=
 |
| Figure 1.1: A E. spelaea feeding on a Durian flower. E. spelaea are almost exclusively the pollinators of Durio spp., therefore without them there will be no durians! (Photo credit and permission: Barbara Maslen and Allen Sheather). |
Table of Contents
1 General Information
Eonycteris spelaea is the only animal that aids the reproduction of the durian plant, an important source of income for Southeast Asian countries. However, its habitat is quickly being depleted due to human intervention. Their diet consists mainly of pollen and nectar and they are important pollinators when searching for trees and shrubs, travelling many kilometers each night. They are nocturnal animals, only feeding later than dusk.
 |
| Figure 1.2: Eonycteris spelaea feeding on nectar of flowers that only open at night. (Photo credit and permission: Nick Baker) |
2 Morphology
2.1 Physical Description
Eonycteris spelaea have small, simple ears, big eyes and does not have a tragus (Figure 2.1). The tongue is long and extendable with rasp-like protrusions and its muzzle is narrow and dog-like (Figure 2).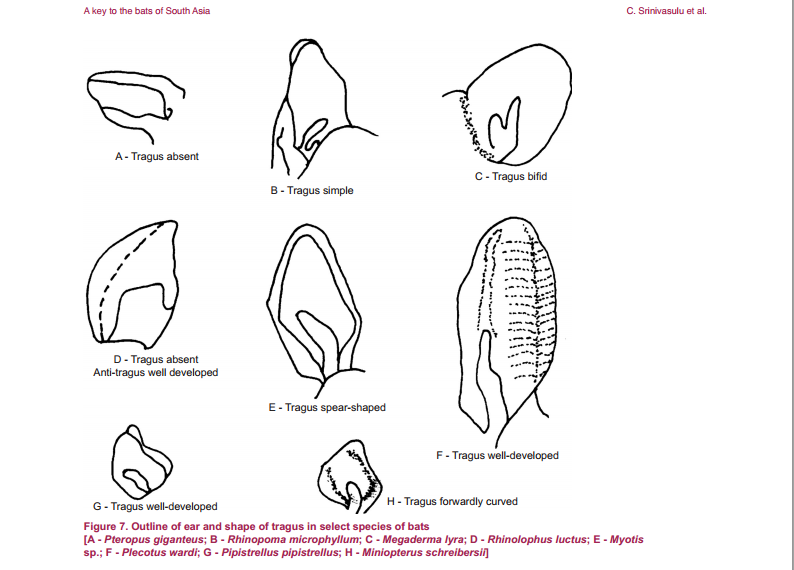 |
| Figure 2.1: Tragus of different species of bats. Tragus is absent from E. spelaea. Diagram credit: (C. Srinivasulu et al, 2001). |
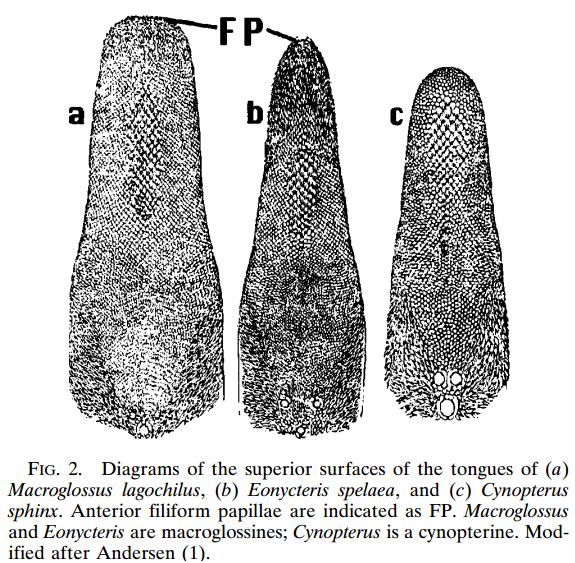 |
| Figure 2.2. One of the unique features of E. spelaea is the tongue, as seen her compared to the tongues of two other species. Diagram credit: (Hollar and Springer, 1997) (permission pending) |
Dorsal pelage is dark brown (dark grey) and the belly is of a lighter shade. Long scent-dispersing “osmetrichia” hairs cover the necks of males and are darker (tinged yellow brown) than the pelage of the head and body. Molariform teeth are considerably reduced and barely extend past the gums. It has a range mass of 35-82g and an average mass of 60g, It has a range length of 8.5-12.5mm, forearm length of up to 7cm and a range wingspan of 60-85mm and an average wingspan of 72.5mm. (Figure 2.2) [1] It has a range basal metabolic rate of 0.881-0.979 cm3 O2/g/hr and an average basal metabolic rate of 0.93 cm3 O2 /g/hr.[2] They are homiothermic, bilaterally symmetrical and males of the species are larger than females, contributing to the sexual dimorphism.[4].
To distinguish Eonycteris, look out for the type of tongue, and absence of the index finger claw.[3] Also, note that they do not possess a tragus. Their tails lengths are generally between 12 and 33mm as well.
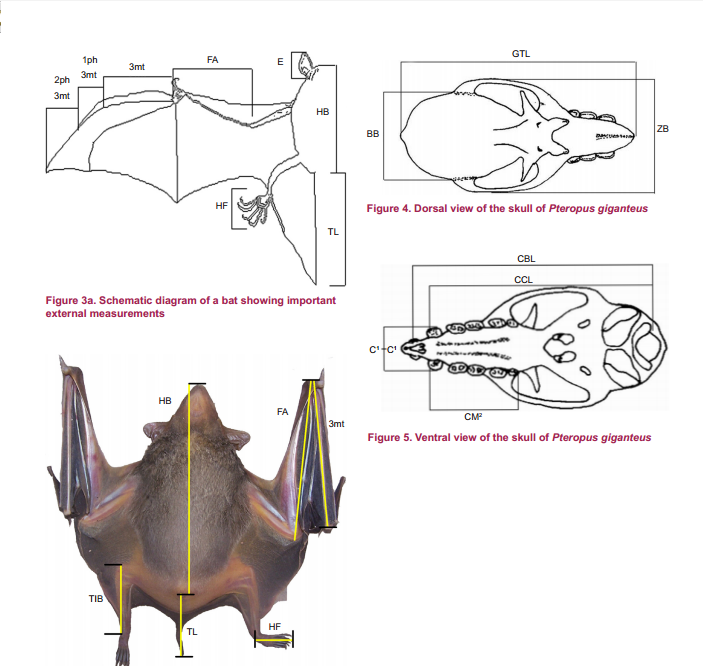 |
| Figure 2.3: The key measurements to a bat's physical morphology with a Pteropus giganteus bat specimen. Eonycteris spelaea is from the same Family and can be measured in a similar way. Diagram credit: (C. Srinivasulu et al, 2001) |
3 Distribution
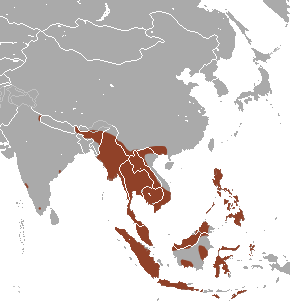 |
| Figure 3.1: Highlighted in brown is the geographic range of E. spelaea. (photo credit: wikipedia) |
It lives in sea-level to elevated subtropical or tropical forest and caves of northern south Asia. They are native in the following countries: Brunei Darussalam; Cambodia; China; India (Andaman Is., Andhra Pradesh, Assam, Karnataka, Manipur, Meghalaya, Nagaland, Nicobar Is., Sikkim, Tamil Nadu, Uttaranchal); Indonesia; Lao People's Democratic Republic; Malaysia; Myanmar; Philippines; Singapore; Thailand; Timor-Leste; Viet Nam. (Figure 3.2)[5]
3.1 Home Range
Although there is little information about Eonycteris spelaea home range, they are known to travel vast distances of 20 to 40 km to forage from their roosts to the trees that flower at night. In family Pteropodidae, it is common to have long flights between roosting and feeding locations.[6]4 Population
 |
| Figure 4.1: There can be as many as 50,000 individuals in a roost and Eonycteris spelaea share its roost with other species too. (Photo credit and permission: Nick Baker) |
5 Habitat and Ecology
5.1 Habitat
Eonycteris spelaea dominantly inhabits caves as roosting sites in tropical regions. However, they are also common in other habitats ranging from agricultural types away from forests and different forest types such as secondary lowland, primary lowland and transitional montane-mossy forests.[11] In the caves, E. spelaea forms close communities and often cohabits with other bats. E. spelaea flies rather slowly, and has adapted to expand its niche to include flowers of many orchard and agricultural crops in its diet.[12] Their range elevation is 50 to 1,250 m and the average elevation is 500 m.[13] In Singapore, their distribution and habitat is not well-researched on, but a population of E. spelaea is found under the Bukit Timah Flyover. (per comm. Coleman) |
| Figure 5.1: Eonycteris spelaea have adapted to the urbanized environment in Singapore and have been spotted roosting under flyovers near forest patches. (Photo credit and permission: Nick Baker) |
6 Ecosystem Roles
The main and most important ecological function of E. spelaea is pollination. This species is a pollinator of many forest tree species including Durians. Without them, durian trees will never be able to bear the fruits that we love! The pollination process occurs when the bat feeds on the nectar of the flower. As its face approaches the flower it is dusted with pollen grains, which will then be transferred to the next flower the bat visits.[14] A possible secondary ecological role is seed dispersal, but consuming fruit as food is not confirmed.
Watch the video to see how fast Enoycteris spelaea are in feeding and pollinating a flower! Video with permission from: Baylis and Fletcher.
7 Reproduction
7.1 Mating Habits
The age range where an E. spelaea male reaches sexual or reproductive maturity is 1 to 2 years. Females reach sexual maturity from 6 months to 1 year. Reports suggest that the females are sole providers of the young before they grow to be independent. After birth, the altricial young will suckle on a nipple and remain attached for 4 to 6 weeks while the mother flies around, after which the young can independently fly for short distances. There is provisioning of food from the mother, as well as protection goes on for about 3 months before the young is completely weaned off.[15]7.2 Mating System
E. spelaea is polygynous, meaning the males freely mate with other individuals throughout their lifetime. There is conflicting information on the cycle or pattern of the female phase of being sexually active as well as birth in E. spelaea females. One of the study reported that females exhibits a pattern characterized by synchronous births and seasonal, bimodal polyestry, the other sources find no synchronicity between females and no seasonal synchronicity. E. spelaea usually have 2 offspring every year, with a gestation period of 3 to 4 months; however, some reports suggest gestation periods that lasts as long as 200 days (between 6 and 7 months). [16]8 Behaviour
Eonycteris spelaea are troglophilic and sociable, especially in their roosts in ceilings of caves with colonies numbering from a dozen to tens of thousands of individuals. Within the colony there is division into sexually segregated groups.[17] The cave nectar bat forage in flocks from 7pm to 2am after their daily torpor.[18] A special behavior that E. spelaea possess is producing of sounds made by wing-clapping when moving in dark environments. This is postulated to be the rudimentary form of echolocation which helps orientate the bat, and alternatively it may just be due to the slower flight of cave nectar bats which helps reduce the impact should they collide with walls or objects in dark caves.[19] Roost caves of E. spelaean are known to be shared with colonies of Rousettus leschenaultia and E. major.
9 Communication and Perception
Eonycteris spelaean do not navigate by echolocation, as is its family Pteropodidae, a group of megabats that has well-developed eyes that provides sufficient vision in twilight caves. Moreover, Pteropodidae species have small, simple ears hence visual perception is their dominant way to sense the environment.[20] Other than visual senses, they might use olfactory senses, considering the presence of “osmetrichia” hair on the back of males which disperses scent over large distances. It is probably used in mating rituals, and in many species the odour in males are stronger than females which indicates the use of olfaction to attract mates.[21] Lastly, E. spelaean might also use sound as they are unique in the production of special wing-clapping sounds, which might either be an earlier form of echolocation.[22] They also use tactile senses to interact with their environment. Most Pteropodidae species locate food by smell.[23]
10 Food Habits
Their diet consists primarily of the nectar and pollen of plant species that flower at night, hence they are nectarivorous generalists and have been documented to feed over 31 plant species. They are hence also classified as herbivores.[24] Two studies found that pollen is found in their stomach and tongue exclusively. In particular, they prefer the nectar and pollen of Oroxylum indicum. Eonycteris spelaea and O. indicum are referred to as an example of coevolution because the flowers evolved to adapt to the head morphology and feeding behaviour of the bat.[25] Other species of plants include Durio zibethinus, Parkia speciosa, Musa acuminata, and Ficus species. [26] The cave nectar bat have shown a particular affinity for a specific species of Agave.
 |
| Figure 10.1: E. spelaea feeding. (Photo credit and permission: Nick Baker) |
11 Predation
This species is likely to fall prey to predation by climbing snakes and nocturnal birds such as owls. Like most other bats, their ability to fly, nocturnality and habit of roosting in inaccessible places is a form of protection from predation. However, there is not much information on the specific predators of this species and their adaptations to evade it.
12 Conservation
12.1 Threats
Overall, this species is not majorly threatened. According to IUCN, they are in the lower risk/least concern category.[27] However, in some parts of South East Asia, land use change such as deforestation for agriculture and other uses poses a threat locally.[28] The increase popularity of cave tourism and using of headlamps by tourists provides
emerging pressure on the species in some caves, for example in Borra Caves, Andhra Pradesh.[29] Bats are nocturnal animals, the intrusion of tourists in the day might disrupt their rest (daily torpor). In countries such as China they are hunted heavily for food.[30]
12.2 Conservation Actions
This species is adaptable, and is present in many protected areas throughout its range. No direct conservation measures are currently needed for the species as a whole. In fact in South Asia, this species, like most other fruit bats in India is considered a vermin. E. spelaea has been recorded from protected areas like Kalakkad-Mundunthurai Tiger Reserve in Tamil Nadu.[31] Despite that, this species should still be monitored for the populations to record any changes in abundance and distribution and also taking it out from the vermin category. This is crucial because E. spelaea is important in its ecosystem function as a pollinator and labelling it as a vermin is fundamentally wrong as there are no damages to crop.On top of that, awareness should be raised on the repercussions of disturbing its cave habitats. This comes after populations in Java and the Lesser Sundas Islands are now endangered due to hunting, habitat destruction and cave disturbance. The subspecies E. spelaea glandifera found in Indonesia, the Philippines and Sulawesi was reported as vulnerable 1992. Although populations of E. spelaea glandifera in the Philippines seem more resistant to habitat changes but are still vulnerable to human disturbances and hunting.[32]
13 Lifespan/Longevity
Little information is known about the lifespan of Eonycteris spelaea in the wild or even in captivity. When it comes to other Pteropodidae species, the longest known lifespan in captivity is the straw-colored fruit bats (Eidolon helvum) which live up to 21.8 years; flying foxes Pteropus, 31.4 years; and rousette fruit bats, 22.9 years.[33]
14 Ecosystem Services
14.1 Importance to Humans: Benefits
The most significant ecosystem services of E. spelaea is the pollination of plants, and by pollinating commercially important species they are very useful to humans.[34] This is especially for durian fruits (Durio spp.), which can contribute up to $120 million (U.S. dollars) to the Southeast Asian economy every year. They are also hunted for food in some countries. In the Philippines, bat guano is collected for use as fertilizer.[35] |
| Figure 14.1: Eonycteris spelaea is an important pollinator that benefits not only the ecosystem, but humans as well when they pollinate and propagate economically viable species of plants such as Durian. (Photo credit and permission: Fletcher and Baylis) |
14.2 Importance to Humans: Threats
Right now, there are no evidence of any adverse effects of E. spelaea on humans.However, bats are known to be vectors of many pathogens and viruses.15 Taxonomy
Binomial name: Eonycteris spelaea (Dobson, 1871)
15.1Common Names
English-Dawn Bat, Common Dawn Bat, Common Nectar Bat, Lesser Dawn Bat15.2 Taxonavigation
| Domain: Eukaryota |
|||||||
| Kingdom:Animalia |
|||||||
| Phylum:Chordata |
|||||||
| Class:Mammalia |
|||||||
| Order:Chiroptera |
|||||||
| Family:Pteropodidae |
|||||||
| Genus:Eonycteris |
|||||||
| Species:Eonycteris spelaea |
15.3 Synonyms
| Eonycteris spelaea, subspecies winnyae |
Maharadatunkamsi & Kitchener, 1997 |
| Eonycteris bernsteini |
Tate, 1942 |
| Eonycteris spelaea, subspecies glandifera |
Lawrence, 1939 |
| Eonycteris spelaea, subspecies rosenbergii |
Jentink, 1889 |
| Macroglossus spelaeus |
Dobson, 1871 |
15.4 First Description
The first description of Eonycteris spelaea, then identified and named as Macroglossus spelaea, was in 1871 by Dobson G E and published under Proceedings of Asiatic Society.
 |
| Figure 15.1: First description of E. spelaea (Macroglossus spelaea) in 1871 by Dobson G E. (Abstract Credit: Biodiversity Heritage Library) |
 |
| Figure 15.1: Nomenclatural change in 1873 by Dobson from M. spelaea to E. spelaea. (Abstract credit: Biodiversity Heritage Library) |
15.5 Phylogeny
In a phylogenetic study on old world bats by Laura J. Hollar and Mark S. Springer in 1997, the "maximum likelihood, minimum evolution, and parsimony (uniform and differential weighting schemes) were used to estimate phylogenetic trees using the three microchiropterans as outgroups. These analyses, including topology-dependent permutation tail probability (T-PTP) tests, bootstrapping, and the Kishino–Hasegawa test, were conducted with PAUP 4.0d49 and 4.0d52 for Power MacIntosh. Bootstrap and T-PTP tests included 500 replications except for the maximum-likelihood analysis with a gamma rate distribution (100 replications)." [36]
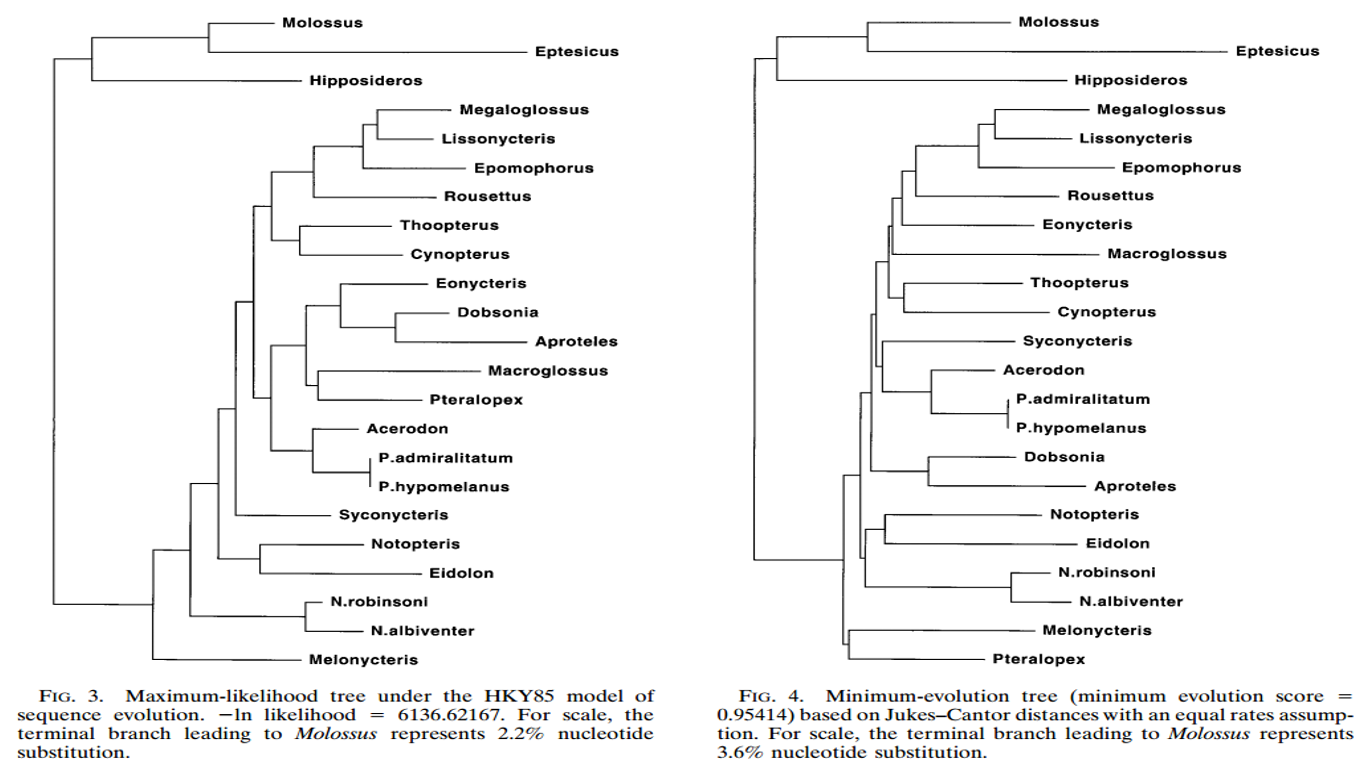 |
| Figure 15.2: Phylogenetic tree of the Family of old world fruit bats. Diagram credit: (Hollar and Springer, 1997) (permission pending) |
 |
| Figure 15.3: A strict consensus tree adapted to five equally strongest parsimonious trees (962 steps) with uniform weighting. The numbers above branches are decay indices. Diagram credit: (Hollar and Springer, 1997) (permission pending) |
16 Glossary
Altricial - "requiring nourishment", refers to a pattern of growth and development in organisms which are incapable of moving around on their own soon after hatching or being
born.
Bat guano – excrement of bats
Bilaterally Symmetrical - having similarity in size, shape, and relative position of corresponding parts
Homiothermic – warm blooded
Molariform - resembling a molar tooth especially in shape
Olfactory - sense of smell
Parsimony - favouring trees that suppose the least evolutionary change to explain observed data
Pelage - the hairy covering of a mammal
Polyestry – more than one breeding season per year
Tactile - sense of touch
Tragus – the ear of bats
Troglophilic – able to spend its whole life in caves
Weaned - To accustom (the young of a mammal) to take nourishment other than by suckling.
17 References
[1]Kunz, T., M. Fenton. 2003. Bat Ecology. University of Chicago Press.
[2] Brian K. McNab. 1989. Temperature Regulation and Rate of Metabolism in Three Bornean Bats. Journal of Mammalogy, 70: 153–161
[3] C. Srinivasulu, Paul A. Racey & Shahroukh Mistry. 2010. A key to the bats (Mammalia: Chiroptera)
of South Asia. Journal of Threatened Taxa. 2(7): 1001–1076
[4]Macdonald, D. 2001. The Encyclopedia of Mammals. New York, New York: Barnes & Nobel Books.
[5] Francis, C., Rosell-Ambal, G., Tabaranza, B., Carino, P., Helgen, K., Molur, S. & Srinivasulu, C. 2008. Eonycteris spelaea. The IUCN Red List of Threatened Species. Version 2014.3. Date Accessed: 11th November 2014.
[6]Neuweiler, G. 2000. The Biology of Bats. New York, New York: Oxford University Press.
[7] P. J. J. Bates; D. L. Harrison Jay F. Storz. 1998. Bats of the Indian Subcontinent. Journal of Mammalogy,
79(4): 1441–1443
[8] P. D. Heideman and L. R. Heaney. Population biology and estimates of abundance of fruit bats (Pteropodidae) in Philippine submontane rainforest. Journal of Zoology. 218(4): 565–586,
[9] Francis, C., Rosell-Ambal, G., Tabaranza, B., Carino, P., Helgen, K., Molur, S. & Srinivasulu, C. 2008. Eonycteris spelaea. The IUCN Red List of Threatened Species. Version 2014.3. Date Accessed: 11th November 2014.
[10] Vanitharani. 2005. Noteworthy representatives of bat species in Agasthiyamalai biosphere reserve, Tamil Nadu, India. Journal of Theoretical and Experimental Biology. 2(2): 47–59
[11] Findley, J. 1993. Bats: a community perspective. New York, New York: Cambridge University Press
[12] A. Smith and Y. Xie eds. 2008. A Guide to the Mammals of China. Princeton University Press.
[13]Reinke, A. 2007. Eonycteris spelaea, Animal Diversity Web. Date Accessed: 14th November 2014.
[14]Allen, G. 1939. Bats. Cambridge, MA: Harvard University Press.
[15]Nowak, R. 1999. Walker’s Mammals of the World. Baltimore and London, Maryland and England: The Johns Hopkins University Press.
[16]Hutchins, M., D. Kleiman, G. Valerius, M. McDade. 2003. Grzimek's Animal Life Encyclopedia, 2nd edition. Farmington Hills, MI: Gale Group.
[17]Reinke, A. 2007. Eonycteris spelaea, Animal Diversity Web. Date Accessed: 14th November 2014.
[18]Brian K. McNab. 1989. Temperature Regulation and Rate of Metabolism in Three Bornean Bats. Journal of Mammalogy, 70: 153–161
[19] Edwin Gould, 1988. MammalogistsWing-Clapping Sounds of Eonycteris spelaea (Pteropodidae) in Malaysia. Journal of Mammalogy, 69: 378–379.
[20] Macdonald, D. 2001. The Encyclopedia of Mammals. New York, New York: Barnes & Nobel Books.
[21]Macdonald, D. 2001. The Encyclopedia of Mammals. New York, New York: Barnes & Nobel Books.
[22] Edwin Gould, 1988. MammalogistsWing-Clapping Sounds of Eonycteris spelaea (Pteropodidae) in Malaysia. Journal of Mammalogy, 69: 378–379.
[23]Kunz, T., M. Fenton. 2003. Bat Ecology. University of Chicago Press.
[24]Hutchins, M., D. Kleiman, G. Valerius, M. McDade. 2003. Grzimek's Animal Life Encyclopedia. 2nd edition. Farmington Hills, MI: Gale Group.
[25] Neuweiler, G. 2000. The Biology of Bats. New York, New York: Oxford University Press.
[26] Edwin Gould. 1978. Foraging Behavior of Malaysian Nectar-Feeding Bats. Biotropica, 10: 184–193
[27] Francis, C., Rosell-Ambal, G., Tabaranza, B., Carino, P., Helgen, K., Molur, S. & Srinivasulu, C. 2008. Eonycteris spelaea. The IUCN Red List of Threatened Species. Version 2014.3. Date Accessed: 11th November 2014.
[28] Molur et al. Status of South Asian Chiroptera. 2002. Date Accessed: 11th November 2014.
[29] C. Srinivasulu, Paul A. Racey & Shahroukh Mistry. 2010. A key to the bats (Mammalia: Chiroptera)
of South Asia. Journal of Threatened Taxa. 2(7): 1001–1076
[30] A. Smith and Y. Xie eds. 2008. A Guide to the Mammals of China. Princeton University Press.
[31] Francis, C., Rosell-Ambal, G., Tabaranza, B., Carino, P., Helgen, K., Molur, S. & Srinivasulu, C. 2008. Eonycteris spelaea. The IUCN Red List of Threatened Species. Version 2014.3. Date Accessed: 11th November 2014.
[32] Francis, C., Rosell-Ambal, G., Tabaranza, B., Carino, P., Helgen, K., Molur, S. & Srinivasulu, C. 2008. Eonycteris spelaea. The IUCN Red List of Threatened Species. Version 2014.3. Date Accessed: 11th November 2014.
[33]Jones, M. 1982. Longevity of captive mammals. Zoological Garten N. F. Jena, 52: 113–128.
[34] Robert Hodgkison, Sharon T. Balding, Akbar Zubaid and Thomas H., 2003.
Fruit Bats (Chiroptera: Pteropodidae) as Seed Dispersers and Pollinators in a Lowland MalaysianRain Forest. KunzSource: Biotropica, 5: 491–502.
[35] Wilson, D. 1997. Bats in Question. Washington and London: Smithsonian Institution Press.
[36] Laura J Hollar and Mark S. Springer, 1997. Old World fruitbat phylogeny: Evidence for convergent evolution and an endemic African clade. Proceedings of the National Academy of Sciences of the United States of America, 94: 5716–5721