|
| Image taken by and used with permission from Nick Baker |
Table of Contents
 |
|
| Distribution | Morphology | Biology | Threats | Taxonomy | Glossary | Additional Info | References
Distribution
 |
| Distribution Map obtained with permission from IUCN |
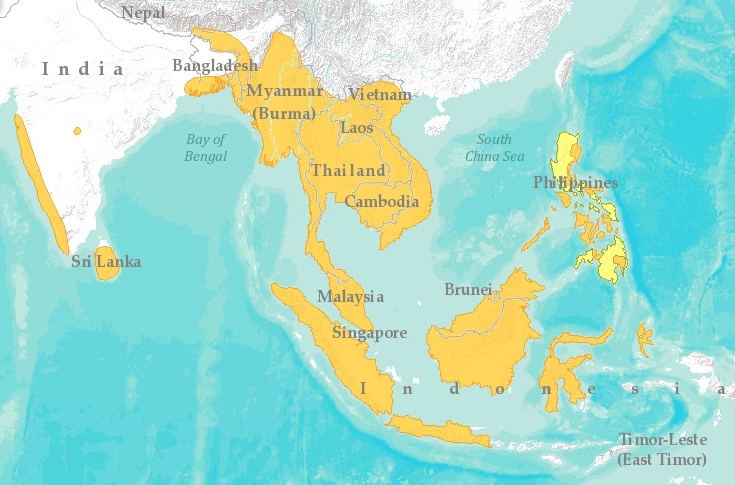
India, Sri Lanka, Burma, Philippines, Riau Islands, Indonesia, Brunei, Sumatra, Malaysia and lastly, Singapore (Pulau Ubin) (Lane, et a.l, 2006)
Habitat
These bats primarily inhabit forests but can be found in caves, large tree hollows, culverts, tunnels, crevices of rock-boulders, and abandoned outbuildings. (Gaikwad, et al., 2012)| Distribution | Morphology | Biology | Threats | Taxonomy | Glossary | Additional Info | References
Morphology
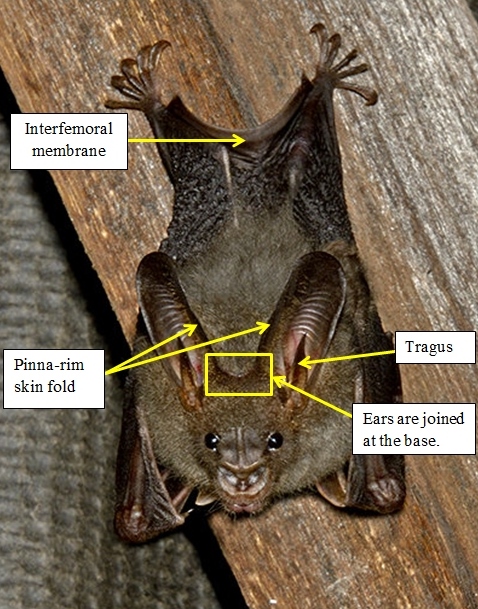
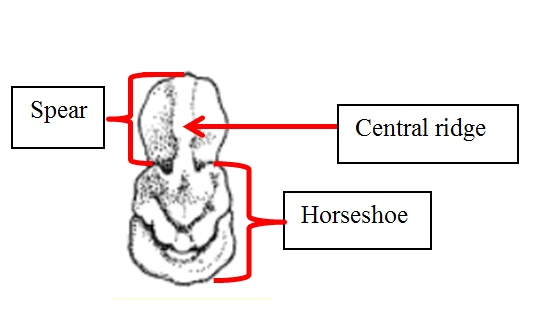
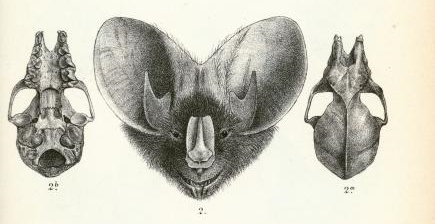
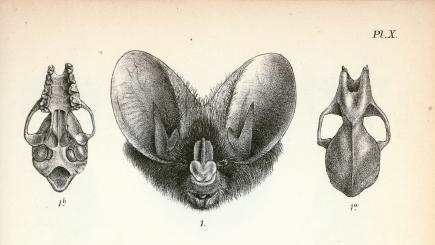
Identification in the field
[for further information, here's a morphological key specific to the different bats in South Asia.]
|
(Gaikwad, et al., 2012), (Csorbn, et al., 1997) (Srinivasulu, et al, 2010) |
||
|
|
|
|
|
| Distribution | Morphology | Biology | Threats | Taxonomy | Glossary | Additional Info | References
Comparison with Megaderma lyra
The distribution of these two species slightly overlap (Sri Lanka and some parts of India).
Anatomy
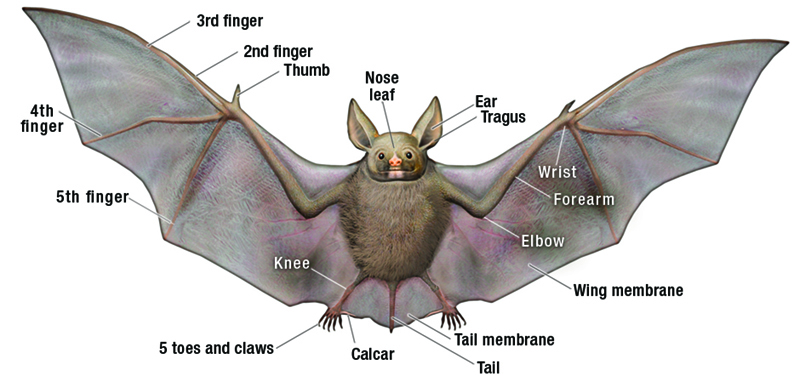 |
| Illustration of basic bat anatomy © Paul Mirocha |
|
|
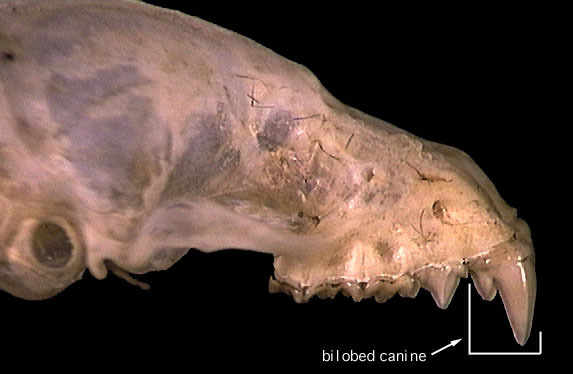
M.spasma is a voracious carnivore with a penchant for insects. The sharp teeth (with the large bilobed canines) are designed to lacerate and rip apart insects and on occasion, frogs and other small vertebrates. (Adams & Pederson, 2013) |
| Distribution | Morphology | Biology | Threats | Taxonomy | Glossary | Additional Info | References
Biology
Diet
 |
| Malayan false vampire bat feeding on moth. CC BY 2.0 |
M.spasma is primarily an insectivore (feeding, in particular, on grasshoppers, cockroaches, beetles and moths). Their in-flight agility and sophisticated echolocation calls allow them to enjoy an astoundingly varied diet; they have been recorded to have fed on vertebrates, including lizards, frogs, fish, birds and small rodents.(Davison, et al., 1992). Adults, on average, eat up to 8 grams of insects per night with pregnant females devouring their body weight in insects per night (Crichton & Krutzsch, 2000). These bats, like most insectivorous bats, play a part in controlling pest populations (Maas, et al., 2013).
Behaviour
Hunting
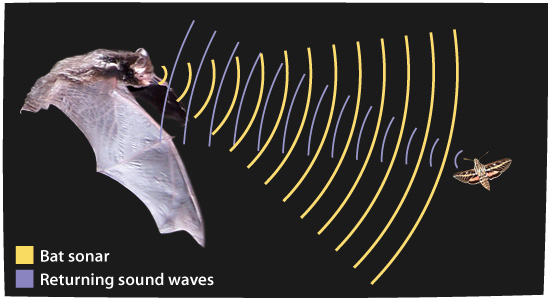
Megaderma spasma, like most Microchopteran bats, uses laryngeal echo-location (high frequency sounds) to navigate and find food. In particular, it emits short, high-frequency sound pulses through its nose (as opposed to its mouth). This is also known as a low-duty cycle call pattern. (Greif & Siemers, 2010) The bats that utilise this approach time their short calls to finish before the echoes return. This is important because these bats contract their middle ear muscles when emitting a call, so as to avoid deafening themselves. The time interval between the emitted call and the returning echo allows them to relax these muscles so they can clearly hear the returning echo.(Airas, 2003)
A sound clip of captive greater false vampire bats (Megaderma lyra): Notice the short pulses of sound and the intervals between calls. (A sound clip of Megaderma spasma could not be found)
This returning echo is picked up by their ears and the message is transmitted to their brain. In this way, they are able to “visualize” the shape and texture of large objects, such as walls and openings of caves as well as of specific targets such as fish, frogs, and insects that they pursue while foraging. The ability of echolocation is particularly well developed in insectivorous bats.(Schmidt, et al., 2000)
When looking for food. they emit approximately 10 pulses per second but when they locate their prey the pulse frequency can rise up to over 150 pulses per second, till they catch their prey (Fenton & Bell, 1981). This style of echolocation is very effective in cluttered environments but it is not easily detected by prey. The large ears of the lesser false vampire bat can, however, pick up sounds from the prey as well as very weak returning echoes.(Obrist, et al.,1993) Armed with a physical structure that enables greater in-flight manoeuvrability, these bats sweep through the trees and shrubs close to the ground, gleaning (or snatching) their prey from tree trunks, branches, leaves, walls or the forest floor, using the interfemoral membrane that stretches between their hind legs (Arita & Fenton, 1997).
When they capture prey it is brought back to the roost before being eaten. If young are present, and are old enough to eat solid food, the parents will feed them before they themselves eat. (Davison et al,1992)
Roosting
Roosting behaviour of M.spasma in Pohara, India. Look out for the clusters of individuals, the chattering and the close-up shot of the interfemoral membrane.
A gentle and sociable bat, the lesser false vampire roosts in small, mixed-sex groups of 3-30 individuals throughout the year. These bats roost primarily in caves or cave-like structures like tilled roofed houses and culverts.(Labonite et al, 2012) When roosting in caves, many bat species constantly chatter with each other as they go about their social life, particularly during twilight hours, just before they take off for foraging. (Wilkinson & South, 2002)
Communication
The bats communicate with social calls that differ considerably from their complex hunting calls, which sweep from frequencies of 130 kHz to 10 kHz extremely rapidly (Pflazer & Kusch, 2003). All of these bats use “low-duty-cycle” echolocation, in which the emitted calls are relatively short in duration and do not overlap in time with the returning echoes. Chattering during roosting or at rest is an essential aspect of social communication in bats. All of the small insectivorous bats rely on complex sounds for most of what they do—to echolocate, to find food, impress another bat, court, show affection, warn others of danger, and fight with each over their preferred mate. They may also indulge in eavesdropping to either gather information on or locate a food source during foraging or be alerted to social activities. (Adams & Pederson, 2013)
Courtship
Male false vampire bats engage in a courtship ritual where they perform a hovering flight, accompanied with song-like vocalisations, aimed at impressing the females. This behaviour is displayed only by the dominant males and can occur at any time of the year. (Gould , 1978) Given the small size of males in the Microbat group, female choice and cooperation is very important in mating . (Brosset, 1962)
Reproduction
Males and females live together throughout the year. They reach sexual maturation within a year of birth (Krutszch, 1979). Megaderma spasma is monoestrous (i.e. breed once a year). They mate between November and January, giving birth in April or June to a single pup after a 150 to 160 day gestation period. The birth of the young is timed to occur before the monsoon season sets in. (Crichton & Krutszch, 2000)
Parental care
In this species, it is unknown if males exhibit parental care. Females, on the other hand, aside from providing nutrition through milk, care for their pups through various ways. They select and maintain a stable thermal environment for the pup, by choosing an appropriate roosting site. Through acoustic signals, she also stimulates the pup’s vocalisation skills and enables the infant to learn adult communication patterns. Maternal olfactory signals and tactile influences (physical interactions between the mother and pup) also aid the development of proper social and locomotor functions in the pup. The females also protect their pups by carrying them in flight. She does this for 2-3 months before the pup is weaned and is able to fly alone. The presence of a continued relationship between the mother and the pup in M.spasma has not been studied yet. (Crichton & Krutszch, 2000)
Infants/Pups
The pups are born altricial (fur-less with their eyes closed) and cannot regulate their temperature very well. They are approximately 10% of their mother’s body weight. Within a few weeks they develop fur and will commence flying at approximately 6 weeks of age. (Nowak,1994)
Each pup has a unique vocal signature that is easily recognised by their mother. Pairs of notes with interpulse intervals ranging around 20- 30 m/sec are common in the vocal repertoire of young bats. Each bat species has a unique frequency pattern for infant calls: The function of these neonatal calls is to promote the reunion of the mother and infant. In Megaderma spasma, although adults emit sonar pulses through the nose, pups are capable of emitting sonar calls through the mouth (these calls are of higher intensity than nasal sounds). During their attempts to reunite with their infants, Megaderma mothers emitted calls clearly audible to humans. (Gould, 1979)
| Distribution | Morphology | Biology | Threats | Taxonomy | Glossary | Additional Info | References
Threats
Predators
Raptors, snakes and predatory mammals (Norberg & Fenton,1988).
Parasites
- Eoctenes spasmae (host-specific to M.spasma) occurs on all parts of the body but particularly in the hollow formed by the head and shoulder blades where it is well protected from host grooming. It never leaves the body, and transference to another host can only occur through body contact. (Marshall, 1982)
- Plasmodium falciparum (malaria): M.spasma serves as a vector. (Duval, et al., 2007)
- Asiolabidocarpus megadermae (mite). (A.Fain, 1980)
- Asiolabidocarpus hipposideros (mite). (A.Fain.1980)
Humans
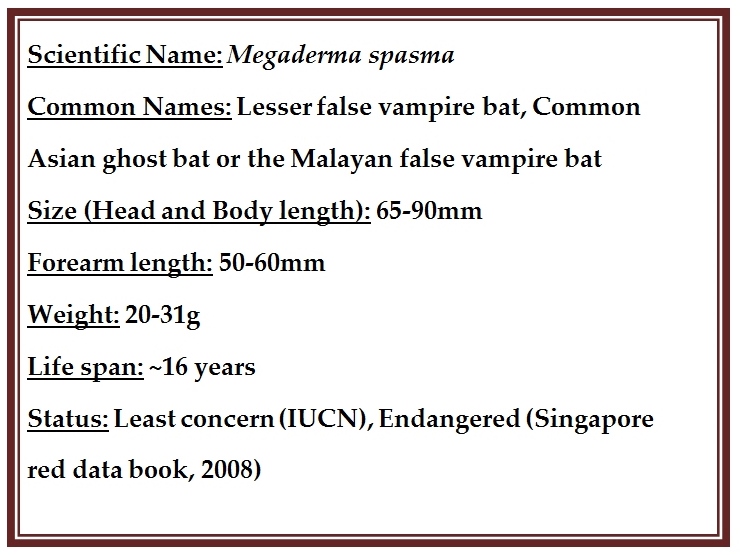
There are about 1,100 species of bats in the world and 30 have been documented in Singapore with only 15 from the Microchiropteran suborder observed (Lane, et al., 2006). Despite the 'Least Concern' status allocated to this species, separate populations of Megaderma spasma are dwindling (the Singapore Red Data Book,published in 2008, lists this species as critically endangered). The primary forest habitats of this species are under threat from deforestation for timber and agricultural usually deforested for timber, firewood and agricultural purposes in South Asia. The rapid increase in land devoted to growing oil palm has resulted in extensive loss of primary forests. In Southeast Asia, there are localized threats to caves, especially due to guano mining and limestone quarrying. Additionally, roosts in large hollow trees and old buildings are under threat due to human interference from being cut down or demolished or cleaned. Despite the contribution of these bats in the control of insect crop pests, persecution of bats is also a threat to the species. (Labonite, et al., 2012)
| Distribution | Morphology | Biology | Threats | Taxonomy | Glossary | Additional Info | References
Taxonomy
What's in a name?
Megaderma spasma was first described by Linnaeus as Vespertilio spasma in 1758 (Linnaeus,1758). In 1864, Allen Harrison proposed the inclusion of a new family, Megadermatidae with 3 new species (Anderson & Wrought, 1907). However, it was not fully recognised by taxonomists till 1907 when a revision on Brazilian bats was published (Miller, 1907). It was only after this that Thomas Oldfield revised the tenth edition of mammals classified by Linnaeus and re-positioned this species under the Megaderma genus (O.Thomas, 1910). Based purely on morphology, Vespertilio as a genus is recognised as having no noseleaf, a well-developed non-bifurcate tragus and shorter ear pinnae while the genus Megaderma is identified by the presence of a distinct noseleaf, a bifurcate tragus and large ear pinnae (Srinivasulu, et al., 2010)
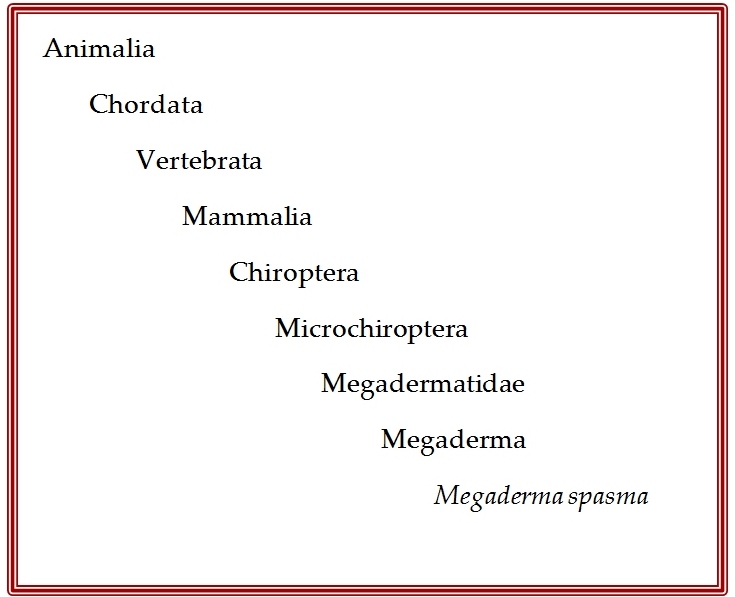
Synonyms:
Vespertilio spasma Linnaeus, 1758
Megaderma horsfieldii Blyth, 1863
Megaderma carimatae
Megaderma lasiae
Megaderma natunae
Megaderma niasense
Megaderma siumatis.
Type specimen
The type specimen used by Linnaeus in 1758 was obtained from Albertus Seba's Cabinet of Natural Curiosities (The current location of this type specimen is unknown). The type specimen used by Thomas Oldfield in his revision was obtained from Indonesia.
Type for Megaderma spasma
Catalog Number: USNM 101632
Collection: Smithsonian Institution, National Museum of Natural History, Department of Vertebrate Zoology, Division of Mammals
Sex/Stage: Male; Adult
Preparation: Skin: Whole
Collector(s): W. Abbott
Year Collected: 1899
Locality: Tambelan Islands, Kepulauan Riau, Indonesia
Image: no image was available
Type status: It is uncertain if this is regarded as a neotype or syntype
Phylogeny
The phylogenetics of Megaderma spasma (or even Megadermatidae) are not very well studied.
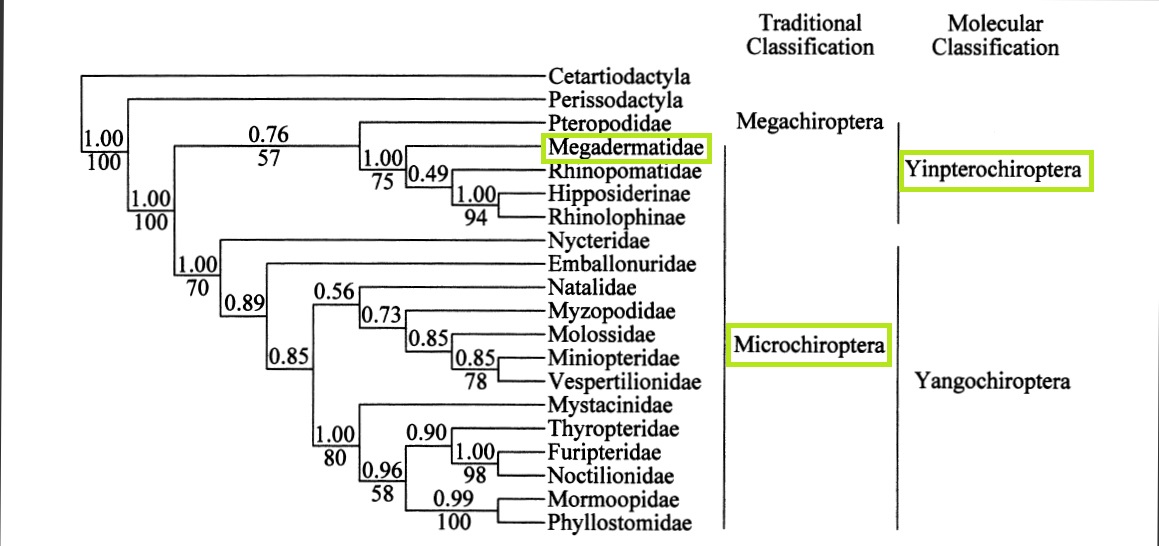
Higher-lever relationships within Chiroptera resulting from unconstrained Bayesian phylogenetics and maximum
likelihood analysis (Busshe & Hoofer, 2004)
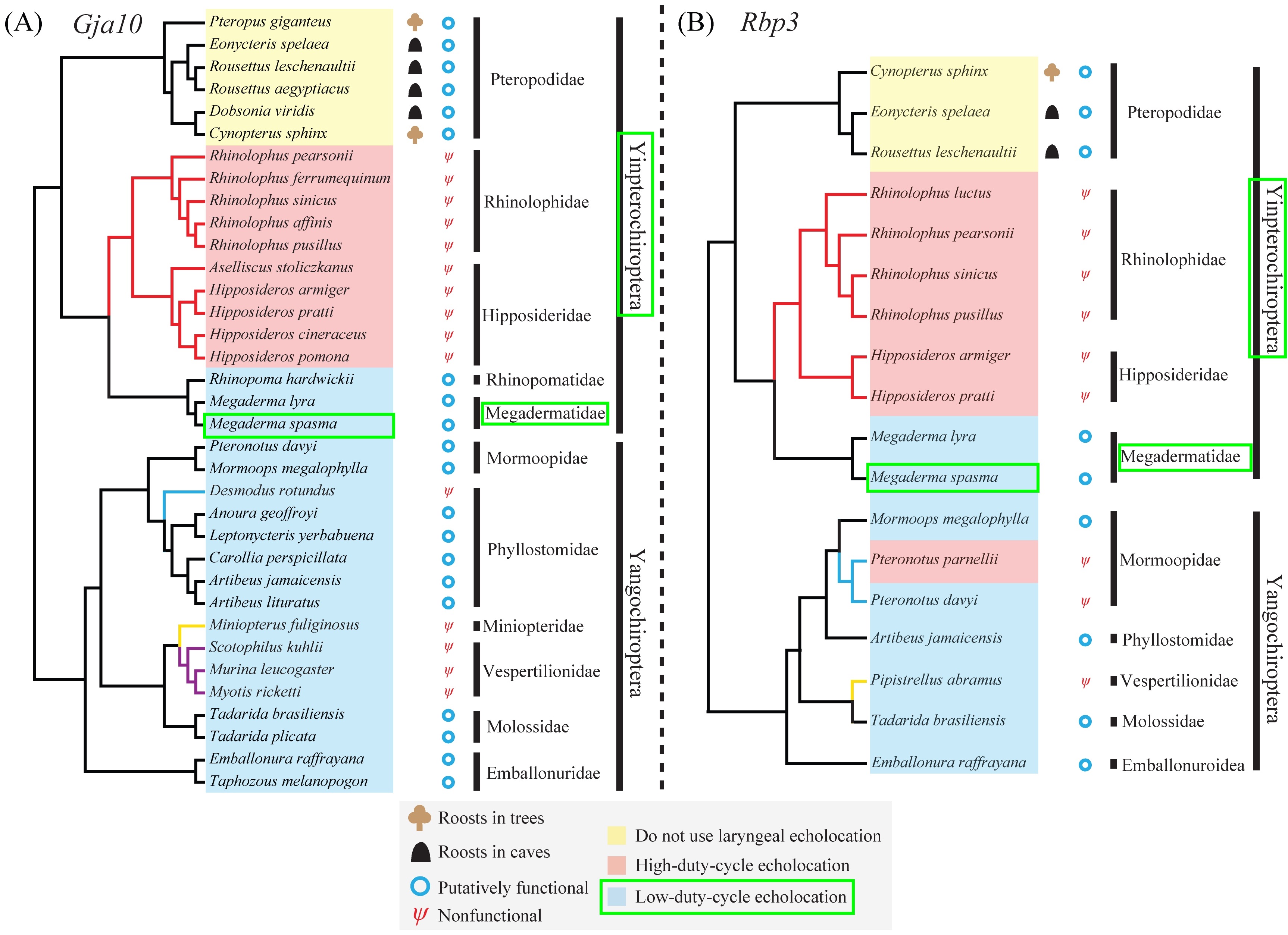
Species topologies of bats showing information on bat phylogeny, gene pseudogenization and echolocation call type, using visual perception genes Gfa10 and Rbp3 (Shen, et al., 2013) : note that Megaderma spasma's allocation to the group (Yinpterochiroptera) doesn't change between the two trees, but its neighbouring taxa change.
Prior to the 1980's, bats were divided into two presumably monophyletic suborders based on their morphological and echolocation characteristics: Microchiroptera (microbats) and Megachiroptera (megabats). Microbats were identified as geographically wide-spread, insectivorous bats capable of laryngeal echolocation while Megabats were identified as frugivorous bats with no echolocating capabilities, restricted mostly to the tropics. (Teeling, et al., 2005) However, recent molecular genetics, based on diverse datasets has revealed that microbats are paraphyletic, thus re-organising the chiropterans into two new recognised suborders: Yinpterochiroptera (consisting of the non-echolocating fruit bats, Pteropodidae, and echolocating insectivorous fruit bats Hipposideridae, Megadermatidae, Rhinopomatidae and Craeseonycteridae) and Yangochiroptera (consists of the remaining twelve echolocating bat families). (Jones, et al.,2002)
(Murray, et al., 2012)
While some traits apparently arose independently in the two suborders (e.g., foliage-roosting, tent building, low intensity echolocation calls, noseleafs, nasal emission of echolocation calls, high duty cycle echolocation behaviour), others appear to have been ancestral (roosting in narrow spaces, laryngeal echolocation, stylohyal-tympanic contact, oral emission of echolocation calls, and small brood size). (Fenton, 2010)
For more information on the phylogenetics of Chiropterans, check out the Tree of Life.
| Distribution | Morphology | Biology | Threats | Taxonomy | Glossary | Additional Info | References
Glossary
Altricial: a condition in which the organism is helpless and requires nourishment and care from a parent.
Biosonar: The sensory system used by bats. It consists of a passive (ears and pinna-skin fold) and active (vocal chords and noseleaf) aspect.
Guano: excrement of cave dwelling bats and seabirds.
Hyoid Appparatus: A structure that supports the larynx.
Interfemoral membrane: the membrane between the hind legs of the bat
Monoestrous: having one estrous cycle per year. i.e. one breeding season per year.
Neonatal: newborn infants
Noseleaf: Modified nasal structure that facilitates laryngeal echolocation.
Pinnae: outer part of an ear
Stylohyal bone: a bone in the hyoid apparatus in bats that directly touches the tympanic bones in bats with laryngeal echolocation
Tragus: small, pointed structure of the external ear
Tympanic bone: a bone in the hyoid apparatus in bats that directly touches the stylohal bone for laryngeal echolocation to occur.
Additional Info
For more information on Megaderma spasma, check out these sites.
http://animaldiversity.ummz.umich.edu/accounts/Megaderma_spasma/
http://www.iucnredlist.org/details/12939/0
http://www.arkive.org/lesser-false-vampire-bat/megaderma-spasma/
http://www.ttu-mbea.org/category/journal-and-capture-data-2010/page/3/
http://www.inaturalist.org/taxa/41321-Megaderma-spasma
http://eol.org/pages/328822/hierarchy_entries/24944582/overview
| Distribution | Morphology | Biology | Threats | Taxonomy | Glossary | Additional Info | References
References
Adams, R. & Pedersen, S., 2013. Bat Evolution, Ecology, and Conservation. Springer
A. Fain., 1980 The Labidocarpine mites (Acari: Chirodiscidae) from oriental bats I. International Journal of Acarology , 6:2, 131-140
Airas, Matti. 2003. Echolocation in bats. Proceedings of spatial sound perception and reproduction. The postgrad seminar course of HUT Acoustics Laboratory, pp. 1-25.
Akbar, Zubaid, L. W. Hong, and S. S. Sastroutomo. 1999. Insectivorous bats as natural predators of insect pests. Biological control in the tropics: towards efficient biodiversity and bioresource management for effective biological control (1999): 34-37
Anderson, K. & R.C.Wrought, 1907. On the bats of the family Megadermatidæ. Journal of Natural History, 19(110).
Arita, H. & Fenton, M., 1997. Flight and Echolocation in the ecology and evolution of Bats. TREE, 12(2).
Brosset, A. 1962. Bats of central and Western India. Journal of Bombay Natural History Society, 59: 608–613.
Bussche, R. A. v. D. & Hoofer, S. R., 2004. Phylogenetic Relationships among Recent Chiropteran Families and the Importance of Choosing Appropriate Out-group Taxa. Journal of Mammology, 85(2).
C. Linnaeus. 1758. Systema Naturae per Regna Tria Naturae, secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis. Tomus I. Laurentii Salvii, Holmiae (Stockholm) 1-823
Crichton, E. & Krutzsch, P., 2000. Reproductive Biology of Bats. s.l.:Academic Press.
Csorbn, G., Fuisz, T. and Kelen, B., 1997. New birds and bats from Pulau Tioman, Malaysia. Malayan Nature Journal, 50: 197-200
Davison, G.W.H. and Zubaid, A. (1992) Food habits of the lesser false vampire, Megaderma spasma, from Kuala Lompat, Peninsular Malaysia. Zeitschrift für Säugetierkunde, 57(5): 310 – 312
Debata, S., Palei, H., Mohapatra, P. and Mishra, A., n.d. 2013 First record of Lesser False Vampire bat (Megaderma spasma, Linnaeus, 1758) from Sundergarh, Odisha, India. Bi-Annual Newsletter of CCINSA and RISCINSA.
Duval, L., Robert, V., Csorba, G., Hassanin, A., Randrianarivelojosia, M., Walston, J., Nhim, T., Goodman, S.M. and Ariey, F. 2007. Multiple host-switching of Haemosporidia parasites in bats. Malaria Journal, 6:57
Fenton, M. B. & Bell, G. P., 1981. Recognition of Species of Insectivorous Bats by Their Echolocation Calls. Journal of Mammology , 62(1).
Francis, C., 1989. A comparison of mist nets and two designs of harp traps for capturing bats. Journal of Mammalogy, 70:4 pp. 865-870
French, B. (1997) False Vampires and Other Carnivorous. Bats, 15(2): 11 – 14
Gaikwad, M. C., Narwade, S. S., Fartade, K. M. & Korad, V. S., 2012. A review of the distribution of bats in southwestern region of Deccan, Maharashtra - India and conservation recommendations. Taprobanica; Journal of Asian Biodiversity, 4(1).
Gould, E., 1978. Rediscovery of Hippsideros ridleyi and Seasonal Reproduction in Malaysian Bats. Biotropica, 10(1).
Gould, E., 1979. Neonatal vocalizations of ten species of Malaysian bats (Megachiroptera and Microchiroptera). American Zoology, 19:481-491
Greif, S. and Siemers, B.M., 2010. Innate recognition of water bodies in echolocating bats. Nature Communications
Jones, K. et al., 2002. A phylogenetic supertree of the bats (Mammalia: Chiroptera). Biological reviews of the Cambridge Philosophical Society
Jones, G. and Teeling, E ., 2006. The evolution of echolocation in bats. Trends in ecology & evolution
Jose, R. P., Labonite, M. A., Bullecer, R. C. & Ancog, A. B., 2012. Indigenous Knowledge and Taxonomy of Bats in Loboc Watershed Forest Reserve, Bohol, Philippines. Biology Education for Social and Sustainable Development , pp. 127-143.
Lane, D.J.W., Kingston, T. and Lee, B.P..-H., 2006. Dramatic decline in bat species richness in Singapore, with implications for Southeast Asia. Biological Conservation
Maas, B., Clough, Y. & Tscharntke, T., 2013. Bats and birds increase crop yield in tropical agroforestry landscapes. Ecology Letters, 16(12).
Miller, G. S. 1907. The families and genera of bats. Bulletin of US National Museum 57: 1–282.
Murray, S. et al., 2012. Molecular phylogeny of hipposiderid bats from Southeast Asia and evidence of cryptic diversity. Molecular phylogenetics and evolution
Norberg, U.M & Fenton,M.B., 1988. Carnivorous bats? Biological Journal of the Linnean Society
Nowak, R. 1994. Walker's Bats of the World. Baltimore and London: The Johns Hopkins University Press.
Obrist, M., Fenton, M., Eger, J. and Schlegel, P., 1993. What ears do for bats: a comparative study of pinna sound pressure transformation in chiroptera. The Journal of experimental biology
Oldfield, Thomas. F.R.S. F.Z.S. 1910 The Mammals of the Tenth Edition of Linnaeeus; an Attempt to fix the Types of the Genera and the exact Bases and Localities of the Species. Proceedings of the Zoological Society of London
P.H.Krutzsch, 1979. Male reproductive patterns in nonhibernating bats. Journal of Reproductive Fertility , Volume 56, pp. 333-344.
Pfalzer, G. and Kusch, J. 2003. Structure and variability of bat social calls: implications for specificity and individual recognition. Journal of Zoology
Schmidt, S., S. Hanke, J. Pillat. 2000. The role of echolocation in hunting terrestrial prey – new evidence for an underestimated strategy in the gleaning bat, *Megaderma lyra*. Journal of Comparative Physiology, 186 (10): 975-988.
Shen, B. et al., 2013. Independent losses of visual perception genes Gja10 and Rbp3 in echolocating bats (order: chiroptera). PloS one
Srinivasulu, A.Racey, P. & Mistry, S., 2010. A key to the bats (Mammalia: Chiroptera) of South Asia. Journal of threatened taxa, 2(7).
Teeling, Springer, Madsen & Bates, 2005. A Molecular Phylogeny for Bats illuminates Biogeography and the Fossil record. Science, 307.
Wang, X. and Müller, R., 2009. Pinna-rim skin folds narrow the sonar beam in the lesser false vampire bat (Megaderma spasma). The Journal of the Acoustical Society of America
Wetterer, A., Rockman, M. and Simmons, N., 2000. Phylogeny of phyllostomid bats (Mammalia: Chiroptera): data from diverse morphological systems, sex chromosomes, and restriction sites. Bulletin of the American Museum of Natural History
Wilkinson, G. S. and J. M. South. 2002. Life history, ecology and longevity of bats. Aging Cell, 1: 124–131.
Zhao, H. et al., 2009. The evolution of color vision in nocturnal mammals. Proceedings of the National Academy of Sciences of the United States of America
Video and Audio
“The Lesser False Vampire Bat, Megaderma spasma, search in Pohara by Dr. V.T.Ingole” by Shirishkumar Patil, YouTube channel, 31 Oct 2011. URL: http://www.youtube.com/watch?v=mx73LWpQ3Dg (accessed on 28 Oct 2013)
“Listen to the call of captive greater false vampire bat” by cdellamore, Soundcloud. URL: https://soundcloud.com/cdellamore/listen-to-the-call-of-captive (accessed on 24 Oct 2013)