Musca domestica (Linneaus 1785)

Table of Contents
Image source: Wikimedia commons, Photographed by: Muhammad Mahdi Karim (Link)
The common housefly, Musca domestica, is easily encountered in areas such as eating joints, freshmarkets, human refuse bins, manure etc. They are commonly associated with poor hygiene standards of a location and is considered as a household pest. This is primarily attributed to the housefly capability of being a disease vector. The housefly is ecologically associated with humans (synanthropic), mainly due to its ability derive benefits (i.e. food and shelter) from living in close relation to humans.
1. DISTRIBUTION AND HABITAT
The housefly is found all over the world, mostly at places where humans reside. They generally occur at areas such as on decaying matter, fecal matter, garbage and sewage.
Potential habitats

Image Source: Fecal matter, Wikimedia commons, Photograph by: Jebulon (Link); Raw Sewage, Photograph by: Tate Rogers (Link); and Decaying food, Flickr, Photograph by: Taz (Link).
2. SIGNIFICANCE & CONTROL OF THE HOUSEFLY
2.1 Effects on Human health
The housefly is notorious for its ability to spread diseases. This is largely attributed to its feeding habit and its structural morphology making it an effective vector in the transmission of diseases. Studies in the early 1900s have shown that as many as 6 million bacteria are found on the surface of the body [1], while more than 100 pathogenic species have been found in the alimentary canal of the flies living for an appreciable amount of time [2].
Pathogens transmitted by houseflies can result in the manifestation of a variety of infections.
Pathogens commonly transmitted by the Housefly and possible infections [4]:
- Campylobacter – main cause of Gastroenteritis (gastrointestinal infection)
- Enterococcus – may cause urinary tract, intra-abdominal, pelvic, soft tissue infection, Bacteremia (blood infection) or Endocarditis (infection of the inner lining of the heart) [3].
- Staphylococcus – causes skin, eye or ear infection
- Pseudomonas
- Klebsiella
- Other viruses
Modes of disease transmission
- Presence of numerous body hair/bristles
Increases surface area available for germs to be picked up from sources of filth such as manure and garbage. So pathogens can be easily transported from a feeding site laden with pathogens to food humans consume.

- Image source: Wikimedia Commons Photography by: USDAgov (Link)
- Pad-like structure (pulvillus) on feet
The pulvillus has numerous bristle-like structures (setae) that secretes a sticky substance[5] Thus providing a suitable medium for pathogens to stick on as houseflies roam on fecal matter, refuse etc., and then transported to other consumables as they feed.

- Image of pulvillus of the tasomere of the housefly with small material attached to the surface as indicated by the arrow [30].
- Regurgitation of food
The regurgitation of food to dissolve solid food during feeding can transport pathogens from one site to another.
- Housefly feeding explanation (Start at 5:35)
- Excretion
Houseflies defecate as they feed, thus they can transmit pathogens from human refuse and fecal matter to food. The hind gut of flies has been shown to harbour a greater number of parasites than any part of the alimentary canal [6]. Thus suggesting that fecal excretions could play a large role in the contamination of food.
2.1.1 Housefly control
Given the pesky nature of houseflies, they are usually harder to swat compared to other insects such as mosquitoes, cockroaches and other insects. Watch the video below to find out why flies are so hard to swat.
Why is it so hard to swat a fly?
Instead of trying to kill a fly by hand, a good way to manage and prevent houseflies from breeding in your home could be done through preventive measures, chemical control and biological control.
Preventive Measures [18]
One of the best ways to prevent houseflies from breeding is to take preventive measures. This necessitates good hygiene practices are key to preventing and infestation of the housefly. One could do so by the following methods:
- Improve environmental sanitation and hygiene
- Install proper toilets and sewage systems
- Regular disposal of biodegradable waste properly
- Installation of fly traps
Chemical control [7]
A popular way to control housefly population is through the use of chemical insecticides which can affect the flies
- Nervous system
- Ability to produce energy
- Cuticle production
- Growth regulator in an insect
- Water balance
These could be done through using the insecticides containing chemicals such as diazinon, carbosulfan, phosphomidon, malathion, cypermethrin and dichlorvos [8].
However, prolonged and repeated usage of could cause houseflies to develop resistance against pesticides, rendering them ineffective in the future. Moreover, insecticides are not specific to houseflies, so other insects are killed as well during processes such as fumigation.
Biological control
One way to overcome potential problems of chemical insecticides, is to control houseflies using biological means. This means to use the housefly's natural enemies such as predators or plant extracts [7].
- Predators
- The hister beetle (Carcinops pumilio) [12]
- Muscid Fly larvae (Ophyra aenescens) [12]
- Macrochelid mite (Macrocheles muscaedomesticae) female [12]
- Parasitoid wasp (Spalangia endius) [13]
- Plant extracts
- Eucalyptus oil [9]
- Grapefruit peel oil (Toxic to adult flies) [10]
- Lemon oil (Toxic to larvae) [11]
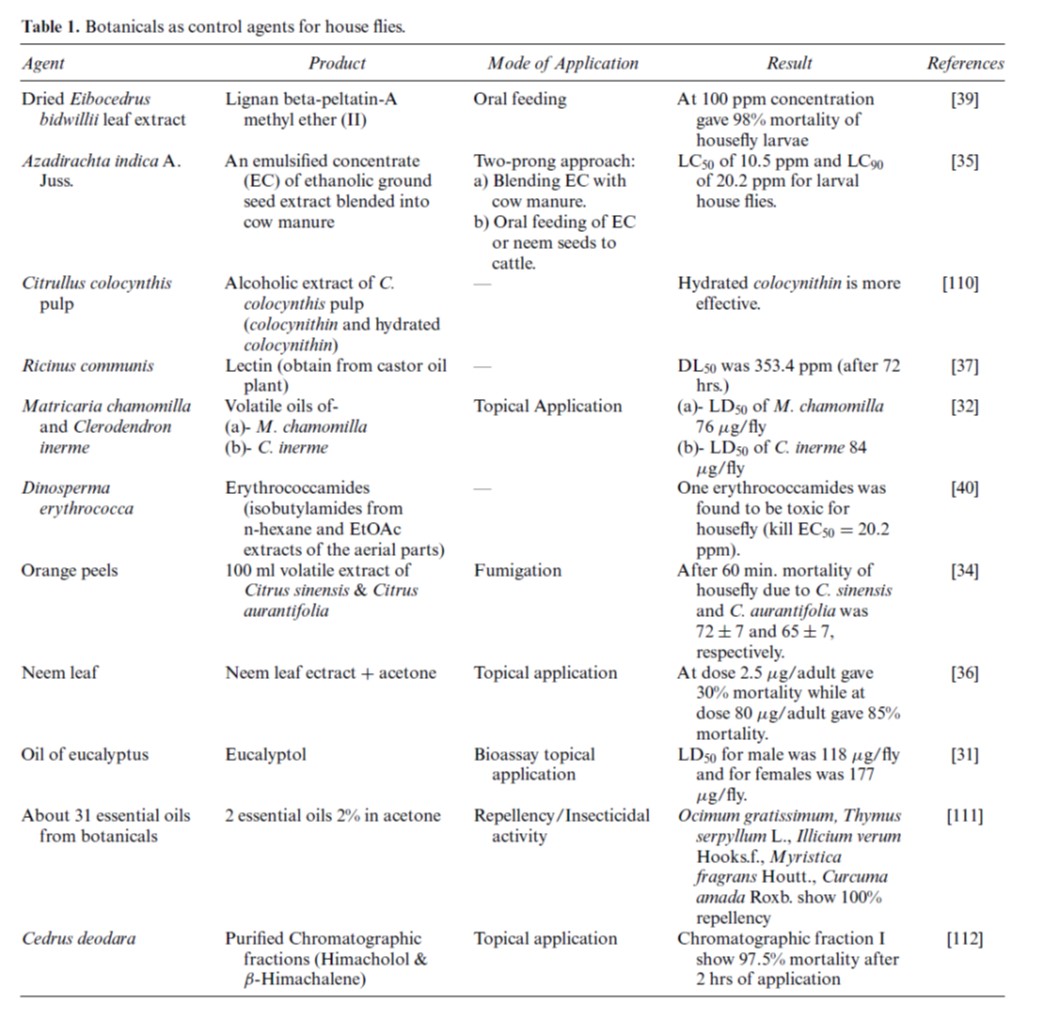
Table of botanicals that can be used as a control agent for houseflies [7].
2.2 Effects on the Environment
Role as a decomposer
Although the housefly is deemed to be undesirable by many due to its status as an effective vector for pathogens. The housefly larvae plays an important role as a decomposer in nutrient cycling. The preference for decomposing sites for oviposition in houseflies and the voracious appetite of its larvae, allows for the rapid breakdown of fecal matter and carrion. This helps prevent the build up of decomposing wastes, and help in unlocking minerals in unwanted matter to be released back into the environment for other organisms to use.
Food resource
The housefly larvae is also a viable food resource for other organisms. A nutritional analysis carried out showed that it contains roughly 71.4% water, 18.6% protein, 5% fats and 5% of salt and carbohydrates [14]. Thus suggesting that they are a viable option for protein acquisition and used as a food resource other organisms. In the face of increasing population growth and greater pressures for more meat products as the middle class rises, maggots are now seen as a potential resource for nutrient acquisition. Recent research of biodegrading cattle manure using maggots has helped reduce organic waste, and also the Nitrogen and Phosphorous contents in them [15]. While nutritional analysis of the housefly larvae revealed values that were as good as high protein food ingredients. This could help mitigate nutrient overload in farms and probably prevent eutrophication of rivers close to farms. The use of maggots as feed for livestock also improves cost effectiveness in farms.
Role as a pollinator
The housefly also plays an important role as a pollinator of Stapeliads, a tribe of flowers that imitate the smell of decomposing organic matter or fecal matter [16]. In a study, the common housefly has been observed to pollinate Huernia longituba, Huernia namaquensis, Caralluma sinaica etc [16].
2.3 Other uses of Housefly
Housefly to monitor disease outbreaks
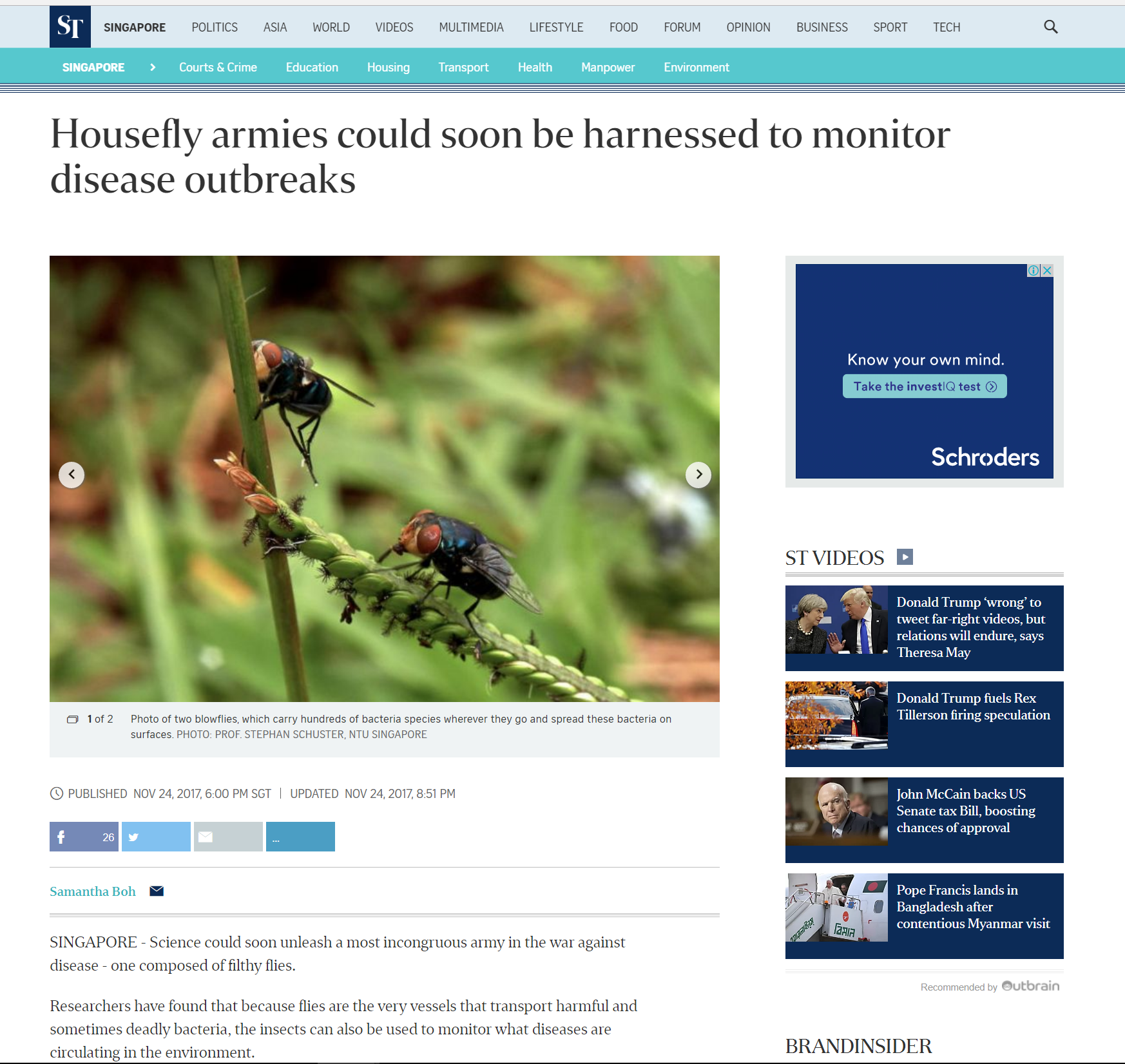
Screenshot of newspaper excerpt on the potential use of houseflies. (Link)
In Singapore, researchers at the Nanyang Technological University (NTU) have found a potential use in the disease transmitting potential of houseflies. According to the study, houseflies have the potential to aid in public health survelliance programmes, by helping to reveal the types of bacteria inhabiting a particular environment [29]. It is suggested that sterile flies bred in the absence of microorganisms could be releasedand pick up bacteria that is present in the environment [29]. The flies are then collected using baits and sequenced for the microbiomes present. Thus potentially giving a rough idea of what pathogens are spreading at the moment [29].
According to Schuster, the use of houseflies as a medium monitor the spread of pathogens have potential benefits to the agriculture sector in understanding what pathogens could be affecting crop growth [29].
3. BIOLOGY
3.1 Morphology and Identification
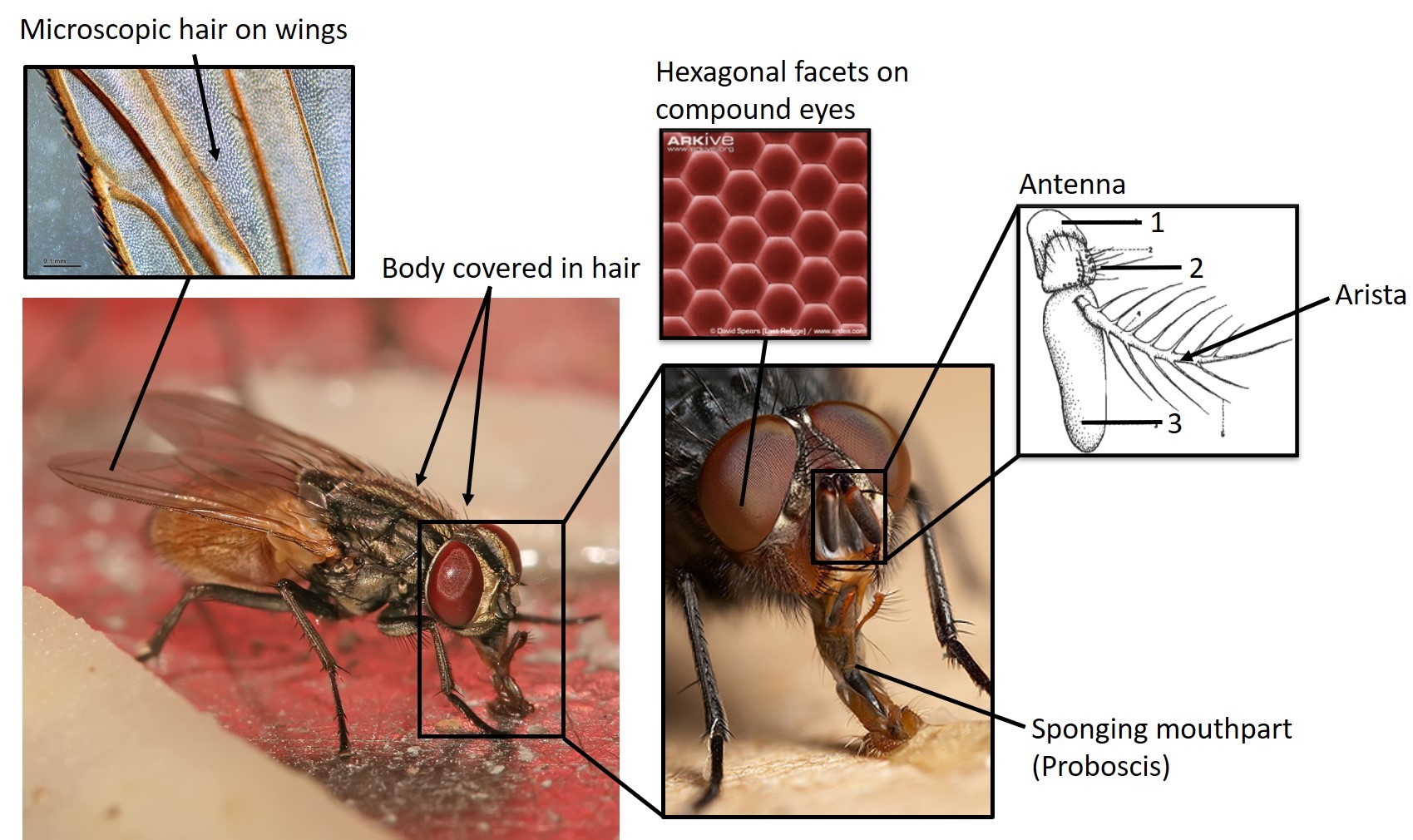
Image compiled by: Chin Zhen Yue
The adult common housefly is approximately 6 to 7 mm long and can be identified by its reddish eyes and sponging mouth parts. Its body is sparsely covered in hair while the wings are covered in microscopic hair [17]. The head consists of a large pair of compound eyes that are slightly bean-shaped and reddish in colour[14]. The compound eyes are segregated into roughly 4,000 roughly hexagonal facets. The facets works like lenses by allowing light to activate the optical units (ommatidium) directly below the facets [14].The antenna are located between the eyes in the facial depression of the housefly[14]. They are separated into three segments, the first segment (1) has a ring-like shape and is quite obscure. The second segment (2) is blackish-brown in colour, is larger than the first segment and has irregular, short and black hairs [14]. The third segment (3) is longest of the three, and bears the arista which is bristle-like and looks like the veins of a leaf. The proboscis, used for feeding, is located at the bottom of the head. The extendable proboscis is normally retracted when the fly is not feeding[14]. The thorax is pale yellowish-gray and has four longitudinal black stripes, while the abdomen is yellowish.
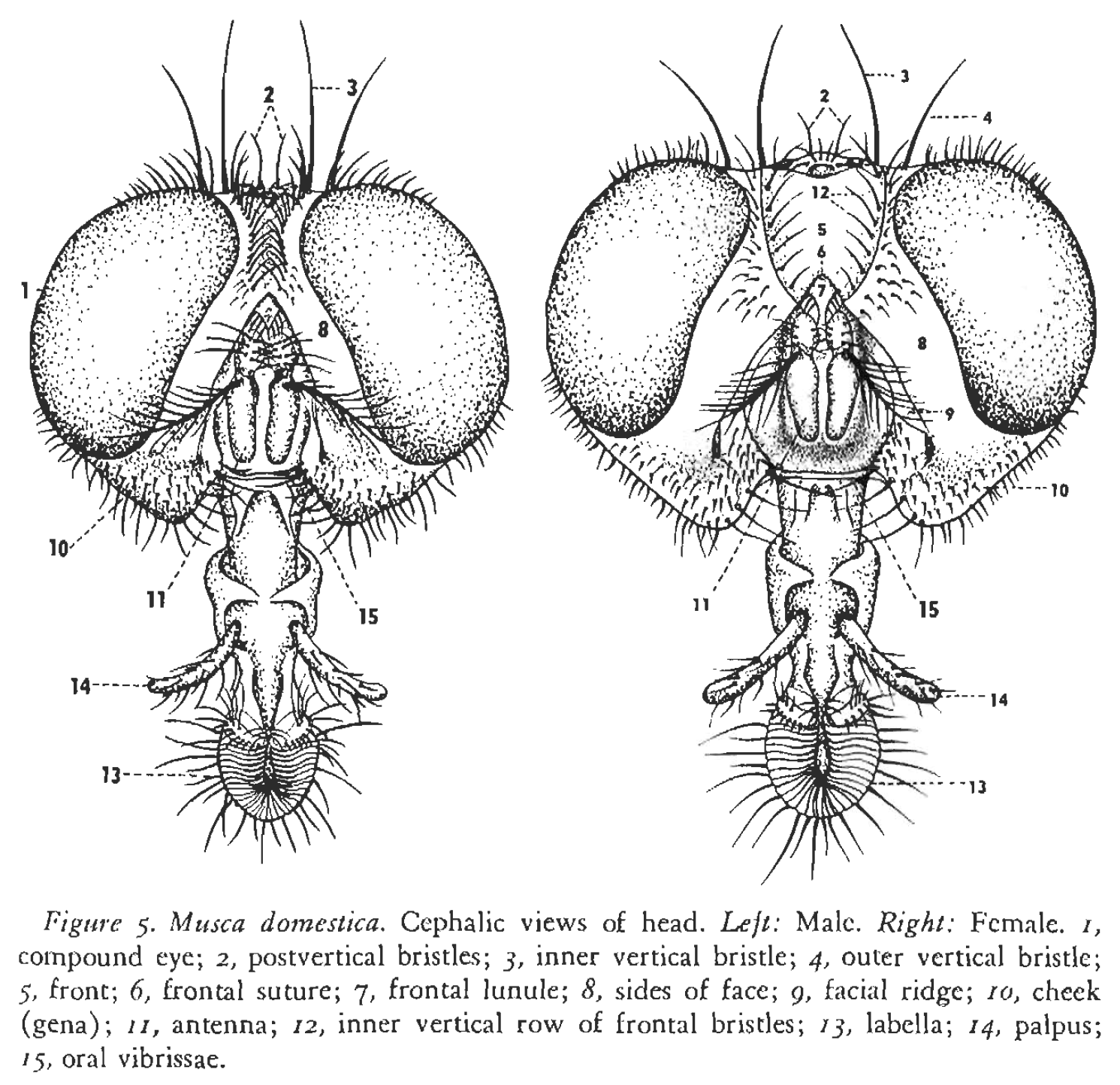
Frontal view of housefly with male on the left and female on the right, showing differences in frontal stripe [14].
They exhibit sexual dimorphism, with the female housefly generally being larger than her male counterpart. The female can be identified by its wider frontal stripe, causing a larger spacing between its eyes compared to the males which have a narrower frontal stripe. In females, the abdomen is yellowish with a gray undertone with a dark mid line, whereas in males, the abdomen is yellowish[18].
It is to note that the identification of Musca domestica is not as easy as it seems, there are several other species of flies that look very similar to Musca domestica as shown in the image below. Therefore, researchers normally identify flies based on their genitalia.
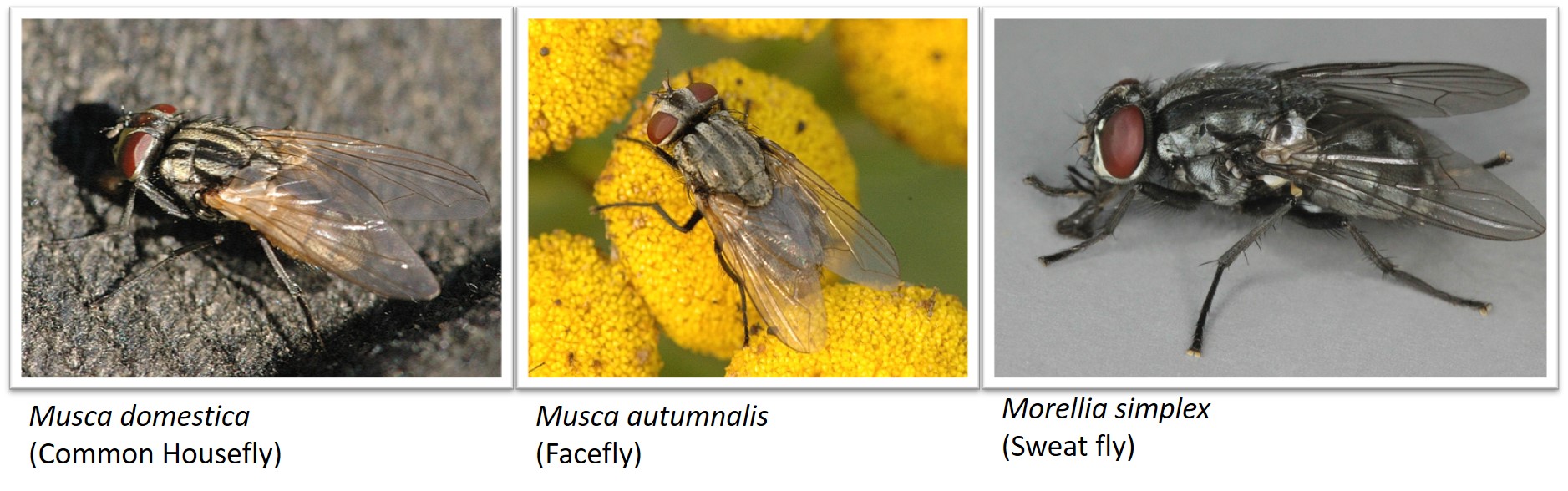
Image source: Musca domestica, Wikimedia commons, Photograph by: James Lindsey (Link); Musca autumnalis, Wikimedia commons, Photograph by: James Lindsey (Link) and Morellia simplex, Wikimedia commons, Photograph by: Janet Graham (Link)
3.2 Life Cycle
The housefly goes through four distinct stages during its life cycle; starting from an egg, to a larva, pupa then adult and can be completed within 7 to 10 days in optimal conditions or up to 2 months when conditions are poor [18] . Annually, the housefly can have more than 20 generations in tropical and subtropical regions, while roughly 10 to 12 generations in temperate regions [18] .
1st Stage: Egg

Image source: [18], Photograph by: Jerry F. Butler, University of Florida. (Link)
The eggs are roughly 1.2 mm long and are laid singly in small batches of between 75 to 150 eggs per batch [18]. The number of eggs produced per female is proportional to the female body size, thus the larger the female, the greater number of eggs she produces. On average, a female housefly can lay up to 500 eggs in a span of three to four days [18]. Most of the time, several flies lay their eggs close to each other leading to large numbers of fly larvae and pupae at the site.
2nd stage: Larval Stage

Image source: Wikimedia commons, Photograph by: Pavel Krok. (Link)
At the larval stage, the larva starts as a 3 to 9 mm creamy-white long maggot that is cylindrical in shape that tapers towards the end [12] . It does not have eyes, legs or other appendages but has a mouth equipped with two black hooks on its head. It is segmented into 13 parts, of which 12 are easily seen[14] . It undergoes three instars (i.e. moults twice times) to become a maggot roughly 7 to 12 mm long. It completes its development after 4 to 13 days at optimum temperatures (23°C - 30°C) or at suboptimal temperatures (12°C - 17°C), 14 to 30 days [14, 18].
3rd stage: Pupae Stage

Colour transition of Housefly pupae [18] Photograph by: Jim Kalisch, University of Nebraska-Lincoln. (Link)
The pupa is oval and is roughly 8 mm long. As the pupa ages, it changes colour from creamy-white to yellow, brown, red then black. Its development is complete after 2 to 6 days at temperatures of 32°C to 37°C, or 17 to 27 days at around 14°C [18] .
4th stage: Adult
The adult housefly emerges from the pupae case by expanding its head [14]. The lifespan lasts between 15 - 25 days, but could live up to two months at cooler temperatures in the availability of suitable food [18].
3.3 Diet and Feeding Habits
Housefly feeding on pizza video.Houseflies are omnivorous and can feed almost on anything as long as it is liquid or can be made into a liquid. Despite so, carbohydrates form an essential part of a housefly diet so as to ensure its longevity. While proteins are necessary for the production and deposition of eggs [14] .
The proboscis is the feeding apparatus of the housefly which functions like a straw for the housefly. Thus, limiting the fly to liquids or else, solid substrates would have to be prepared for ingestion. Houseflies can feed via normal suction and filtration of liquid food, where food is drawn by the suction power caused by muscle contraction. For solids, houseflies make use of the prestomal teeth present in the labella by scraping the food, then moistening the food using saliva or regurgitated food to produce a solution that can be ingested by sucking subsequently [14] .
3.4 Courtship and Mating Behaviour

Image Source: Wikimedia commons, Photograph by: Muhammad Mahdi Karim
Mating begins when a male captures a female, takes place on an object and lasts for a few seconds to a few minutes [14]. After capture, the male would use its front leg (fore tarsi) to stroke the head of the female, stimulating the female to extend her ovipositor to connect with the terminal end of the penis, which descends from the genital atrium of the male. The stroking of the female using the fore tarsi, also allows the male to determine the species, sex and mating status of its potential mate [5]. The houseflies mate while facing the same direction with the male on top of the female (superimposed position). This position observed is due to the position of the genitalia of muscoid groups. However, other positions can be observed if the male is much smaller than the female or if it loses balance [14].
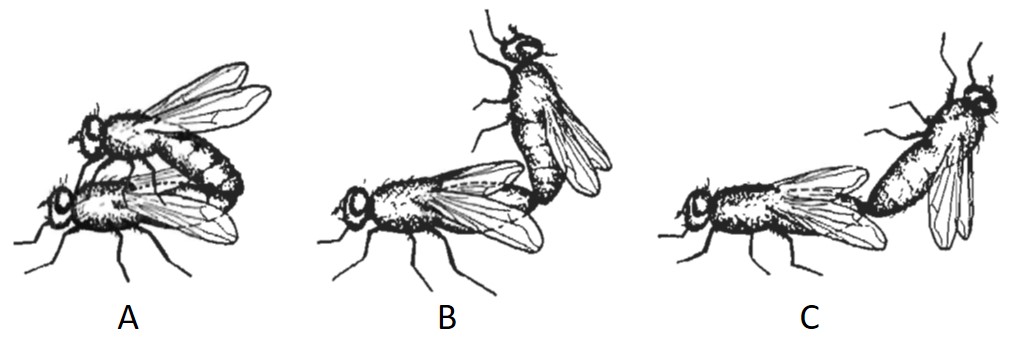
Housefly intercourse. A) Normal position; male atop the female, facing the same direction. B) Position observed when male is small. C) Position observed when male loses stability. [18]
Housefly mating
3.5 Oviposition site
Within four to eight days after copulation, females begin laying their eggs on suitable substrates. Compared to other species of insects, the female housefly is not exceptionally particular with its nesting site. The only drawback of a less suitable medium just yields smaller houseflies [14].
The versatile housefly can oviposition on various mediums to yield viable offspring. For example, oviposition sites have been recorded on mattress with urine, vegetables, rubber etc [14]. However, research have suggested that houseflies preferentially favour any medium undergoing the process of decomposition; which are most commonly animal manure, kitchen refuse and carrion [14].
Potential breeding grounds

Image Source: Fecal matter, Wikimedia commons, Photograph by: Jebulon (Link); Raw Sewage, Photograph by: Tate Rogers (Link); and Decaying food, Flickr, Photograph by: Taz (Link).
4. PHYLOGENY AND SYSTEMATICS
4.1 Description

Original description of Musca domestica in Systema Naturae in Latin [19].
Musca domestica was first described by Linnaeus in 1758 in the 10th edition of the Systema Naturae. The picture shows the original description of Musca domestica which includes the diagnosis, bilbliography, distribution and biology [20]. To delimit the different orders, Linnaeus used wings and subsequently used mouthparts to describe genera [20]. He describes the genus Musca to be: “Os proboscide carnosa: labiisl lateralibus: Palpi nulli" which means, “mouth with a fleshy proboscis: with two lateral lips: palpi absent”[20]. Subsequently, the genus was divided into 100 species using the characters of the body vestiture, and the antenna and arista [20].
However, the original description Musca domestica by Linneaus in 1785 of was later found out to be likely described from two other species of the Muscidae family that was found in Linneaus's collection box. Given the general description prescribed by Linneaus, it is assumed that one of the two specimens were used, since it was labelled with a specific name and the number in Systema Naturae (1758). Haliday suggests that the remarks "Habitat in Europae domibus, etiam Americae. Larvae infimo Equino. Pupae parallele cubantes." in the original description, and the name used by Linneaus, suggested that he had the common housefly in mind [21]. Thus, the use of domestica for the common housefly is still used to respect the spirit of Linneaus [21].
4.2 Type Information
The syntypes Linneaus used for the original description of Musca domestica in 1758 are considered to be ‘lost’. Although there were two specimens found under the collection name Musca domestica in Linneaus’s Musca no. 69 in Diptera box 12, the specimens were later identified to be Morellia simplex (Loew, 1857) and Muscina assimilis (Fallén, 1824) [20].Today, the type specimens of Musca domestica are composed of holotypes, lectotypes and syntypes specimens of synonymised names. For example, the holotypes, Musca contigua, Musca determinata and Musca vicaria described by Walker (1856) [22] were synonymised to Musca domestica Linneaus [20]. There are 7 holotype specimens (3 male; 4 female) located in the Natural History Museum (London), 1 male holotype specimen at the Museum National d'Histoire Naturelle, Paris and 1 male holotype specimen at the Universitetets Zoologiske Museum, Copenhagen.
4.3 Synonyms
There are several names that have been synonymised to Musca domestica.
| Species name |
Author, year |
|---|---|
| Ascaris conosoma |
Jordens, 1802 |
| Degeeria dawsoni |
Rainbow, 1897 |
| Degeeria obscura |
Pont, 1968 |
| Musca analis |
Macquart, 1843 |
| Musca antiquissima |
Walker, 1849 |
| Musca atrifrons |
Bigot, 1888 |
| Musca aurifacies |
Robineau-Desvoidy, 1830 |
| Musca aurulans |
Robineau-Desvoidy, 1830 |
| Musca australia |
Sherborn, 1922 |
| Musca australis |
Macquart, 1843 |
| Musca basilaris |
Macquart, 1843 |
| Musca campicola |
Robineau-Desvoidy, 1863 |
| Musca chilensis |
Macquart, 1843 |
| Musca consanguinea |
Rondani, 1848 |
| Musca contigua |
Walker, 1853 |
| Musca cuthbertsoni |
Patton, 1936 |
| Musca determinata |
Walker, 1853 |
| Musca divaricata |
Awati, 1916 |
| Musca divisa |
Meigen, 1975 |
| Musca flavifacies |
Bigot, 1888 |
| Musca flavinervis |
Thomson, 1869 |
| Musca flavipennis |
Bigot, 1888 |
| Musca frontalis |
Rondani, 1868 |
| Musca fulvescens |
Robineau-Desvoidy, 1830 |
| Musca harpyia |
Harris, 1841 |
| Musca multispina |
Awati, 1916 |
| Musca nancauriensis |
Schiner, 1868 |
| Musca oceanica |
Le Guillou, 1842 |
| Musca pampasiana |
Bigot, 1888 |
| Musca pellucens |
Meigen, 1835 |
| Musca rivularis |
Robineau-Desvoidy, 1863 |
| Musca rufifrons |
Macquart, 1843 |
| Musca rufiventris |
Macquart, 1846 |
| Musca sanctae-helenae |
Macquart, 1848 |
| Musca sordidissima |
Walker, 1864 |
| Musca soror |
Robineau-Desvoidy, 1830 |
| Musca stomoxidea |
Robineau-Desvoidy, 1830 |
| Musca taitensis |
Macquart, 1843 |
| Musca tiberina |
Sacca, 1947 |
| Musca umbraculata |
Fabricius, 1805 |
| Musca vaccina |
Robineau-Desvoidy, 1863 |
| Musca vagatoria |
Robineau-Desvoidy, 1830 |
| Musca vicaria |
Walker, 1853 |
| Musca vicina |
Macquart, 1851 |
| Sphora australis |
Robineau-Desvoidy, 1830 |
| Sphora nigricans |
Robineau-Desvoidy, 1830 |
4.4 Phylogeny
The family Muscidae commonly known as “House flies” mostly due to the prevalence of Musca domestica worldwide. The family is composed of more than 5000 species and 200 genera [23]. Based on morphological[24-26] and molecular[27, 28] studies over the years, the family Muscidae is well supported to be monophyletic.However, classification of groups within the Muscidae are still widely contested today. Presently, eight subfamilies are recognised (Achanthipterinae, Muscinae, Azeliinae, Atherigoninae, Cyrtoneurininae, Mydaeinae, Phaoninae and Coenosiinae) [27].
A comprehensive study using three nuclear genes and four mitochondrial genes across 84 species from 40 genera, concluded the subfamily muscinae to be monophyletic [27]. The study revealed a strong node support (Bootstrap value: 97 and Jackknife: 95) for the split between the subfamilies, Atherigoninae + Coenosiinae + Mydaeinae + Phaoniinae and Muscinae + Azeliinae. Although a low bootstrap value suggests weak node support for the split between Muscinae and Azeliinae, the node supports within the Muscinae and Azeliinae families show relatively strong support.
Regarding Musca domestica, there is strong support that it is a sister species to Musca autumnalis and Musca confiscata.
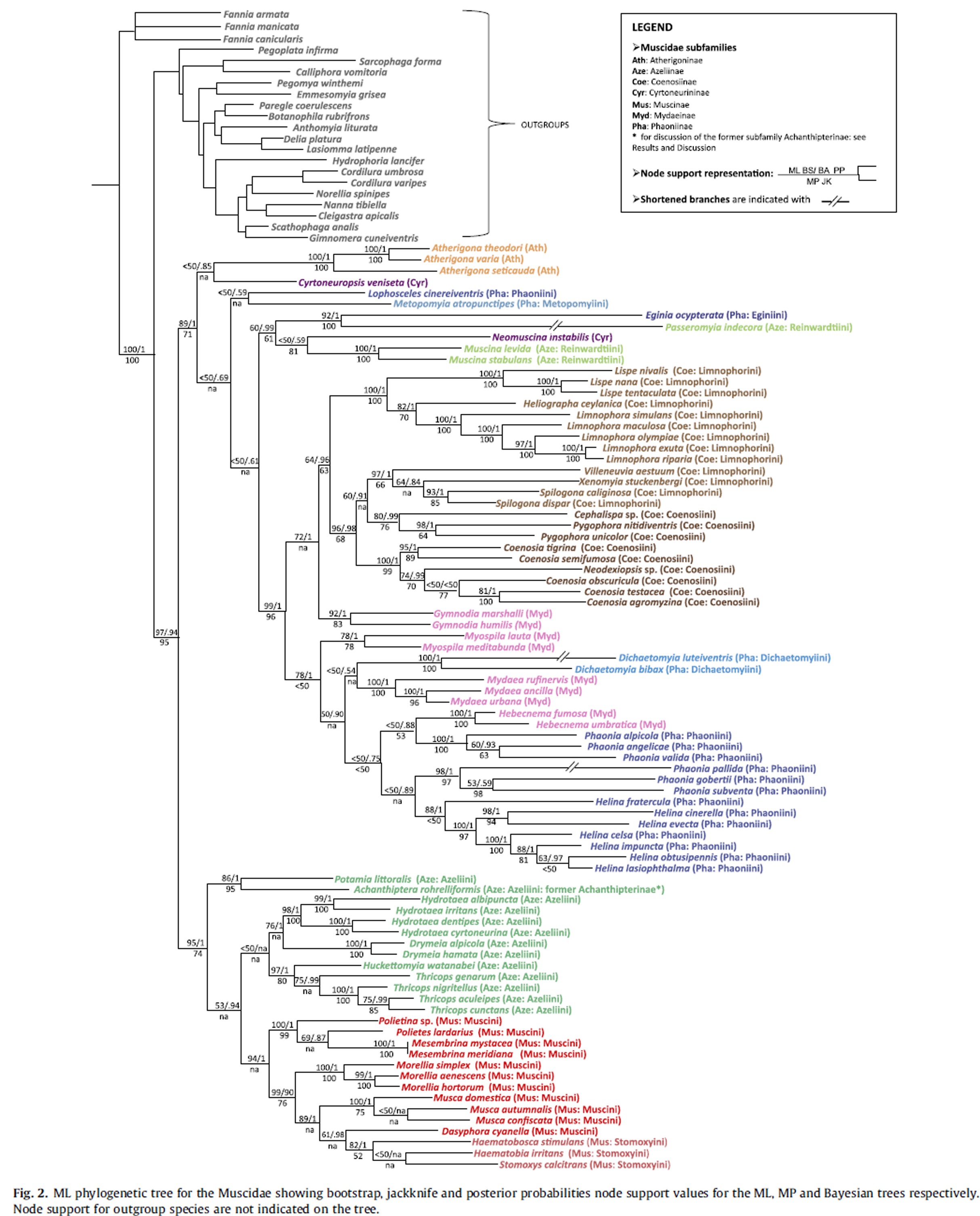
Phylogenetic tree for the Muscidae [27].
4.5 Taxonomic Hierarchy
Kingdom: AnimaliaPhylum: Arthropoda
Subphylum: Hexapoda
Class: Insecta
Subclass: Pterygota
Order: Diptera
Suborder: Brachycera
Family: Muscidae
Subfamily: Muscinae
Tribe: Muscini
Genus: Musca
Species: Musca domestica
REFERENCES
1. Esten, W.M. and C.J. Mason, Sources of bacteria in milk. 1908.2. Nayduch, D. and R.G. Burrus, Flourishing in Filth: House Fly–Microbe Interactions Across Life History. Annals of the Entomological Society of America, 2017. 110(1): p. 6-18.
3. Gilmore, M., et al., Enterococcal Disease, Epidemiology, and Implications for Treatment--Enterococci: From Commensals to Leading Causes of Drug Resistant Infection. 2014.
4. Bahrndorff, S., et al., Bacterial Communities Associated with Houseflies (Musca domestica L.) Sampled within and between Farms. PLoS ONE, 2017. 12(1): p. e0169753.
5. Dahlem, G.A., Chapter 125 - House Fly: (Musca domestica) A2 - Resh, Vincent H, in Encyclopedia of Insects (Second Edition), R.T. Cardé, Editor. 2009, Academic Press: San Diego. p. 469-470.
6. Ahmadu, Y., O. Goselle, and L. Ejimadu, Microhabitats and Pathogens of Houseflies (Musca domestica): Public Health Concern. Electronic Journal of Biology, 2016. 12(4).
7. Malik, A., N. Singh, and S. Satya, House fly (Musca domestica): a review of control strategies for a challenging pest. Journal of Environmental Science and Health Part B, 2007. 42(4): p. 453-469.
8. Khalequzzaman, M., et al., Toxic, repellent and attractant properties of some insecticides towards the house fly (Musca domestica L.). J Biol Sci, 2002. 2: p. 672-676.
9. Sukontason, K.L., et al., Effects of eucalyptol on house fly (Diptera: Muscidae) and blow fly (Diptera: Calliphoridae). Revista do Instituto de Medicina Tropical de São Paulo, 2004. 46(2): p. 97-101.
10. Palacios, S.M., et al., Efficacy of essential oils from edible plants as insecticides against the house fly, Musca domestica L. Molecules, 2009. 14(5): p. 1938-1947.
11. Shalaby, A.A., et al., Insecticidal properties of citrus oils against Culex pipiens and Musca domestica. Journal of the Egyptian Society of Parasitology, 1998. 28(2): p. 595-606.
12. Ceden, C., R. Stinner, and R. Axtell, Predation by predators of the house fly in poultry manure: effects of predator density, feeding history, interspecific interference, and field conditions. Environmental Entomology, 1988. 17(2): p. 320-329.
13. Broski, S.A. and B. King, Effects of Size and Age of the Host Musca domestica (Diptera: Muscidae) on Production of the Parasitoid Wasp Spalangia endius (Hymenoptera: Pteromalidae). Journal of economic entomology, 2016. 110(1): p. 282-287.
14. West, L.S., The housefly. 1951: Comstock Publishing Company; New York.
15. Hussein, M., et al., Sustainable production of housefly (Musca domestica) larvae as a protein-rich feed ingredient by utilizing cattle manure. PloS one, 2017. 12(2): p. e0171708.
16. Meve, U. and S. Liede, Floral biology and pollination in stapeliads—new results and a literature review. Plant Systematics and Evolution, 1994. 192(1-2): p. 99-116.
17. Hewitt, C.G., The House-Fly: Musca Domestica Linn: Its Structure, Habits, Development, Relation to Disease and Control. 2011: Cambridge University Press.
18. Sanchez-Arroyo, H. and J.L. Capinera, House fly, Musca domestica Linnaeus (Insecta: Diptera: Muscidae).
19. Linnaeus, C., Systema Naturae, Ed. 10, Vol. 1. 824 pp. Salvii, Holmiae, 1758.
20. Thompson, F.C. and A.C. Pont, Systematic database of Musca Names (Diptera): A catalog of names associated with the genus-group name Musca Linnaeus, with information on their classification, distribution, and documentation. Vol. 20. 1993: Koeltz Scientific Books.
21. Pont, A.C., The Linnaean species of the families Fanniidae, Anthomyiidae and Muscidae (Insecta: Diptera). Biological journal of the Linnean Society, 1981. 15(2): p. 165-175.
22. Walker, F. and W.W. Saunders, Insecta Saundersiana: Or Charcters of Undescribed Insects in the Collection of William Wilson Saunders. Vol. 1: Diptera. 1856: John Van Voorst.
23. Pape, T. & Thompson, F.C. (editors). 2013. Family tables. In: Pape, T., Thompson, F.C. (Eds.), Systema Dipterorum, Version 1.5. work records (not peer-reviewed material). http://www.diptera.org/, accessed on 19 November 2017
24. Stuttgart, S.M.F.N.i. and W. Hennig, Vorarbeiten zu einem phylogenetischen System der Muscidae (Diptera: Cyclorrhapha). 1965.
25. McAlpine, J., Phylogeny and classification of the Muscomorpha. Manual of nearctic Diptera, 1989. 3: p. 1397-1518.
26. Michelsen, V., Revision of the aberrant New World genus Coenosopsia Diptera: Anthomyiidae, with a discussion of anthomyiid relationships. Systematic Entomology, 1991. 16(1): p. 85-104.
27. Kutty, S.N., et al., The Muscoidea (Diptera: Calyptratae) are paraphyletic: evidence from four mitochondrial and four nuclear genes. Molecular Phylogenetics and Evolution, 2008. 49(2): p. 639-652.
28. Kutty, S.N., et al., Molecular phylogeny of the Calyptratae (Diptera: Cyclorrhapha) with an emphasis on the superfamily Oestroidea and the position of Mystacinobiidae and McAlpine's fly. Systematic Entomology, 2010. 35(4): p. 614-635.
29. Ratan, A., et al., The microbiomes of blowflies and houseflies as bacterial transmission reservoirs. Scientific Reports, 2017. 7(1): p. 16324.
30. Sukontason, K., et al., Ultrastructure of adhesive device in fly in families calliphoridae, muscidae and sarcophagidae, and their implication as mechanical carriers of pathogens. Parasitology research, 2006. 98(5): p. 477-481.