, 1935) accepted as Echinophyllia orpheensis (Veron & Pichon, 1980- synonym)
Original description

Madrepora foliaceous smoothed aggregate, lifting subdistinctis stars, plates and spinulosis, walkways concave
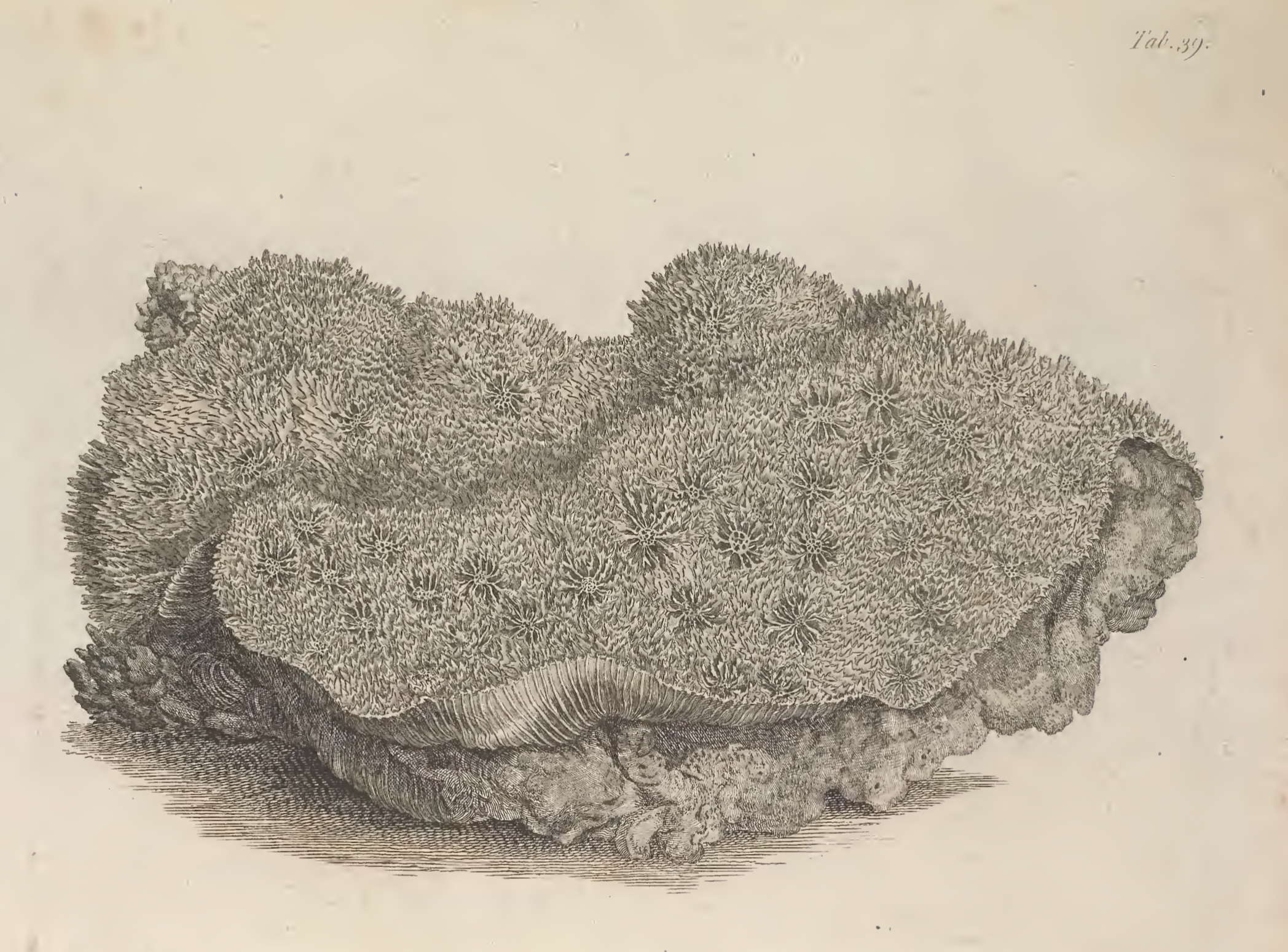
Type information
Echinophyllia aspera (Ellis & Solander, 1786: 156, pl. 39) is the type species for its genus Echinophyllia; holotype: GLAHM 104004 (dry speci- men); type locality: ‘Oceano Indiæ orientalis’ (Ellis & Solander, 1786: 156) [50] .
Phylogeny
This section is heavily referenced from Huang et al's recent paper on the taxonomic classification of the reef coral family Lobophyllidae [51] .Family
The history for E. aspera's family classification is very complicated. Genus Echinophyllia was established by Klunzinger in 1879 for the type species Madrepora aspera under the family "Fungidae". It was thought to be closely related to Halomitra, Mycedium and Echinopora but only Halomitra is in Fungiidae while the latter two are nested within Merulinidae. In 1935, Wells stated the Physophyllia, and by familial association, Echinophyllia is not in Fungiidae. In 1943, Echinphyllia was classified under Pectiniidae established by Vaughan & Wells even though there was little doubt the Echinphyllia was distinct from fungiids (characterised by fenestrate septa). Since then, this classification had become convention. In 1975, Chevalier observed that the septal tooth ornamation is strong and similar to those in 'Mussidae' (also equivalent to Lobophyllidae) being increasingly irregular distally. [52]The long held convention was challenged by Fukami et al.'s study using molecular data in 2004. More extensive genetic sampling of Echinophyllia in recent years lead to the agreement that Echinophyllia and Oxypora are sister genera (subclass F+G) nested within the Lobophyllidae clade. The remaining living genera in Pectiniidae are nested within Merulinidae and Pectiniidae has been synonymized. The placement of Echinophyllia under Pectiniidae was long held and appeared stable, thus it was a surprise when it resembled an outgroup. Multiple sources online still listed E. aspera was still listed under the family Pectiniidae. Huang et al.'s character analysis shows that Echinophyllia display similar tooth base and tip outline as other lobophylliids, but with the apex enlarging into a multiracial bulb by branching into multidirectional tips [53] .
Genus
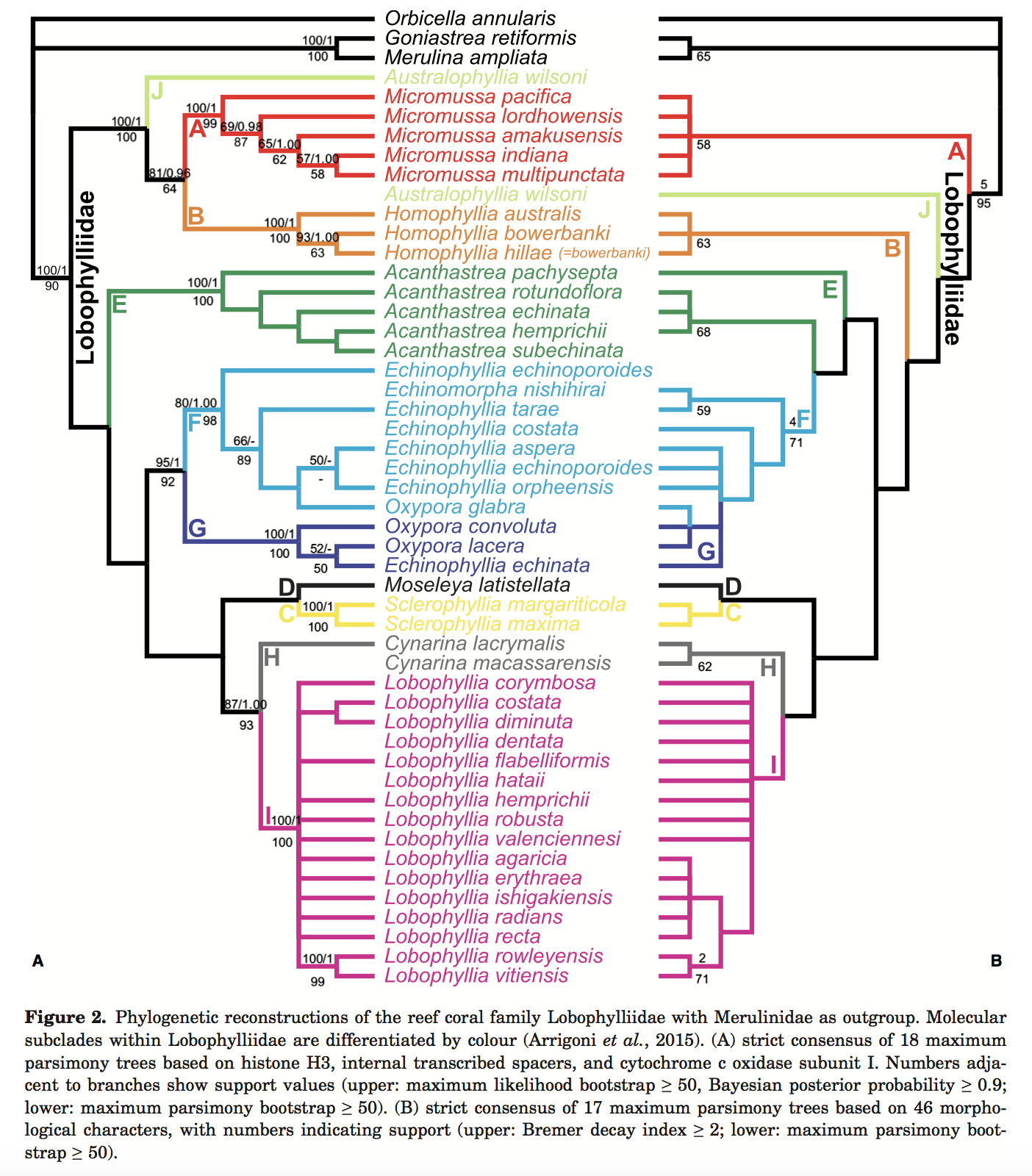
Huang et al. did a detailed species level analysis of 44 out of 54 living Lobophyllidae species based on an integrative analysis of colony, corallite and subcorallite morphology with molecular sequence data. Coral skeletal features were examined at three distinct levels- macromorpholoy, micromorphology, and microstructure and a morphological matrix comprising of 46 characters were built. Data were analysed using maximum parsimony and transformed onto a robust molecular phylogeny inferred using two nuclear (histone H3 and internal transcribe spacers) and one mitochondrial (cytochrome c oxidase subunit I) DNA loci. The results suggest that micro morphological characters exhibit the lowest level of homoplasy within Lobophylliidae. There are insufficient data to redefine Oxypora, Echinophyllia, and Echinomorpha, thus they maintain their placement within Lobophyllidae [55] .
A tree was constructed based on morphological traits (right) and compared with the phylogenetic tree (left). Maximum parsimony scores are indicated by the numbers on the bottom of the branch. By morphological data, there is no doubt that F+G forms a clade. Phylogenetic data shows that the genera Echinophyllia and Oxypora are both paraphyletic groups. In simple words, genus delimitation is messy.
Morphological tree
The placement of Echinomorpha, Echinophyllia, and Oxypora species in both subclass F and G is tentative. The morphological phylogenetic analysis based on the 47-taxon by 26-character dataset found 17 more parsimonious trees with a length of 108. The strict consensus tree is shown above. The molecular clades F and G, which comprise species of the paraphyletic genera Echinophyllia, are not found on the morphological tree.
Molecular tree [57]
For micromorphology, tooth tip form a multiracial bulb (character 24) in F+G, which less than or equals to 6 teeth per septum (character 27) in E+F+G. The only microstructure trait diagnostic of subclasses is weak costa centre clusters (character 39), a synapomorphy for F+G.
It is clear that the evolutionary history of genera Oxypora and Echinophyllia is complex. Species are not split by genus identity into the 2 subclasses F and G. It has been found that Oxypora species form a monophyletic group nested within a paraphyletic Echinophyllia. Currently, the conflict between molecular and morphological at a stems from the convergent macro-morphological features that group Oxypora species together, as all the subcorallite characters observed are invariable amongst Echinophyllia and Oxypora. This needs mores studies with greater sampling to better characterise intra-and inter-specific variation to unveil phylogenetically informative traits at the micromorphological and microstructural levels.
On the molecular tree, sister group relationships are supported for genus pairs of Echinophyllia and Oxypora. This is shown in analysis of micro morphological characters such as the shape of tooth tip having a multiracial bulb in both Echinophyllia and Oxypora and the number of teeth per septum is less than or equals to 6 in Echinophyllia, Echinomorpha and Oxypora.
There are no unambiguous apomorphies for Echinophyllia on either the molecular or morphological tree. 3 Oxypora species are nested amongst the 5 Echinophyllia species in subclass F+G on the molecular phylogeny (fig 2a) and these general are not reciprocally monophyletic on the morphological tree (fig 2b). The clade comprising three genera (Echinomorpha, Echinophyllia and Oxypora) is well supported with a bootstrap value of 71 and decay index of 4 and by multiple synapomorphies.
The sister relationship between Echinophyllia and Oxypora is further supported by the presence of alveoli (small pits on the exotheca forming at points of insertion of new septocostae. However, within the Echinophyllia group, it is confusing too. Echinophyllia echinata and Echinophyllia tarae are morphologically similar to Echinomorpha nishihirai mainly because they all possess a prominent central (polymorphic) corallite. However, this affinity is not so well supported by either molecular or morphological data.
The unexpected split of Echinophyllia into the molecular clades F and G, not accompanied by consistent morphological variation, indicates that the Echinophyllia–Oxypora dichotomy ought to be tested with more comprehensive taxonomic and genetic sampling of Oxypora.
Species
Species-level delimitationThe two closely related coral genera Echinophyllia and Oxypora made it interesting and frustrating for species delimitation. Arrigoni et al. applied three species delimitation approaches to assess species status in 14 recognised species of Echinophyllia and Oxypora in the Indo-Pacific. 109 colonies through the distribution range of these 2 genera were collected and genetically characterised using two mitochondrial and two nuclear genes.
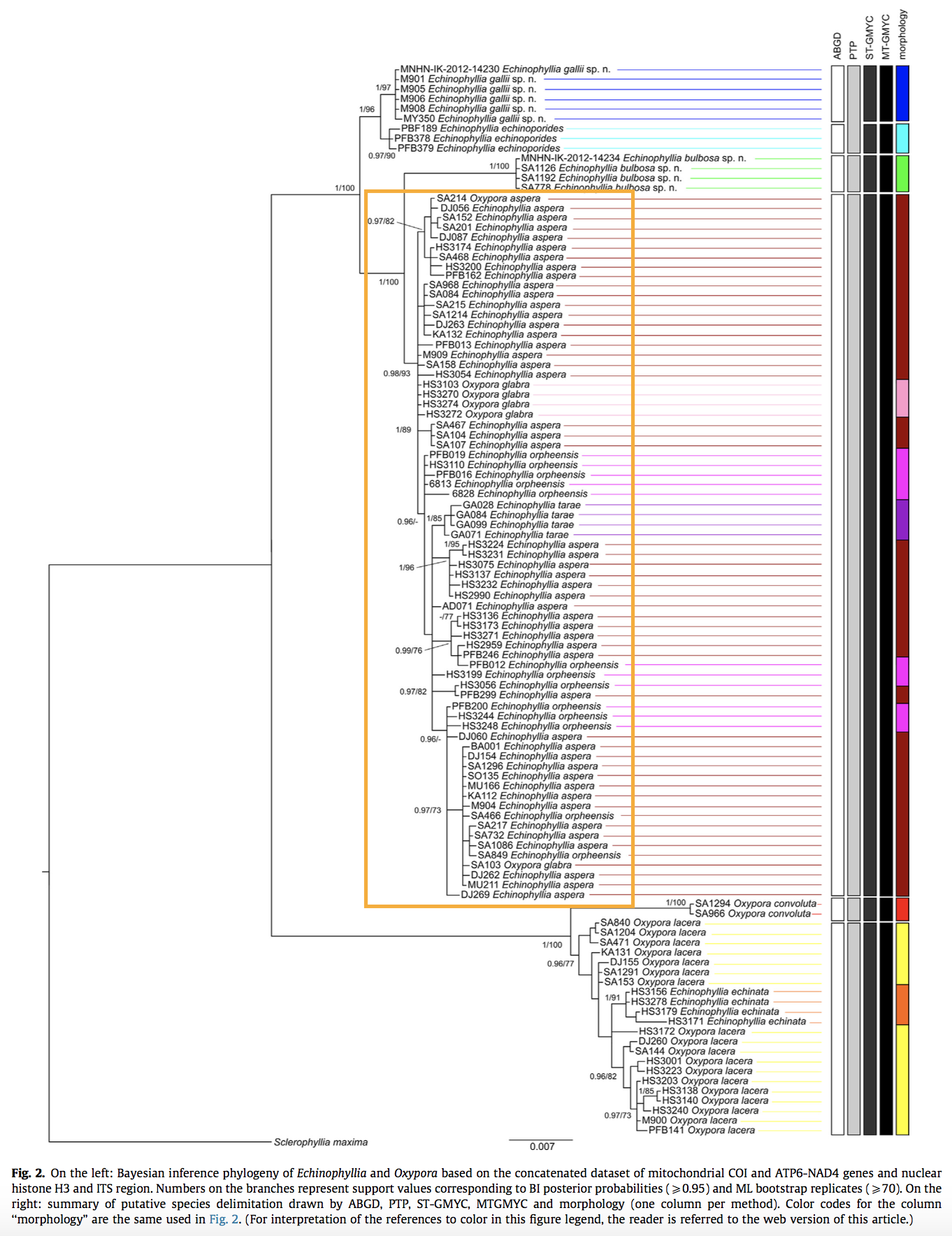
In Echinophyllia clade, E. aspera falls under a species complex composed of 4 different morphospecies- E. aspera, E. orpheesis, E. Tarae, and O. glabra. [59] Even delimiting the species-- Echinophyllia aspera is hazy. Till now, there is no change to its species status nor resolution to its high genetic variation.
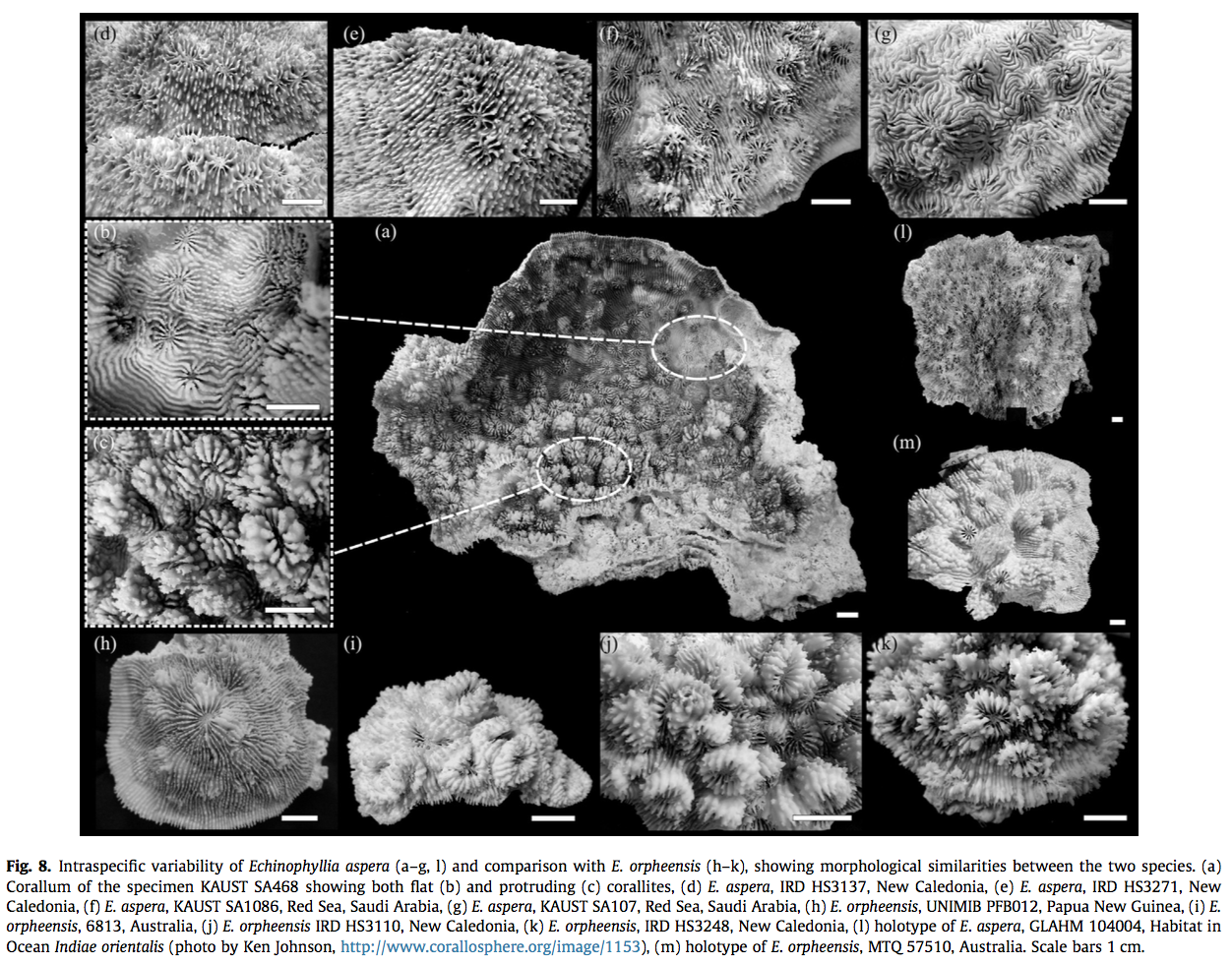
Even within colonies of E. aspera, it exhibits continuous variation in macromorphological features. Extensive sampling of E. aspera showed that colonies showed a great deal of variation in colony shape, calice dimension, calice walls, number of septa, development of paliform lobes, and thickness of costosepta. It is significant to note that morphological polymorphisms were both inter- (Fig. 8a– c) and intracolony (Fig. 8d–g) and no consistent geographical pattern could be identified.
A possible reason could be there are not enough markers to distinguish between these species. Another possible reason could be that E. aspera is not a real species.
Fossil records
The fossil record of this family atas to the Oligocene (32-25 Ma B.P.). Echinophyllia has the oldest fossil record of the extant genera, who fossil record (from Indonesia) dates to the Miocene. [1]Age range: 20.43 to 0.0Ma
DNA barcode
The National Center for Biotechnology Information contains nucleotide and protein sequences for E. aspera, which can be found here
 |
| Representative barcode sequence, the centroid of all available sequences for this species |
References
- ^ Golden Eye Chalice (Echinophyllia aspera). (2017). Aquarium Depot. Retrieved 3 December 2017, from https://aquariumdepot.com/golden-eye-chalice-echinophyllia-aspera/
- ^ Australian Watermelon Chalice (Echinophyllia aspera). (2017). Aquarium Depot. Retrieved 3 December 2017, from https://aquariumdepot.com/australian-watermelon-chalice-echinophyllia-aspera/
- ^ Echinophyllia aspera, flat lettuce coral. (2017). Sealifebase.org. Retrieved 3 December 2017, from http://www.sealifebase.org/summary/Echinophyllia-aspera.html[28] Burke L, K Reytar, M Spalding & A Perry. 2011. Reefs at Risk Revisited. World Resources Institute, Washington DC. 114pp.
- ^ Flat Lettuce Coral - Echinophyllia aspera - Encyclopedia of Life. (2017). Eol.org. Retrieved 15 November 2017, from http://www.eol.org/pages/200391/data
- ^ Sheppard, A., Fenner, D., Edwards, A., Abrar, M. & Ochavillo, D. (2014). Echinophyllia aspera. The IUCN Red List of Threatened Species 2014: e.T133172A54206928. http://dx.doi.org/10.2305/IUCN.UK.2014-1.RLTS.T133172A54206928.en. Downloaded on 03 November 2017.
- ^ Guest, J., Tun, K., Low, J., Vergés, A., Marzinelli, E., & Campbell, A. et al. (2016). 27 years of benthic and coral community dynamics on turbid, highly urbanised reefs off Singapore. Scientific Reports, 6(1). http://dx.doi.org/10.1038/srep36260
- ^ Type the content of your reference here.
- ^ Erftemeijer, P., Riegl, B., Hoeksema, B., & Todd, P. (2012). Environmental impacts of dredging and other sediment disturbances on corals: A review. Marine Pollution Bulletin, 64(9), 1737-1765. http://dx.doi.org/10.1016/j.marpolbul.2012.05.008 [2]
- ^ Sheppard, A., Fenner, D., Edwards, A., Abrar, M. & Ochavillo, D. (2014). Echinophyllia aspera. The IUCN Red List of Threatened Species 2014: e.T133172A54206928. http://dx.doi.org/10.2305/IUCN.UK.2014-1.RLTS.T133172A54206928.en. Downloaded on 03 November 2017.
- ^ Flat Lettuce Coral - Echinophyllia aspera - Encyclopedia of Life. (2017). Eol.org. Retrieved 15 November 2017, from http://www.eol.org/pages/200391/data
- ^ Flat Lettuce Coral - Echinophyllia aspera - Encyclopedia of Life. (2017). Eol.org. Retrieved 15 November 2017, from http://www.eol.org/pages/200391/data
- ^ Huang, D., Tun, K., Chou, L., and Todd, P. (2009). An inventory of zooxanthellate sclerectinian corals in Singapore including 33 new records (pdf). Raffles Bulletin of Zoology Supplement No. 22: 69-80.
- ^ Huang, D., Tun, K., Chou, L., and Todd, P. (2009). An inventory of zooxanthellate sclerectinian corals in Singapore including 33 new records (pdf). Raffles Bulletin of Zoology Supplement No. 22: 69-80.
- ^ Echinophyllia aspera. Corals of the World - Photos, maps and information about corals and reefs. (2017). Coral.aims.gov.au. Retrieved 15 November 2017, from http://coral.aims.gov.au/factsheet.jsp?speciesCode=0136
- ^ Echinophyllia aspera. Corals of the World - Photos, maps and information about corals and reefs. (2017). Coral.aims.gov.au. Retrieved 15 November 2017, from http://coral.aims.gov.au/factsheet.jsp?speciesCode=0136
- ^ Richmond, R.H. and C.L. Hunter (1990) Reproduction and recruitment of corals: comparisons among the Caribbean, the Tropical Pacific, and the Red Sea. Marine Ecology Progress Series 60(1):185-203.
- ^ Flat Lettuce Coral - Echinophyllia aspera - Encyclopedia of Life. (2017). Eol.org. Retrieved 15 November 2017, from http://www.eol.org/pages/200391/data
- ^ Sheppard, A., Fenner, D., Edwards, A., Abrar, M. & Ochavillo, D. (2014). Echinophyllia aspera. The IUCN Red List of Threatened Species 2014: e.T133172A54206928. http://dx.doi.org/10.2305/IUCN.UK.2014-1.RLTS.T133172A54206928.en. Downloaded on 03 November 2017.
- ^ Bongaerts, P., Sampayo, E., Bridge, T., Ridgway, T., Vermeulen, F., & Englebert, N. et al. (2011). Symbiodinium diversity in mesophotic coral communities on the Great Barrier Reef: a first assessment. Marine Ecology Progress Series, 439, 117-126. http://dx.doi.org/10.3354/meps09315
- ^ Bongaerts, P., Sampayo, E., Bridge, T., Ridgway, T., Vermeulen, F., & Englebert, N. et al. (2011). Symbiodinium diversity in mesophotic coral communities on the Great Barrier Reef: a first assessment. Marine Ecology Progress Series, 439, 117-126. http://dx.doi.org/10.3354/meps09315
- ^ Eyal, G., Wiedenmann, J., Grinblat, M., D’Angelo, C., Kramarsky-Winter, E., & Treibitz, T. et al. (2015). Spectral Diversity and Regulation of Coral Fluorescence in a Mesophotic Reef Habitat in the Red Sea. PLOS ONE, 10(6), e0128697. //http://dx.doi.org/10.1371/journal.pone.0128697//
- ^ Eyal, G., Wiedenmann, J., Grinblat, M., D’Angelo, C., Kramarsky-Winter, E., & Treibitz, T. et al. (2015). Spectral Diversity and Regulation of Coral Fluorescence in a Mesophotic Reef Habitat in the Red Sea. PLOS ONE, 10(6), e0128697. //http://dx.doi.org/10.1371/journal.pone.0128697//
- ^ reefphilippines.com • View topic - Echinophyllia aspera. (2017). Reefphilippines.com. Retrieved 3 December 2017, from http://www.reefphilippines.com/forums/viewtopic.php?f=18&t=13437
- ^ Richmond, R.H. and C.L. Hunter (1990) Reproduction and recruitment of corals: comparisons among the Caribbean, the Tropical Pacific, and the Red Sea. Marine Ecology Progress Series 60(1):185-203.
- ^ Tomascik, T., Mah, A., & Nontji, A. (1997). The ecology of the indonesian seas. Part 1 [et] 2. [Oxford]: Oxford University Press.
- ^ Richmond, R.H. and C.L. Hunter (1990) Reproduction and recruitment of corals: comparisons among the Caribbean, the Tropical Pacific, and the Red Sea. Marine Ecology Progress Series 60(1):185-203.
- ^ Okubo, N., Mezaki, T., Nozawa, Y., Nakano, Y., Lien, Y., & Fukami, H. et al. (2013). Comparative Embryology of Eleven Species of Stony Corals (Scleractinia). Plos ONE, 8(12), e84115. http://dx.doi.org/10.1371/journal.pone.0084115
- ^ __
Chou, L. (2016). Rehabilitation Engineering of Singapore Reefs to Cope with Urbanization and Climate Change Impacts. Journal Of Civil Engineering And Architecture, 10(8). http://dx.doi.org/10.17265/1934-7359/2016.08.009 - ^ Leal, M., Ferrier-Pagès, C., Petersen, D., & Osinga, R. (2014). Coral aquaculture: applying scientific knowledge to ex situ production. Reviews In Aquaculture, 8(2), 136-153. http://dx.doi.org/10.1111/raq.12087
- ^ Ruppert, E.E., R.S. Fox and R.D. Barnes (2004) Invertebrate Zoology. A functional evolutionary approach. 7th Ed. Brooks/Cole, Thomson Learning learning, Inc. 990 p.
- ^ Tomascik, T., Mah, A., & Nontji, A. (1997). The ecology of the indonesian seas. Part 1 [et] 2. [Oxford]: Oxford University Press.
- ^ Coral Reefs of Singapore. (2017). Coralreef.nus.edu.sg. Retrieved 3 December 2017, from http://coralreef.nus.edu.sg/singapore.html
- ^ Guest, J., Tun, K., Low, J., Vergés, A., Marzinelli, E., & Campbell, A. et al. (2016). 27 years of benthic and coral community dynamics on turbid, highly urbanised reefs off Singapore. Scientific Reports, 6(1). http://dx.doi.org/10.1038/srep36260
- ^ Sheppard, A., Fenner, D., Edwards, A., Abrar, M. & Ochavillo, D. (2014). Echinophyllia aspera. The IUCN Red List of Threatened Species 2014: e.T133172A54206928. http://dx.doi.org/10.2305/IUCN.UK.2014-1.RLTS.T133172A54206928.en. Downloaded on 03 November 2017.
- ^ Ng, C.S.L., Toh, T.C., and Chou, L.M. (2013). Current status of coral reef restoration in Singapore. The Asian Conference on Sustainabil- ity, Energy and the Environment Official Conference Proceedings 2013, pp. 546–558.
- ^ Sheppard, A., Fenner, D., Edwards, A., Abrar, M. & Ochavillo, D. (2014). Echinophyllia aspera. The IUCN Red List of Threatened Species 2014: e.T133172A54206928. http://dx.doi.org/10.2305/IUCN.UK.2014-1.RLTS.T133172A54206928.en. Downloaded on 03 November 2017.
- ^ Bridge, T., Fabricius, K., Bongaerts, P., Wallace, C., Muir, P., Done, T., & Webster, J. (2011). Diversity of Scleractinia and Octocorallia in the mesophotic zone of the Great Barrier Reef, Australia. Coral Reefs, 31(1), 179-189. http://dx.doi.org/10.1007/s00338-011-0828-
- ^ Coral Reefs of Singapore. (2017). Coralreef.nus.edu.sg. Retrieved 3 December 2017, from http://coralreef.nus.edu.sg/singapore.html
- ^ TAN, A. (2017). Coral nurseries bloom under special care in waters off the coast. The Straits Times. Retrieved 3 December 2017, from http://www.straitstimes.com/singapore/coral-nurseries-bloom-under-special-care-in-waters-off-the-coast
- ^ Ng, C.S.L., Toh, T.C., and Chou, L.M. (2013). Current status of coral reef restoration in Singapore. The Asian Conference on Sustainabil- ity, Energy and the Environment Official Conference Proceedings 2013, pp. 546–558.
- ^ TAN, A. (2017). Rescued corals thrive on sea walls. The Straits Times. Retrieved 3 December 2017, from http://www.straitstimes.com/singapore/environment/rescued-corals-thrive-on-sea-walls
- ^ Papua New Guinea, PNG, Milne Bay Province, Coral Diversity, Scleractinian Corals. (2017). Biophysics.sbg.ac.at. Retrieved 3 December 2017, from http://biophysics.sbg.ac.at/png/png3.htm
- ^ Flat Lettuce Coral - Echinophyllia aspera - Encyclopedia of Life. (2017). Eol.org. Retrieved 15 November 2017, from http://www.eol.org/pages/200391/data
- ^ Papua New Guinea, PNG, Milne Bay Province, Coral Diversity, Scleractinian Corals. (2017). Biophysics.sbg.ac.at. Retrieved 3 December 2017, from http://biophysics.sbg.ac.at/png/png3.htm
- ^ Papua New Guinea, PNG, Milne Bay Province, Coral Diversity, Scleractinian Corals. (2017). Biophysics.sbg.ac.at. Retrieved 3 December 2017, from http://biophysics.sbg.ac.at/png/png3.htm
- ^ Purkis, S., & Riegl, B. (2012). Coral Reefs of the Gulf: Adaptation to Climatic Extremes (Coral Reefs of the World). Springer Netherlands.
- ^ Echinophyllia aspera. Corals of the World - Photos, maps and information about corals and reefs. (2017). Coral.aims.gov.au. Retrieved 15 November 2017, from http://coral.aims.gov.au/factsheet.jsp?speciesCode=0136
- ^ Papua New Guinea, PNG, Milne Bay Province, Coral Diversity, Scleractinian Corals. (2017). Biophysics.sbg.ac.at//. Retrieved 3 December 2017, from http://biophysics.sbg.ac.at/png/png3.htm
- ^ J. R. Guest, J. Low, K. Tun, B. Wilson, C. Ng, D. Raingeard, K. E. Ulstrup, J. T. I. Tanzil, P. A. Todd, T. C. Toh, D. McDougald, L. M. Chou & P. D. Steinberg. (2017) Coral community response to bleaching on a highly disturbed reef
- ^ Huang, D., Arrigoni, R., Benzoni, F., Fukami, H., Knowlton, N., & Smith, N. et al. (2016). Taxonomic classification of the reef coral family Lobophylliidae (Cnidaria: Anthozoa: Scleractinia). Zoological Journal Of The Linnean Society, 178(3), 436-481. http://dx.doi.org/10.1111/zoj.12391
- ^ Huang, D., Arrigoni, R., Benzoni, F., Fukami, H., Knowlton, N., & Smith, N. et al. (2016). Taxonomic classification of the reef coral family Lobophylliidae (Cnidaria: Anthozoa: Scleractinia). Zoological Journal Of The Linnean Society, 178(3), 436-481. http://dx.doi.org/10.1111/zoj.12391
- ^ Huang, D., Arrigoni, R., Benzoni, F., Fukami, H., Knowlton, N., & Smith, N. et al. (2016). Taxonomic classification of the reef coral family Lobophylliidae (Cnidaria: Anthozoa: Scleractinia). Zoological Journal Of The Linnean Society, 178(3), 436-481. http://dx.doi.org/10.1111/zoj.12391
- ^ Huang, D., Arrigoni, R., Benzoni, F., Fukami, H., Knowlton, N., & Smith, N. et al. (2016). Taxonomic classification of the reef coral family Lobophylliidae (Cnidaria: Anthozoa: Scleractinia). Zoological Journal Of The Linnean Society, 178(3), 436-481. http://dx.doi.org/10.1111/zoj.12391
- ^ Huang, D., Arrigoni, R., Benzoni, F., Fukami, H., Knowlton, N., & Smith, N. et al. (2016). Taxonomic classification of the reef coral family Lobophylliidae (Cnidaria: Anthozoa: Scleractinia). Zoological Journal Of The Linnean Society, 178(3), 436-481. http://dx.doi.org/10.1111/zoj.12391
- ^ Huang, D., Arrigoni, R., Benzoni, F., Fukami, H., Knowlton, N., & Smith, N. et al. (2016). Taxonomic classification of the reef coral family Lobophylliidae (Cnidaria: Anthozoa: Scleractinia). Zoological Journal Of The Linnean Society, 178(3), 436-481. http://dx.doi.org/10.1111/zoj.12391
- ^ Huang, D., Arrigoni, R., Benzoni, F., Fukami, H., Knowlton, N., & Smith, N. et al. (2016). Taxonomic classification of the reef coral family Lobophylliidae (Cnidaria: Anthozoa: Scleractinia). Zoological Journal Of The Linnean Society, 178(3), 436-481. http://dx.doi.org/10.1111/zoj.12391
- ^ Huang, D., Arrigoni, R., Benzoni, F., Fukami, H., Knowlton, N., & Smith, N. et al. (2016). Taxonomic classification of the reef coral family Lobophylliidae (Cnidaria: Anthozoa: Scleractinia). Zoological Journal Of The Linnean Society, 178(3), 436-481. http://dx.doi.org/10.1111/zoj.12391
- ^ Arrigoni, R., Berumen, M., Chen, C., Terraneo, T., Baird, A., Payri, C., & Benzoni, F. (2016). Species delimitation in the reef coral genera Echinophyllia and Oxypora (Scleractinia, Lobophylliidae) with a description of two new species. Molecular Phylogenetics And Evolution, 105, 146-159. http://dx.doi.org/10.1016/j.ympev.2016.08.023
- ^ Arrigoni, R., Berumen, M., Chen, C., Terraneo, T., Baird, A., Payri, C., & Benzoni, F. (2016). Species delimitation in the reef coral genera Echinophyllia and Oxypora (Scleractinia, Lobophylliidae) with a description of two new species. Molecular Phylogenetics And Evolution, 105, 146-159. http://dx.doi.org/10.1016/j.ympev.2016.08.023
- ^ Echinophyllia aspera | Taxonomy Browser | BOLDSYSTEMS. (2017). Boldsystems.org. Retrieved 15 November 2017, from http://www.boldsystems.org/index.php/Taxbrowser_Taxonpage?taxid=29916