Pangio muraeniformis (de Beauford, 1933) Spotted Eel-loach
Table of Contents

Fig 1. Pangio muraeniformis from Singapore (c) Choy Heng Wah
Foreword
This page is intended to serve as a resource for aquarists on the ecology and maintenance of Pangio spp. in captivity. It helps to elucidate the taxonomy of a particular species, P. muraeniformis - the sole representative of the genus in Singapore, and will aid in identifying it. This species also has the distinction of being described from specimens obtained from this country as well. Little literature has been published on the ecology of the genus. As such, much of the information available on this page is a combination of the author's and other aquarists own experiences with these fish. Pangio muraeniformis is exceedingly rare in the Singaporean ornamental trade - much of the information/videos available on this page pertains to other members in the genus, though the keen aquarist can have a good chance seeking them out directly from the ornamental fish exporters in the country. However, all members of the genus have a similar enough ecology and habits that the information unless otherwise specified will be relevant to P. muraeniformis.All photographs on this page have been obtained with the permission of their creators/owners. Please contact the image owners directly, as the images have only been granted for use on this page.
General Description of the Eel-loaches
The Pangio eel-loaches are small (5 to 10cm long), eel-like cypriniforms (related to barbs, rasboras, loaches, carp) distributed from India south through South-east Asia and into the Indonesian islands of Sumatra, Borneo and Java. Often two to three species of Pangio co-occur in a single locality (Tan & Ng, 1999), with up to seven species within a single drainage basin in Kalimantan. Singapore was historically home to two species of eel-loaches. The banded eel-loach Pangio semicincta and the spotted eel-loach Pangio muraeniformis. However, the former though common throughout Peninsula Malaysia, Sumatra and Borneo is now extirpated since the loss of the swamp forests at Mandai in the 1960s (Giam et al., 2011). Pangio muraeniformis is presently the sole representative of the eel-loaches in Singapore and its ecology and maintenance in captivity is the focus of the rest of this page.Consequences of the ornamental fish trade
These fishes are popular in the aquarium trade, where they are often known as 'coolie' loaches: an unfortunate name derived from the first species described in the genus - Pangio kuhlii. Though traded in their thousands, the status of most Pangio species is incompletely known with most of them listed as Data Deficient (DD) or not evaluated by the IUCN (Internation Union for Conservation of Nature). Pangio muraeniformis has not yet been evaluated by the IUCN and its status in the wild is incompletely known.It is currently thought that the trade in wild-caught individuals of small, schooling, invertebrate-eating fish such as P. muraeniformis is sustainable (Ng & Tan, 1997). However, these fishes are also dependent on forests for their survival. The current rates of deforestation in south-east Asia are accelerating; habitat loss could deplete and isolate populations of this species to an extent where commercial collections for the ornamental fish trade could conceivably lead to population collapse and extinctions (Giam et al., 2011).
As aquarists, it is important to be aware of where and how our fish are collected and be mindful of supporting only sustainable enterprises. Asking your local importer about the source of their fishes would be a good first step.Many sustainable collections of ornamental fish species rely on indigenous people to procure and deliver the fish to the middlemen - injecting much needed revenue into impoverished communities; an endeavor worth supporting. Sustainably sourced fish also tend to be slightly more expensive than their counterparts in the trade because of the longer supply chains (pers. obs.). It is highly encouraged that aquarists show preference for sustainably sourced fish to push to make this the norm in the industry. To many of us, these sustainable ventures are definitely worthwhile and we are only too glad to know that some of what we pay for own beloved fish goes back to incentivising and perpetuating the conserving their wild counterparts.

Fig 2. Pangio semicincta a species now extirpated in Singapore but common throughout the rest of its range (c) Nonn Panitvong
Etymology
The species epitaph is derived from the Latin ‘muraena’; a reference to the generic name for Moray-eels which have a body-form that resembles that of the present species.Nomenclature
This species was originally included in the genus Acanthophthalmus which Kottelat (1987) demonstrated to be a synonym for Cobitis. He chose the replacement name Pangio Blyth, 1860.Synonyms
Acanthophthalmus muraeniformis de Beaufort, 1933 – original name, genus misappliedPangio muraeniforme (de Beaufort, 1933) - misspelled
Acanthophthalmus shelfordii (non Popta, 1903) - misapplied
Pangio shelfordi (non Popta, 1903) – misapplied
From Catalogue of Life
Pangio muraeniformis
Diagnosis
Pangio is distinguished from all other Cobitid loach genera by its very slender to anguilliform (eel-like) body, compressed to very compressed, by the position of the dorsal fin whose origin is conspicuously behind the pelvic origin (vs. in front, above or slightly behind) and more vertebrae (45-71 vs. 28-43) (Kottelat & Lim, 1993).
Fig 3. Pangio anguillaris from Thailand, note worm-like elongated body and proportionally small head (c) Nonn Panitvong

Fig 4. Lepidocephalus macrochir a typical cobitid, note sturdier body and head (c) Choy Heng Wah
Within the genus, P. muraeniformis (Fig 6) is most similar to P. shelfordii (Fig 5) (a species from Borneo). Both species are rather rare in the Singaporean aquarium trade though are more prevalent overseas. They are separated from all other congeners by the possession of a series of dark blotches on the side of the body, connected to or alternating with a similar number of dark saddles on the back (Kottelat & Lim, 1993).

Fig 5. Pangio shelfordii from Borneo (c) Tan Heok Hui

Fig 6. Pangio muraeniformis collected from Singapore, Peninsula Malaysia and Riau Islands (c) Tan Heok Hui
Separating both species from each other can be challenging, but is possible. In P. muraeniformis, (Fig. 7) the lateral, black blotches are finer, forming an almost contiguous line along the body, bordered ventrally by a row of paler, irregular mottling extending, from a point between the head and vertical of pelvic fin to caudal base. However, the ventral mottling may be exceedingly faint in stressed specimens and may be an unreliable indicator when individuals are observed in a shop or newly purchased. Black saddles are usually replaced by median patches of small, brown blotches; though in some specimens the saddles may still be retained, though not sharply marked (Kottelat & Lim, 1993).

Fig 7. Pangio muraeniformis, collected from Nee Soon Swamp Forest (c) Tan Heok Hui; annotations added by Movin Nyanasengeran
In Pangio shelfordii (Fig 8) the lateral blotches tend to be less fine, vertically elongated and much darker at the midline of the body. The black saddles on the back are very pale and contiguous leaving only a thin line between them. This species also lacks the row of mottling below the lateral blotches.

Fig 8. Pangio shelfordii, collected from Borneo (c) Tan Heok Hui; annotations added by Movin Nyanasengeran
Coloration
Base body colour is pale yellow to orange. Blotches on back and on sides of the fish are dark brown to black. Underside is conspicuously lighter than dorsal surface. Caudal and dorsal fins streaked with black spots along fin rays and brownish to black patch on caudal peduncle. (Refer to diagnosis for precise pattern of blotches on back and sides)Native Distribution
Pangio muraeniformis as presently defined appears restricted to the Malay Peninsula, Singapore and the Riau Islands (Kottelat & Lim, 1993). Pangio muraeniformis was considered a synonym of the Bornean eel-loach (P. shelfordii) in this paper; P. muraeniformis is split from this taxon in later publications (Kottelat & Whitten, 1996; Bohlen et al., 2011) with P. shelfordii now restricted to the Sungei Sarawak drainage in Borneo (Bohlen et al., 2011).Sexing
In Pangio muraeniformis, males have a thickened second pectoral ray which is turned upwards - this trait is absent in females. Females are heavier bodied than the males. Sometimes when gravid, it is possible to view light green eggs through the body wall.Size
Largest official specimen measured 49.2mm (CMK 7381) from head to caudal peduncle (Kottelat & Lim, 1993). Larger specimens have been noted at Nee Soon Swamp Forest (pers. obs.).Biology
Feeding Habits
This species is a micropredator feeding on worms and small crustaceans. Its diet in Singapore remains undocumented. Pangio spp. sift through the substrate with its mouth and gills from which prey items are extracted and consumed. Though the fish take mouthfuls of substrate and expel the sediment out of their gills while feeding, this behaviour can only be seen when housed in aquaria with exceedingly fine substrate. Wild-caught specimens in the aquarium trade feed readily on frozen bloodworm, daphnia and processed food that settles on the substrate (pers. obs.).Video 1. Pangio semicincta foraging in soil. Note the twin puffs of substrate exiting the gills as the loach burrows into the substrate looking for food. (c) YouTube User: Movin Nyanasengeran.
Behaviour
Pangio eel-loaches are found in large aggregations in the wild and prefer a similar arrangement in captivity. A minimum of six individuals (more is ideal) should be housed together - if held in smaller numbers they tend to be retiring and will barely be seen; only coming out when the aquarium lights are off. If held in sufficient numbers, it is possible to witness a group of eel-loaches packing themselves under pieces of wood or in the substrate with just their heads sticking out. It is recommended that ample cover in the form of aquatic plants, wood and non-reactive rocks be used as part of the tank decor for the eel-loaches to hide in when alarmed. Having inadequate cover can lead to shy, stressed fish that rarely come out into the open.Habitat
The type locality of species is Singapore, from a stream flowing alongside Thompson Road (Kottelat, 2012). Specimens have also been recorded from the leaf litter or dense vegetation in silty or sandy, forest streams of moderate current, with tannin-stained (tea-coloured) water in the Endau drainage in Johor, Malaysia (Ng & Tan, 1999) as well as in silty, slow-flowing streams in Nee Soon Swamp forest, Singapore (Li et al., 2016; pers. obs.). The waters of these streams tends to be soft (poor in dissolved minerals) and acidic and the species should ideally be maintained in such conditions in captivity.
Fig 9. Pangio muraeniformis was found in this pool (Nee Soon swamp forest). Very thick mats of leaf litter and roots. The pH was 5.4 and flow rate was negligible. (c) Low Bi Wei
Locally, P. muraeniformis is restricted to the Nee Soon and Upper Seletar drainages (Li et al., 2016). In these areas they are found in forest streams and pools with thick accumulations of leaf litter and acidic waters (pH 4.5 - 6.0) (pers. obs.).
Breeding
Breeding behaviour in the wild remains undocumented. In captivity, males pursue the female across the tank before spawning takes place. The fish are egg scatterers and will spawn while swimming across the surface of the aquarium. The eggs are mildly adhesive and will stick to wood, plants and other available surfaces. Hatching takes place after 2-3 days. This species may spawn in schools as is observed in captivity. Indeed, this species is known to be difficult to spawn in the aquarium and successful breeding attempts have not been documented unless the fish are held in large groups (> 10 individuals).Video 2. Pangio oblonga breeding behaviour. (c) YouTube User: Daniella Alexandersson.
Conservation Status
Pangio muraeniformis has not been assessed by the International Union for Conservation of Nature (IUCN), but is listed as Endangered in Singapore’s Red Data Book – chiefly due to its localized distribution nationally, though it appears fairly common where it does occur (Li et al., 2016).Biotope Ideas (for interested aquarists!)
Biotope aquaria are created to simulate the natural habitats of particular species. The fish, plants, substrate, water chemistry, and furnishings are similar to those found in a natural setting. This section will function as an aid for interested aquarists who wish create a biotope aquarium around Pangio muraeniformis it will highlight various localities, habitat descriptions, fish and plants that co-occur with P. muraeniformis at each site.1. Bintan Island (Riau archipelago)
Video 3. Video of stream habitat in Bintan Island (YouTube User: DiscoveryPlanetuk)
Description: Muddy stream covered in thick accumulations of leaf litter and woody debris
Plant: Cryptocoryne griffithii
Fish: Rasbora einthovenii, Desmopuntius hexazona, Trigonostigma heteromorpha, Sundadanio axelrodii
2. Singkep Island (Riau archipelago)
Video 4. Video of stream in Singkep Island (YouTube User: DiscoveryPlanetuk)
Description: Medium forest stream with dark tannin-stained water, large amounts of woody debris and leaf litter, substrate is silty
Plants: Mats of Cryptocoryne griffithii
Fish: Trigonopoma gracile, Rasbora einthovenii, Barbodes banksi, Nemacheilus selangoricus, Sundadanio atomus, Desmopuntius hexazona, Rasbora kalochroma,
3. Nee Soon Swamp Forest (Singapore)

Fig 10. Small pool with eel-loaches in Nee Soon Swamp Forest (c) Low Bi Wei
Description: Large pool with slow-flowing water, thick mats of leaf litter and woody debris, roots of terrestrial plants growing into the stream, fine, silty substrate
Plants: mosses growing into pool, mats of Cryptocoryne griffithii
Fish: Trigonostigma heteromorpha, Rasbora einthovenii, Betta pugnax, Boraras maculatus, Barbodes lateristriga, Barbodes banksi, Desmopuntius hexazona, Parakysis longirostris, Nemacheilus selangoricus, Macrognathus maculatus
4. Endau-Rompin drainage (Johor, Malaysia) (from Tan & Ng, 1999)
Description: Sandy stream with thick leaf litter
Plants: Mats of Cryptocoryne affinis
Fish: Akysis microps, Pangio cuneovirgata, Pangio doriae, Pangio semicincta, Pangio malayana, Pangio piperata, Homaloptera nebulosa, Homaloptera nigra, Homaloptera tweediei, Pseudomystus fuscus, Glyptothorax major
Recommendations:
Dark sand or soil substrate can be used as the substrate. Ketapang (Terminalia cattapa) leaves can be used both for the leaf litter and to darken the water through the addition of tannins. Dried palm leaves can be used to break the monotony of the leaf litter and add cover for the fish. Most available Cryptocoryne species can be substituted for the abovementioned species. Though note that in natural habitats soil often forms a layer on the leaves of these plants and can be maintained in aquaria to increase realism. Though absent in the natural habitats some floating plants (i.e. duckweed, Azolla) can be used to break up the light entering the tank and artfully add contrast to the aquascape.
Minimum tank sizes for any of the above habitats should be a minimum of 60 by 30 cm. Filtration need not be strong and current kept to a minimum. Most commercial foods will be accepted by the aquarium inhabitants outlined above, and water changes of 20% carried out once a week.
Phylogeny & Sytematics
Taxonavigation
Order: Cypriniformes Family: Cobitidae (Loaches) Genus: Pangio| Taxonomic Hierarchy |
|
| Kingdom |
Animalia |
| Phylum |
Chordata |
| Class |
Actinopterygii |
| Subclass |
Neopterygii |
| Infraclass |
Teleostei |
| Order |
Cypriniformes |
| Superfamily |
Cobitoidea |
| Family |
Cobitidae |
| Subfamily |
Cobitinae |
| Genus |
Pangio Blyth, 1860 |
| Species |
Pangio muraeniformis (de Beaufort, 1933) |
From: Integrated Taxonomic Information System (ITIS)
Type Specimen
The syntype series was collected streams in the area of Thomson Road, Singapore (1.328151°,103.840929°), on the 16th of May, 1912. It was identified by L.F. de Beauford and originally described as Acanthophthalmus muraeniformis in 1933. (Global Species Information Facility)
Phylogeny
Combined data from the mitochondrial cytochrome b gene and control region were used to estimate the phylogenetic relationships of the loaches using maximum parsimony, neighbour joining and Bayesian methods have demonstrated that cobitids form a separate lineage from the botiid loaches (Tang et al., 2006), leading to the elevation of Cobitinae and Botiinae as subfamilies within Cobitidae to the families Cobitidae and Botiidae.Pangio was traditionally divided into four main species groups (Kottelat & Lim, 1993): The kuhlii group, defined by a pattern of dark bars across the head and body (Burridge, 1992). The oblonga-group with ‘a plain body, 8-9 pectoral rays, 45-51 vertebrae and conspicuous adipose dorsal and ventral keels on caudal peduncle’ (Kottelat & Lim, 1993). The shelfordii-group (including P. muraeniformis), characterised by four instead of three pairs of labial barbels (Burridge, 1992); ‘dark bar at caudal base, rows of spots on caudal fin, and a slender caudal peduncle’ (Kottelat & Lim, 1993). And the anguillaris group possessing a ‘high vertebral count and vermiform body’ (Kottelat & Lim, 1992). Males species in this group possess strongly curved pectoral fins (Kottelat & Lim, 1993). However, recent genetic evidence lumps the oblonga and kuhlii groups together - as the latter is nested within the former. And recognises three species groups as valid instead: the shelfordii, anguillaris and kuhlii-oblonga groups.

Fig 11. Pangio cuneovirgata was a member of the kuhlii group. Now known to no longer be monophyletic, and is instead part of the kuhlii-oblonga group (c) Nonn Panitvong
Fig 12. Pangio oblonga, a member of the paraphyletic oblonga-group now lumped together into the kuhlii-oblonga group (c) Steve Grant


Fig 13. (Left) Pangio muraeniformis is a member of the shelfordii-group, note the four pairs of barbels. (c) Choy Heng Wah
Fig 14. (Right) Pangio semicincta on the other hand, has only three sets of barbells (c) Mark Duffill

Fig 15. Pangio anguillaris the name-giver of the anguillaris-group is unusually elongate due to the high number of vertebrae (c) Nonn Panitvong
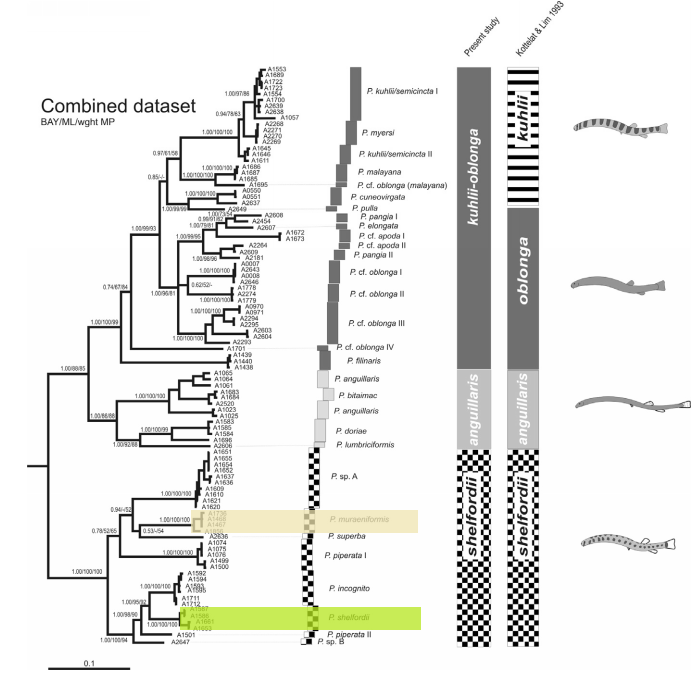
Fig 16. Phylogeny of Pangio loaches and species groups resulting from a Bayesian/Maximum Likelihood/Weighted Parsimony analysis of the combined dataset (sequence data from both mitochondrial cytocrome b and the nuclear recombination-activating gene 1 (RAG1) ) and a comparison with the species groups at defined by Kottelat & Lim, 1993 (Image from Bohlen et al., 2011). Pangio muraeniformis is in beige; Pangio shelfordii is in green.
The above phylogeny (Fig 16.) is a result of a Bayesian/Maximum Likelihood/Weighted Parsimony analysis of the combined dataset (sequence data from both mitochondrial cytocrome b and the nuclear recombination-activating gene 1 (RAG1) ). Trees of all three different methods converged and all methods have high bootstrap support, suggesting that the tree presented above is robust.The data only support the monophyly of the shelfordii and anguillaris-groups (Bohlen et al., 2011; Fig 15.), demonstrating that members of the kuhlii-group are instead nested within the oblonga-group, making the latter group paraphyletic. The authors suggest grouping Pangio into three monophyletic kuhlii-oblonga, shelfordii, and anguillaris groups instead.
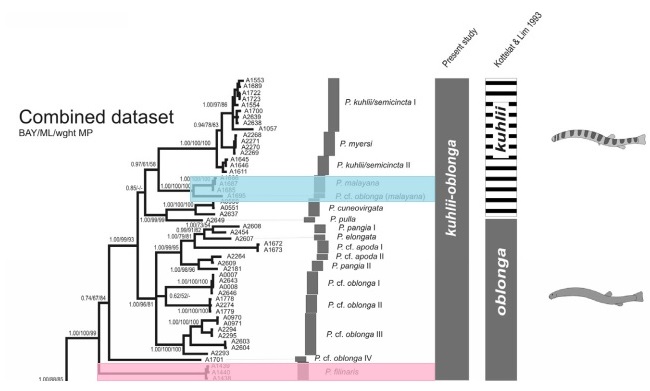
Fig 17. Phylogeny of kuhlii-oblonga groups of Pangio, same gene sequences used as Fig 16. The branch containing Pangio cf. oblonga and Pangio malayana is highlighted blue while the basal-most of the phylogeny with Pangio filinaris is highlighted pink.
Pangio cf. oblonga (an all-brown individual) was found to cluster most closely with the banded P. malayana. This suggests that coloration, a trait that Burridge and Kottelat & Lim used to separate the kuhlii and oblonga groups is an unreliable indicator of phylogenetic relationships, especially when compared to morphological structures such as the number of barbels or vertebrae. The authors of the paper tentatively include Pangio cf. oblonga within Pangio malayana which if correct, suggests that many banded kuhlii-oblonga species may display variability in coloration, and that further study might be necessary to accurately separate species in the field.
Based on Fig 17. it appears that the plesiomorphic (ancestral) condition for the kuhlii-oblonga species was a plain brown body, void of any barring. All the basal-most branches in the phylogenetic tree are of species traditionally traditionally included in the oblonga group (all brown coloration). It appears that barred coloration appeared in stages, with the more basal barred species (P. pulla, P. cuneovirgata & P. malayana) only having bars on the dorsal portion of their bodies, above the lateral line. More advanced species (species that branch off later on) have bars that pass through the lateral line (P. kuhlii/semicincta & P. myersi).

Fig 18. Pangio filinaris, most basal members of the kuhlii-oblonga group are plain brown suggesting that this is the plesiomorphic condition (c) Thomas Frank

Fig 19. Pangio malayana, this species has bands only across the top of its body; some members of this species may lack barring entirely. (c) Steve Grant

Fig 20. Pangio myersi has bands that pass from the top to the bottom of its body (c) Mark MacDonald
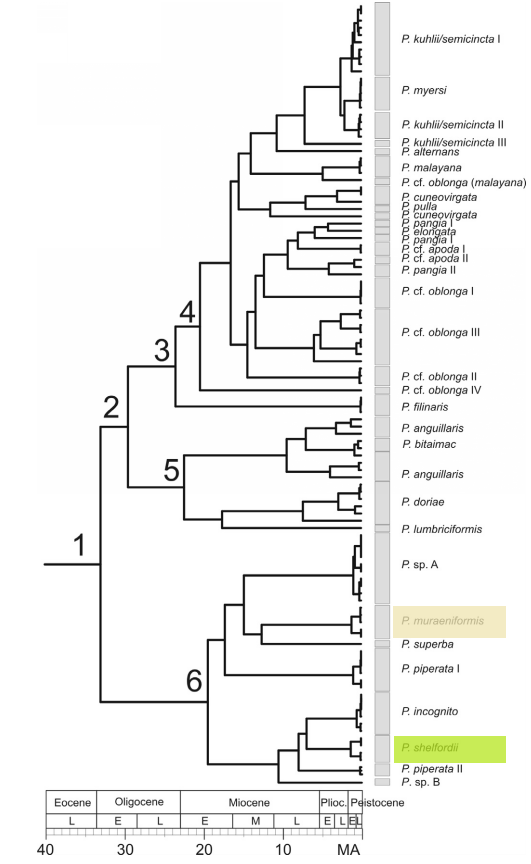
Fig 21. Ultrametric calibrated tree of Pangio dating cladogenetic (speciation) events across the evolutionary history of the genus. Pangio muraeniformis is highlighted beige while Pangio shelfordii is highlighted green. Bar at bottom of the diagram indicates age of lineage (MA = million years)
The above diagram (Fig 21.) demonstrates that P. muraeniformis and P. shelfordii though historically lumped into a single species (Burridge, 1992; Kottelat & Lim, 1993) are indeed two species that have had separate evolutionary histories for approximately 18 million years ago (MYA), since the early Miocene (Node 6, Fig 21.). Higher global sea levels during that time period have been suggested as a possible vicariance event that led to the separation of these two lineages (Bohlen et al., 2011).
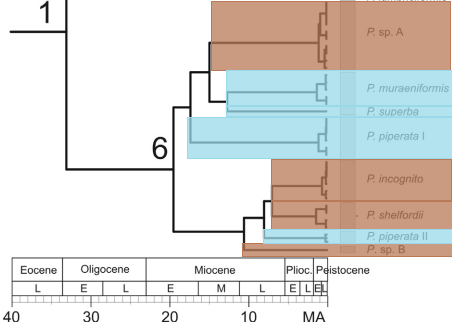
Fig 22. Ultrameric calibrated tree of the Pangio shelfordii group dating cladogenetic events across evolutionary history. Species occurring on Borneo are highlighted brown while those occurring on Peninsula Malaysia are highlighted blue.
The distribution of the shelfordii group species has interesting biogeographical implications. Most taxa in the shelfordii lineage occur in Borneo while those in the muraeniformis lineage are chiefly distributed over Peninsula Malaysia (Fig 22.). However, P. piperata II of the muraeniformis lineage occurs in Borneo while P. sp. A of the shelfordii lineage occurs on Peninsula Malaysia. Pangio piperata II diverges from the other Peninsula Malaysian species around 15 MYA, a time period when the sea level repeatedly lowered allowing eel-loaches the cross to Borneo (Inger & Voris, 2001). Pangio sp. A, diverges from the other shelfordii lineage species approximately 8 MYA during which exceptionally low sea levels left Sundaland dry and reestablished land bridges between Borneo and Peninsula Malaysia allowing a population of Bornean eel-loaches to migrate to Peninsula Malaysia and establish a small population there (Bird et al., 2005).
References
- Bird, M. I., Taylor, D., & Hunt, C. (2005). Palaeoenvironments of insular Southeast Asia during the Last Glacial Period: a savanna corridor in Sundaland?. Quaternary Science Reviews, 24(20), 2228-2242.
- Bohlen, J., Šlechtová, V., Tan, H. H., & Britz, R. (2011). Phylogeny of the southeast Asian freshwater fish genus Pangio (Cypriniformes; Cobitidae). Molecular phylogenetics and evolution, 61(3), 854-865.
- Burridge, M., (1992). Systematics of the Acanthophthalmus kuhlii complex (Teleostei: Cobitidae), with the description of a new species from Sarawak and Brunei. Copeia 1992, 172–186.
- Giam, X., Ng, T. H., Lok, A. F., & Ng, H. H. (2011). Local geographic range predicts freshwater fish extinctions in Singapore. Journal of Applied Ecology, 48(2), 356-363.
- Global Species Information Facility: http://www.gbif.org/occurrence/607789231/verbatim (Accessed 7th November 2016)
- Inger, R. F., & Voris, H. K. (2001). The biogeographical relations of the frogs and snakes of Sundaland. Journal of Biogeography, 28(7), 863-891.
- Kottelat, M. (1987). Nomenclatural status of the fish names created by JC van Hasselt (1823) and of some cobitoid genera. Japanese Journal of Ichthyology, 33(4), 368-375.
- Kottelat, M. (2012). Conspectus cobitidum: an inventory of the loaches of the world (Teleostei: Cypriniformes: Cobitoidei). The Raffles Bulletin of Zoology, 26(S1), 1-199.
- Kottelat, M., & Lim, K. K. (1993). A review of the eel-loaches of the genus Pangio (Teleostei: Cobitidae) from the Malay Peninsula, with descriptions of six new species. The Raffles Bulletin of Zoology, 41(2), 203-249.
- Kottelat, M., & Whitten, T. (1996). Freshwater fishes of Western Indonesia and Sulawesi: additions and corrections. Hong Kong: Periplus Editions.
- Li, T., Chay, C. K., Lim, W. H., & Cai, Y. (2016). The fish fauna of Nee Soon Swamp Forest, Singapore. Raffles Bulletin of Zoology, 32, 56-84.
- Ng, P. K., & Tan, H. H. (1997). Freshwater fishes of Southeast Asia: potential for the aquarium fish trade and conservation issues. Aquarium Sciences and Conservation, 1(2), 79-90.
- Ng, H. H., & Tan, H. H. (1999). The fishes of the Endau drainage, Peninsular Malaysia with descriptions of two new species of catfishes (Teleostei: Akysidae, Bagridae). Zoological Studies-Taipei-, 38(3), 350-366.
- Tang, Q., Liu, H., Mayden, R., & Xiong, B. (2006). Comparison of evolutionary rates in the mitochondrial DNA cytochrome b gene and control region and their implications for phylogeny of the Cobitoidea (Teleostei: Cypriniformes). Molecular Phylogenetics and Evolution, 39(2), 347-357.