Table of Contents
Species name: Megabalanus coccopoma (Darwin, 1854)
 |
| The non-native titan acorn barnacle, Megabalanus coccopoma in Florida. Photograph courtesy: Janson Jones |
Did you know...
......They are not clams or snails. They are more similar to crabs and shrimps.
......They have the longest penis length relative to body size in the animal kingdom.
......They were firstly described by Charles Darwin in 1854.
......They are invasive species.
......They respond to competition by growing to a very large size, which makes them less prone to predation and a candidate for human consumption.
1- Overview
1.1 Classification
Biota > Animalia (Kingdom) > Arthropoda (Phylum) > Crustacea (Subphylum) > Maxillopoda (Class) > Thecostraca (Subclass) > Cirripedia (Infraclass) > Thoracica (Superorder) > Sessilia (Order) > Balanomorpha (Suborder) > Balanoidea (Superfamily) > Balanidae (Family) > Megabalaninae (Subfamily) > Megabalanus (Genus)
1.2 Etymology
Megabalanus literally means 'large barnacle.' Its Dutch name 'grote roze zeepok' means big pink acorn-shell. Commonly known as megabalanaid barnacle. In literature, they can be found with the following names:
- Megabalanus californicus (Pilsbry, 1916)
- Megabalanus stultus (Darwin, 1854)
- Megabalanus tanagrae (Pilsbry, 1928)
- Megabalanus tintinnabulum (Linnaeus, 1758) [1]
1.3 Brief summary
The titan acorn barnacle Megabalanus coccopoma, is native to Pacific coasts of Central and South America. Although not native to the Atlantic, its range extends along the U.S. Atlantic coast and in the Gulf of Mexico. This balanid (acorn barnacle family) is very large in size, pink in color and growing to over 5 cm in height and width [2]. When identifying balanomorph barnacles, its morphological features are helpful. In addition to its shell morphology, anatomical characters are used to identify the subfamily and genus levels. Coloration can be helpful, but it is of secondary importance to distinguish M. coccopoma from other subfamily and genus groups [3].
 |
| Megabalanus coccopoma growing on Asian green mussel. As a reference, the Asian green mussel is 7.7 cm in length. |
2- Invasion Biology
2.1 What are invasive species?
Invasive species are the plants or animals those aggressively establish themselves in a new space over the species already in the space they enter (Alpert et al. 2000) [4]. Although it is known that non-native species can provide a limiting resource or habitat, replace a native species, and provide an escape route from enemies or competitors, their potential invasibility is still considered to be one of the greatest threats to biodiversity and community structure [5, 6]. Below is a figure explaining what alien invasion is in detail. The pictured organisms are potential invasives that are found in Singapore.
 |
| What is alien invasion? Source: NParks, WildLife Singapore, National University of Singapore |
- Why are invasive alien species considered bad?
- How do invasive alien species get introduced?
2.2 Distribution and invasion history
The titan acorn barnacle or Megabalanus coccopoma is an opportunistic species and is part of the fouling community on ships hulls and other hard substrates. It originally only occurred along the west coasts of Central- and South-America. Its dispersal capabilities are well known, since its establishment ranges from the western Atlantic (Brazil and the northern Gulf of Mexico to the Carolinas), northwestern Europe and the western Indian Ocean (Mauritius) [10]. The first time this species was observed in Europe was in 1851, on the hull of a ship docked at Le Havre, France. In Belgium, the titan acorn barnacle was first observed on buoys on the coast in 1997 [11]. The most probable introduction vectors can be named as ship hull fouling and ballast water transport. Below, Table 1 provides the year and the locations for the known history of titan acorn barnacle invasion.
Table 1: Known history of invasion of Megabalanus coccopoma [12]
| Year |
Location |
| 1980s |
South Brazil |
| 1987 |
San Diego, California, USA [13], Belgium [14] |
| 2002 ~2006s |
Louisiana, N. Florida, Georgia, N. Carolina, East USA |
| 2007 ~2009s: |
Japan |
2.3 Potential Economic Impacts of Invasion
Boat hulls props, and drive shafts, and coastal navigation buoys are readily colonized by settling Megabalanus coccopoma [1]. These organism attach on surfaces by gluing themselves. Ships need to use extra fuel to overcome the drag caused by accumulations of these animals on hulls. Combating this problem is in fact a multi-million dollar industry for marine paint countries. Furthermore, usage of heavy metal paints and synthetic compounds cause damage to the environment. Thus in case they are established, costs associated with removing animals from such structures may be expensive [15]. Factors such as rapid growth, and large size suggest M. coccopoma has the potential to become an economically important nuisance species in Florida [16].
2.4 Potential Biodiversity and Ecological Impacts
Once introduced, eradication of these species is nearly impossible. The real cost is a large annual budget for pest management. For instance, cost of eradication of Mytilopsis sallei from Darwin Harbour is almost equal to US$1.6m excluding manpower costs. Furthermore, management of zebra mussels in the Great Lakes can cost up to US$100m/yr [17]. If combating with invasive species is by means of toxic and chemical based materials, it may increase the risk of bioaccumulation of toxic substances in the environment, thus reducing fitness of native biodiversity, resulting in native habitats becoming more susceptible to invasions.
 |
| The non-native titan acorn barnacle, Megabalanus coccopoma. Photograph courtesy: Cedric Audibert |
2.5 What is so special about Singapore?
Being a global hub for shipping and trade, and providing a crossroad between biogeographical regions, Singapore is vulnerable to the introduction of invasive species that enter the tropical Southeast Asia region [18, 19]. According to Yamaguchi et al. (2009), several species of barnacle, including A. amphitrite, Megabalanus tintinnabulum and Megabalanus coccopoma have been dispersed by shipping throughout the subtropic and tropic waters of the world due to increasing marine traffic [20]. Two sessile alien barnacles, Megabalanus coccopoma and Amphibalanus variegatus were already observed on vessels coming into the port of Singapore, although not in local fouling communities [21]. Hutchings et al. (2002) hypothesized that high biodiversity and low endemicity offer fewer niches for NIS to become established and become invasive (also known as biotic resistance hypothesis). On the other hand, stressed marine ecosystems and man-made environments are vulnerable for invasions into a new region [22]. Regarding to its wide and continuous dispersal capability and history of introduction in many different regions of the world, they may be well established in Singapore waters as well.
 |
| A busy day in Port of Singapore |
According to the following study, Introduced Species In Singapore: An Overview, by Assistant Professor Darren Yeo of the National University of Singapore and Ms Cheryl Chia of National Parks Board (NParks) (2010), Singapore does not have a centralized national body that is responsible for the coordination and management of invasive species, nor does it have any regulation that deals specifically with invasive species issues [23]. Invasive alien species are very costly to control. The most efficient and low-cost solution to tackle with potential invasive species is early detection and stopping them from becoming permanently established. It, however, requires a body of regulations and legislations. A list of marine invasive species that are found on shipping in Singapore waters are provided below, in Table 2.
Table 2: Alien marine invasive species detected as biofuoling on shipping in Singapore waters [24]. M. coccopoma is marked in yellow.
| Species |
Family |
Probable origin |
| Cirripedia |
||
| Amphibalanus variegatus |
Balanidae |
Indo west-Pacific |
| Megabalanus coccopoma |
Balanidae |
Eastern Pacific |
| Decapoda: Bracyhura |
||
| gen. n., sp. n. |
Acidopsidae |
Unknown |
| Glabropilumnus seminudus |
Pilumnidae |
East Indo-West Pacific |
| Pilumnus cf. schellenbergi |
Pilumnidae |
Ausrtalia and New Guinea |
| Carupa tenuipes |
Portunidae |
Indo-West Pacific |
| Thalamitoides quadridens |
Portunidae |
Indo-West Pacific: oceanic |
| Chlorodiella laevissima |
Xanthidae |
Indo-West Pacific: oceanic |
| Liomera cinctimana |
Xanthidae |
Indo-Pacific |
| Liomera monticulosa |
Xanthidae |
Xanthidae Indo-West Pacific: oceanic |
| Liomera rubra |
Xanthidae |
Indo-West Pacific: oceanic |
| Liomera tristis |
Xanthidae |
Indo-West Pacific: oceanic |
| Platypodia tomentosa |
Xanthidae |
West Pacific |
| Pseudoliomera helleri |
Xanthidae |
Indo-West Pacific: oceanic |
| Xanthias punctatus |
Xanthidae |
Indo-west central Pacific |
| Stomatopoda |
||
| Gonodactylaceus randalli |
Gonodactylidae |
Oceanic western Pacific and northern Australia |
3- Life History and Population Biology
3.1 Age, Size, Lifespan
The titan acorn barnacle grows to around 5 cm in height and width. Non-native M. coccopoma found in South Carolina can attain a body mass 100 times greater than that of native barnacle species, according to Tibbets et al (2007) [25]. Juveniles to adults are sessile, means that they cannot migrate themselves but be transported with movement of ships or floating materials. On the other hand, larvae can disperse naturally [12].
3.2 Feeding
It is a suspension feeder, means that by extending its cirri (modified legs) from the aperture at the top of the shell, it can catch plankton [26]. Below you can see the titan acorn barnacle's feathery foot trying to approach the food!
3.3 Reproduction
Reproduction of this particular species is not well known. In general, that of barnacles involves the deposition of sperm into the mantle cavities of adjacent animals via an elongated intermittent tube. Fertilization occurs internally [27]. Larvae are planktonic. After passing through several stages over the course of about three weeks, they settle and undergo metamorphosis. Individual animals are hermaphrodites [28]. Below, you can find the video of mating barnacles!
Mating barnacles from Casey Dunn on Vimeo.
Barnacles are shown to adapt reproductive mechanisms to their surroundings. Former Stony Brook University Department of Ecology and Evolution student J. Matthew Hoch studied barnacle penis morphology. Some of his findings were published in Marine Biology. Barnacles within a sparse population had more of the folds in the penis, showing that they had bigger fully stretched length. Those barnacles having a high population density had fewer folds, indicative of their relatively lower ability to stretch. Furthermore, barnacles on wave exposed coasts can grow larger and their penises grow thicker too. These barnacles have to have thicker penises for more support, making them less prone to be destroyed in the wave action and more likely to produce successful mating attempts. Hence, his findings provide important insights into how both wave action and population density can have significant impacts on penis morphology [29]. Furthemore, since they are sessile in adulthood and unable to self mate, a lengthy penis increases a barnacle's odds of spreading its seed. They regrow their penises each year, just before their brief mating season.Below are the pictures taken from the former study.
 |
| On exposed shores, it's better for barnacles to grow shorter, thicker penises (Image: J. Matt Hoch) |
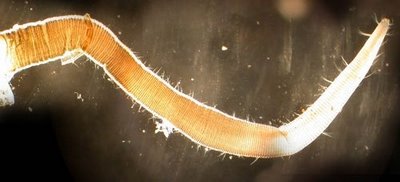 |
| In calm waters, barnacles grow longer, flexible penises with greater reach (Image: J. Matt Hoch) |
3.4 Habitat
They are key components in many marine ecosystems, and found abundantly in the upper rocky intertidal regions. Besides the rocky regions, they tend to settle on new man-made structures such as cables, buoys and the hulls of boats, and also on the shells of bivalve and molluscs. They can also be found on living crabs and snails, one snail can provide habitat for more than one of the barnacles on its shell, which can be considered quite large compared to its own size [30]! They prefer highly saline waters [31]. Under their optimal conditions, such as in cold temperate to tropical and high saline environment, they can grow rapidly [25]. Below, you can find a compilation of acorn barnacle photos [32].
3.5 Phylogeny
A phylogenetic tree is used to show the evolutionary relationships among different biological species by means of their genetic and/or morphological similarities and differences. Various tree optimality criteria such as Maximum Parsimony (MP), Maximum Likelihood (ML), and Bayesian Inference (BI) have been provided for constructing a tree. Below is a phylogram (or phylogenetic tree) showing the relationships of the three distinct species of Megabalanus genus with each other. The first picture (below) shows the phylogram of BI tree indicating the splitting of M. coccopoma, M. rosa and M. volcano as distinct species. M. rosa is in the same lineage as M. coccopoma, and M. volcano is in divergent lineage, respectively. Using five populations from Panama, Brazil, Australia, Japan and Ship hull, Yamaguchi et al (2009) also showed that the haplotype analysis of M. coccopoma provides a single haplotype network with low interspecific genetic variety (second picture, below) [20].
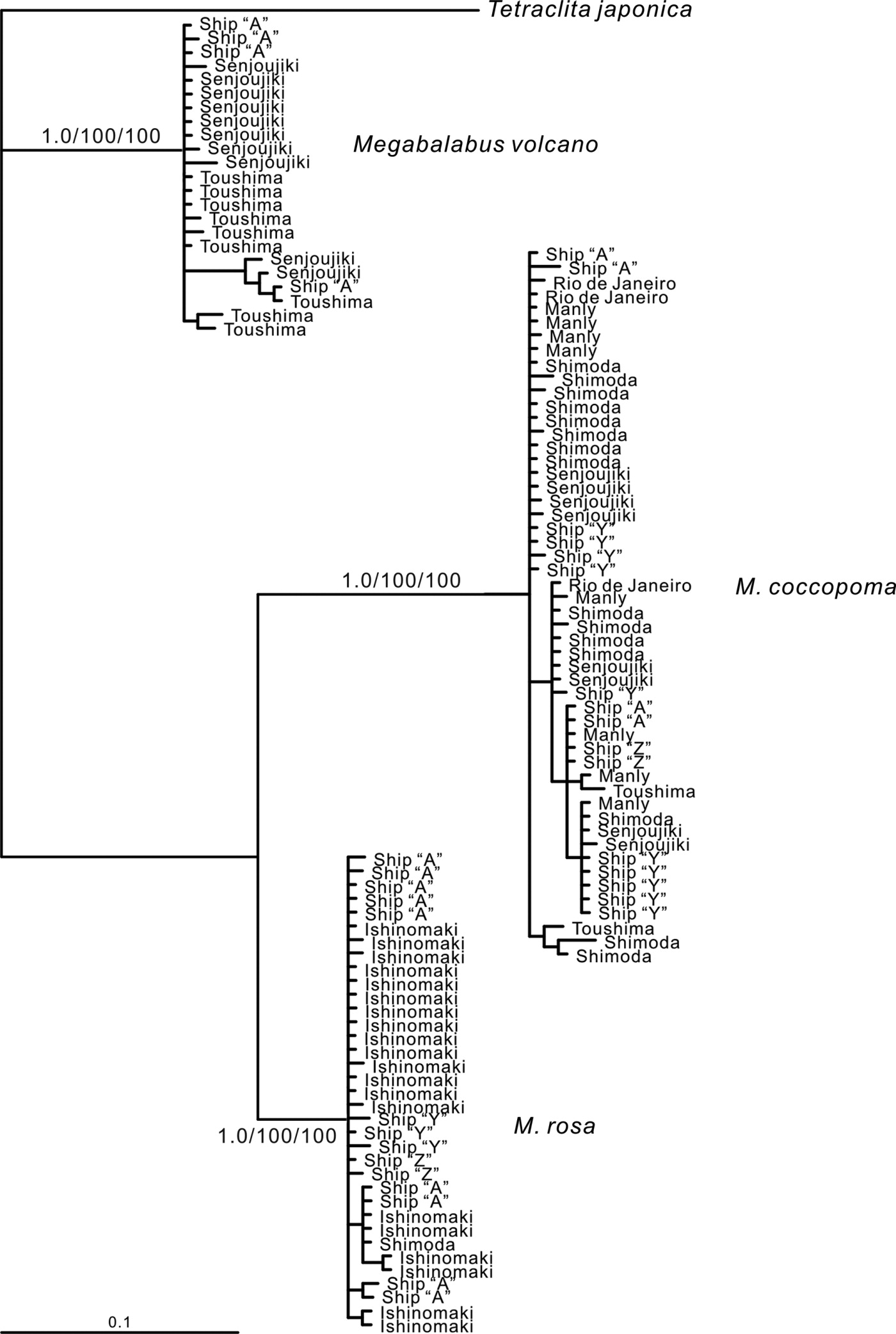 |
| Pylogram derived from Bayesian Inference (BI) tree showing three distinct species of genus Megabalanus |
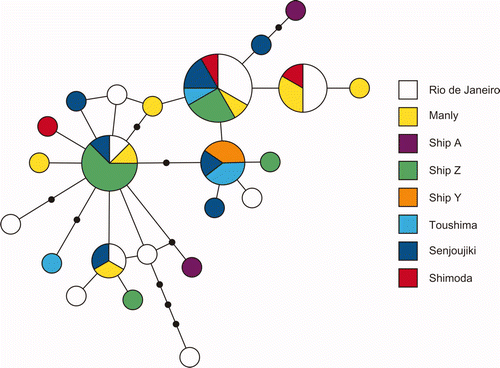 |
| Haplotype network of COI sequence data of M. coccopoma. The area of a circle is proportional to the number of observed individuals. |
3.6 Threats and Status
The larvae are subject to planktonic predators. There are not much studies conducted on the reproduction or larvae development, nor on their conservation status. Since Megabalanus coccopoma is recognized as invasive species, its characteristics overlap with that of other species in invasiveness. They can be detected by their common characteristics such as broad native range and rapid dispersal [33, 34]. Hence, they are neither endangered nor protected species [35].
 |
| An artistic approach to M. coccopoma by Jonathan Delafield Cook. |
4- References
1- http://eol.org/pages/127700/names. Retrieved 2013-11-13.
2- Kerckhof, F. 2002. Barnacles (Cirripedia, Balanomorpha) in Belgian waters, an overview of the species and recent evolutions, with emphasis on exotic species. Bull. Kon. Belg. Inst. Natuurwet. Biologie 72 (Suppl.): 93-104.
3- Perreault, R.T. 2004. An exotic tropical barnacle, Megabalanus coccopoma (Darwin, 1854), in Louisiana: Its probable arrival and environmental implications. Proc. Louisiana Acad. Sci., 66:13-16.
4- Alpert, P., Bone, E., and Holzapfel, C. 2000. Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspectives in Plant Ecology, Evolution and Systematics. Vol. 3/1, pp. 52–66
5- Wilcove D.S., Rothstein D., Dubow J., Phillips A., Losos E. 1998. Quantifying threats to imperilled species in the United States. BioScience, 48:607 – 15.
6- Mack R.N., Simberloff, D., Lonsdale, W.M., Evans H., Clout, M. and Bazzaz F.A. 2000. Issues in Ecology. Biotic invasions: causes, epidemiology, global consequences, and control. Ecological Applications. 10 (3): 689-710.
7- Vitousek, P.M., D’Antonio, C.M., Loope, L.L., and Westbrooks, R. 1996. Biological invasions as global environmental change. American Scientist 84:468–478
8- Bruno J.F., Fridley J.D., Bromberg K.D. and Bertness M.D. 2005. Insights into biotic interactions from studies of species invasions. In: Sax DF, Stachowicz JJ and Gaines SD (eds). Species Invasions: Insights into Ecology, Evolution, and Biogeography, pp 13–40. Sinauer Associates, Sunderland, Massachusetts.
9- http://iyb2010singapore.blogspot.sg/2010/03/bad-biodiversity-invasive-alien-species.html#.UpPnYMQ3BDw. Retrieved 2013-11-13.
10-Yamaguchi T., Prabowo R.E., Ohshiro Y., Shimono T., Jones D., Kawai H., Otani M., Oshino A., Inagawa S., Akaya T., Tamura I. The introduction to Japan of the Titan barnacle, Megabalanus coccopoma (Darwin 1854) (Cirripedia: Balanomorpha) and the role of shipping in its translocation. Biofouling 2009;25:325-33.
11- http://www.marinespecies.org/aphia.php?p=taxdetails&id=149682. Retrieved 2013-11-20
12- http://www.pices.int/publications/presentations/PICES-2010/2010-S12/S12-1400-Yamaguchi.pdf. Retrieved 2013-11-20
13- Newman, W.A., and R.R. McConnaughey. 1987. A tropical eastern Pacific barnacle, Megabalanus coccopoma(Darwin), in southern California, following El Niño 1982-83. Pacific Science 41:31-36.
14- Kerckhof, F. and Cattrijsse, A. 2001. Exotic Cirripedia (Balanomorpha) from buoys off the Belgian coast. Senckenb. Marit. 31 (2): 245-254.
15- http://mangrove.nus.edu.sg/guidebooks/text/2123.htm. Retrieved 2013-11-13.
16- Crickenberger S., Moran A. 2013. Rapid range shift in an introduced tropical marine invertebrate. PLoS One. 8 (10) :e78008.
17- Schultz, M.P. 2007. Effects of Coating Roughness and Biofouling on Ship Resistance and Powering. Biofouling Vol 23(5): 331-341
18- Kaluza, P., Koelzsch, A., Gastner, M.T., Blasius, B. 2010. The complex network of global cargo ship movements. Journal of the Royal Society Interface 7: 1093-1103
19- Hutchings, P. A., R. W. Hilliard & S. L. Coles, 2002. Species introductions and potential for marine pest invasions into tropical marine communities, with special reference to the Indo-Pacific. Pacific Science, 56: 223–233
20- Yamaguchi, T., Prabowo, R.W., Ohshiro, Y., Shimono, T., Jones, D., Kawai, H., Otano, M., Inagawa, S., Akaya, T., Tamura, I. 2009. The introduction to Japan of the Titan barnacle, - Megabalanus coccopoma (Darwin, 1854) (Cirripedia: Balanomorpha) and the role of shipping in its translocation. Biofouling 25:325–333.
21- In the Wrong Place - Alien Marine Crustaceans: Distribution, Biology and Impacts. Series: Invading Nature - Springer Series in Invasion Ecology, Vol. 6. Galil, Bella S.; Clark, Paul F.; Carlton, James T. (Eds.) 2011, XVI, 716p.
22- Hutchings, P.A., Hilliard, R.W., and Coles, S.L. 2002. Species Introductions and Potential for Marine Pest Invasions into Tropical Marine Communities, with Special Reference to the Indo-Pacific. Pacific Science, Vol 56 (2): 223-233.
23- Yeo, D.C.J., Chia, C.S.W. 2010. Introduced Species in Singapore: An Overview. COSMOS. 06:01, 23-37.
24- Yeo, D. C. J., Ahyong, S. T., Lodge, D. M., Ng, P. K. L., Naruse, T. & Lane, D. J. W. 2009. Semi-submersible oil platforms: Understudied and potentially major vectors of biofouling-mediated invasions. Biofouling, 26: 179–186.
25- Tibbetts, J.H. 2007. Knocking Back Biological Invaders. Coastal Heritage, South Carolina Sea Grant Consortium 21:3-11.
26- http://eol.org/pages/127700/details. Retrieved 2013-11-13.
27- http://www.sms.si.edu/irlspec/Megabalanus_coccopoma.htm. Retrieved 2013-11-03.
28- Masterson, J. 2007."Megabalanus coccopoma". Smithsonian Marine Station at Fort Pierce. http://www.sms.si.edu/irlspec/Megabalanus_coccopoma.htm. Retrieved 2013-11-03.
29- Hoch, J. M. 2010. Effects of crowding and wave exposure on penis morphology of the acorn barnacle, Semibalanus balanoides Marine Biology, 157, 2783-2789
30- http://www.wildsingapore.com/wildfacts/crustacea/othercrust/ciriipedia/balanus.htm. Retrieved 2013-11-03.
31- http://www.nature.nps.gov/water/marineinvasives/assets/PDFs/Megabalanus_coccopoma.pdf. Retrieved 2013-11-13.
32- http://www.flickr.comsearch/show/?q=Balanus&w=54527470%40N00&s=rec. Retrieved 2013-11-13.
33- Lodge, D.M. 1993a. Biological invasions: lessons for ecology. Trends Ecol. Evol. 8:133–37.
34- Perrins, J., Fitter, A. and Williamson, M. 1993. Population biology and rates of invasion of three Impatiens species//. Journal of Biogeography, 20, 33–44.
35- Crickenberger, S., Moran, A. 2013. Rapid range shift in an introduced tropical marine invertebrate. PLoS One. 8 (10). e78008.