Portia labiata (Thorell, 1887)
White-moustached PortiaTable of Contents

Figure 1: Male Portia labiata (Photo credit: Hirzi Hussain under Creative Commons Attribution license)
(1) Introduction
Portia labiata is a jumping spider from family Salticidae, which is the largest family of spiders. The key characteristic to distinguish a jumping spider is to look at their eyes. Jumping spiders are unique among spiders in that they are visual 'specialists', having two large, prominent frontal eyes that are specialized for high spatial resolution (1 ,2). Their excellent vision even rivals those of primates (9, 10, 11)! This makes them capable stalker-hunters (1, 2). A common tactic is to detect mobile prey, chase and stalk, and lunge (3). What is interesting about Portia is its diverse hunting tactics. The genus Portia is referred to as 'eight-legged cats' due to their highly versatile hunting tactics just like a lion. P. labiata is known to use different tactics for different types of prey. Let's explore what kind of tactics they used to hunt for web-building spiders, insects and eggs!(2) Hunting behaviour of Portia labiata
When Portia labiata invades a spider web, it is displayed with three choices, the host spider, insects trapped on the web and the host spider's eggs, depending on the availability. |
| Figure 2: Male Portia labiata. Large Anterior Median (AM) eyes, pedipalps and chelicerae are clearly shown (Photo credit: Hirzi Hussain under Creative Commons Attribution license) |
2.1 Hunting behaviour on web-building spiders
Portia labiata are araneophagic, which means that they prey on other spiders. They usually walk onto the web of web-building spider slowly and performs silk manipulating behaviour that produce vibrations on the web. The spider on the web would approach P. labiata, thinking that a prey is trapped on the web. As a result, the web-building spider is lured within P. labiata's attack range, which is around one or two body lengths. This is known as aggressive mimicry. If the spider remains unresponsive, P. labiata will continue to vibrate the silk while stalking on the web. P. labiata also leap onto webs of web-building spiders and chase spiders that move out of web (4).Another strategy that they use is to probe for a silk-thread of the web and severe with its chelicerae (4). Firstly, P. labiata often stand at the edge of the web and make very slow and gentle probes, with the leg stopping as soon as it contacted a thread (4). Thus, the web is vibrated minimally and there will be no response from the web-building spider (4). Next, the thread is released and P. labiata move slightly away and severe it with its chelicerae (4). This will continue, often for many hours, before P. labiata leap towards the spider through the thread-free space it had established (4).
An account of how a P. labiata captures a web-building spider:
"Once, a female P. labiata was observed to enter an orb web of Nephilengys malabarensis (Araneidae), stalk slowly across the sticky spirals while vibrating, and lunge at and capture the host spider at the hub." (4)
2.2 Smokescreen behaviour
Portia labiata are intelligent hunters. They opportunistically take advantage of background noise such as wind blowing on web as a smokescreen against the web-building spiders (5). How this smokescreen behaviour works is that the natural disturbances made by wind will mask the P. labiata's stalking movement and thus the prey are not able to detect P. labiata moving closer to it (5). Even when there is an insect trapped on the web and struggling, P. labiata performs smokescreen behaviour in synchrony with the insect's struggling vibrations (5). They adopt this specialised behaviour only against spiders and not against other types of prey (5).Smokescreen behaviour is also adopted by other animals that hunt, such as lions. It was known that lions not only hunt more often at night, but they also prefer to hunt when cloud cover blocks the moon, reducing the night vision of their prey. (6) This shows that animals are able to incorporate environmental conditions for their hunting strategy.
2.3 Trial-and-error Strategy
Portia labiata uses a trial-and-error method as part of its strategy to derive appropriate signals for different types of prey. This is how they do it. P. labiata first presents the prey with an array of different vibratory signals. When the prey spider responded to the signals, P. labiata will narrow the signals and continue to produce the signal that elicited the response.This is an account of a predation trial conducted in lab where the trial-and-error strategy is used.
"A Portia moved slowly onto the edge of a web, fixated its antero-medial eyes on P. variabilis c. 120 mm away, and moved its legs and palps to make a variety of vibratory signals. After c. 10 min, the P. variabilis oriented and moved a few millimetres toward Portia just after Portia had plucked slowly (c. 2/s) with its left palp for 1-2 s. From this time until P. variabilis was captured c. 8 min later, Portia plucked repeatedly (22 successive bouts) with its palp at c. 2/s for 1-2 s, but did not perform any other vibratory behaviours. P. variabilis usually approached a few millimetres each time Portia plucked. When the P. variabilis was less than the Portia's body length (c. 4 mm) away, Portia lunged and grabbed it." (17)
Video 1: How Portia moves around in the alien web and catching its prey (Credit: Wildvisuals)
2.4 Detour behaviour
Another stalking strategy demonstrated by P. labiata is the detour behaviour (5, 7, 8). They do not stalk directly towards the prey spider (7, 8). Instead, they take a circuitous route so as to reach an advantageous position to attack the prey (7, 8). When doing that, they often do not look at the prey and may lose visual contact (7, 8). Before starting a detour, they will perform a behaviour called scanning, in which they will stay roughly in a spot and examines the environment with its highly developed eyes (8, 12,14). After scanning, they generally choose the correct route to reach their prey even if it takes up to an hour to detour (8,12, 15). Initiating a detour suggests that they may plan ahead on how to reach the prey and also remember the prey's location (13). Unbelievable right? Behaviours such as these were amazing especially when their small brain only consists of two enlarged ganglion (cluster of nerves) (16).Here's a few interesting articles that explores how the tiny brain is able to accomplish such amazing feat: Mapping the tiny brain of the aristocrat of arachnids, The Amazing Spider Brain: A Great Mystery In a Tiny Head and How much Brain Can You Pack Into A Spider Head? .
2.4 Hunting behaviour on insects
Portia labiata do eat insects, but the bulk of their diet is made up of web-building spiders (3). They stalk insects and usually attacks by lunging and picking up (4). Thye picked up an insect by reaching out and touching the insect with its forelegs (4). They might have also used its forelegs to test the insect's chemical defenses before making contact with its mouth parts (4). They can also leap on insects when these insects are not trapped on their webs (4). Large, vigorously moving insects that were stuck in webs were rarely captured (4). They will sometimes wait for a many hours until the struggling has subsided (4). If the Portia waits, the insect becomes less dangerous, but the insect might also escape through the struggles (4). They may also opportunistically feeds on insects entrapped in another spider's web (kleptoparasitism) (3).2.5 Feeding on spider's egg
While on an alien web, P. labiata may seize and open a spider's egg case and feed on the eggs (oophagy) (4). Holes were made by chewing and by expelling saliva, and then digestive fluids were injected, and finally the liquified contents of the case were extracted (4).2.6 Using of Venom to subdue prey
The venom of Portia labiata seems to be specifically potent to their primary prey, spiders (4). Spiders are paralysed rapidly when attacked but generally, insects do not (4). After being injected by the venom through its fangs, the prey may go into convulsions and become paralysed after 10-30 seconds (4). Even during the short interval while the venom was taking effect, P. labiata could remain at a safe distance (4). Large prey that succumbed less quickly to venom was often stabbed repeatedly until it was safe to seize (4).2.7 Getting killed during hunting
Despite having an edge over its prey attributed from its highly versatile hunting tactics and highly developed vision, P. labiata may be injured or killed by its intended prey (4). Below is a video that shows a Portia's misfortune while trying to attack a St Andrew Cross spider much bigger than it (Video 2).Video 2: How Portia labiata gets killed by a St Andrew Cross spider (Credits: Wildvisuals)
2.8 Cryptic behaviour of Portia labiata
Part of the reason why the prey of Portia labiata are not able to detect the presence of its predator is because they are well-camouflaged. When they are inactive, they assumed a characteristic cryptic rest posture while on silk or a leaf surface (3). They tucked their legs in close to the body and their pedipalps beside the chelicerae. Morphologically, Portia resembled detritus with the markings, tufts of hairs and long legs (18). The cryptic rest posture enhanced this resemblance (3).When moving, they have a slow, mechanical locomotion that makes it difficult to recognise P. labiata. They pause often at irregular intervals, moving their pedipalps jerkily up and down and move each appendage out of time with the others (4). This resembles light flickering through the forest canopy and striking on a piece of detritus (4).
(3) Distribution and Habitat
3.1 Distribution
Portia labiata can be found across South-East Asia (18).Figure 2: Distribution map of Portia labiata (distribution points from materials examined in Wanless, 1978, created by Teo Huey Yee)
3.2 Habitat
They are often found on less dense forest where there is higher ambient light levels (4). They can also be found in highly disturbed areas such as oil palm plantations (4). Webs of P. labiata were often fastened to flexible stems and leaves on shrubs and lower branches of trees (4).(4) Basic Anatomy of a Spider
|
|
||||
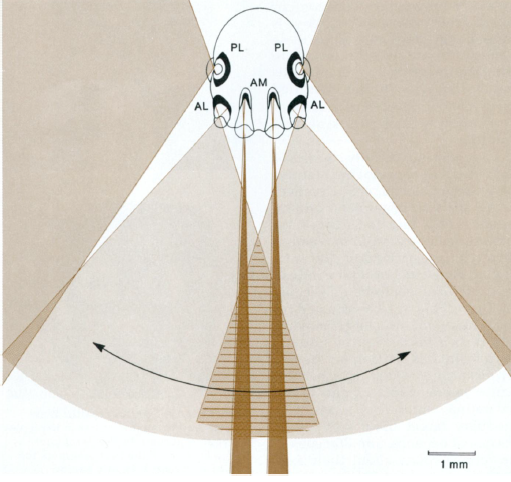
Figure 5: A horizontal frontal section shows the shape, position, and fields of the Anterior Median (AM), Anterior Lateral (AL) and Posterior Lateral (PL) eyes. AM fields operate up to to a distance of about 30 cm. The fields of view of the AL eyes overlap in front, forming a visual triangle shared with the AM eyes (striped area). The Posterior Median (PM) eyes are omitted here. Diagram from Forster, 1982.
(5) Morphology

Figure 6: A comparison between a male (left) and female (right) Portia labiata. (Photo credit: Chang Chia-Chen)
Table 1: Comparison of morphology structures between male and female P. labiata (18)
| Characters (looking at basic Anatomy) |
Male |
Female |
| Body length |
5-7.5mm |
7-10mm |
| White moustache |
Absent |
Present below its eyes (Figure 5) |
| Carapace |
Orange-brown, lighter in eye region. Brown black hairs |
Orange brown, lighter in eye region. White hairs and long brown ones in eye region |
| Eyes |
Anterior Median (AM) eyes fringed by orange hairs. Dark hairs behind AM. |
Anterior Median (AM) eyes fringed by orange hairs. Dark hairs behind AM. |
| Clypeus |
Orange-brown with black markings. Light orange-brown hairs with fine whitish hairs centrally |
Transverse, crescent-shaped band of short white hairs with a marginal fringe of long whitish ones |
| Abdomen |
Brownish with lighter markings. Orange-brown hairs |
Mottled brown and black. Golden, whitish and black hairs with a series of tufts composed of brownish or creamy brown hairs with white tip |
| Chelicerae |
Orange-brown with brown-black markings. Sparse fine light yellowish orange hairs |
Dark orange-brown. Sparse clear long white hair with transverse white haired bands proximally |
| Palps |
Orange-brown to dark brown. Orange and white hairs |
Light yellow with dark brown spots. Fringed with long white hairs |
| Legs |
Dark brown |
Numerous long white hairs on underside of femora |
Juvenile P. labiata resembled the adult females.
See additional information below for detailed descriptions.
(6) Biology
6.1 Life Span
Adult females live longer than males (3). After maturation, females could live for around 6 months while males could live 4 months (3).6.2 Moulting
When P. labiatia are going to moult in 2-4 days, they reduce their activity and stop feeding (3). During this period, they will hang upside down from the underside of a piece of leaf debris or from small small horizontal silk platforms (3).6.3 Web building
Portia labiata is both a web-builder and a cursorial hunter moving about to hunt for prey (3).Portia spin two types of webs. Females spin both types of webs while males spin Type 1 web only (4).
Type 1 webs are silk platforms suspended horizontally (4). These platforms are usually 1-3 times the body length of the spider (4).
Type 2 webs are larger 3-dimensional silk networks (4). Structure is highly varied but web was basically funnel-like (4). Usually, a dead leaf or other detritus, such as a clump of dirt or a piece of bark,was suspended near the top centre of the Type 2 web (4).
Adult males and subadult females were observed to cohabit in Type 2 webs, standing close to each other (4).
|
|
6.4 Reproduction
An adult female is able to produce more than 50 eggs per batch (3). After mating, females lay eggs around every 26 days (3). Before laying the eggs, the female have to make a detrital egg sac (4). Female will spin a thick sheet of silk onto the surface of the leaf (or some other object), covering an area similar to or slightly larger than that of a Type I web (4). Next, the eggs were oviposited on the centre of the sheet of the web (4). The eggs and the first layer of silk were then covered by a less thickly woven second sheet (4).(7) Etymology
Portia derived its name from Porcia, the feminine form of the Roman family name Porcius (19). William Shakespeare named the heroine of his play 'The Merchant of Venice' (1596) as Porcia (19). In the play, Porcia acted as a lawyer's apprentice, who disguised herself as a man in order to defend Antonio in court (19). It was perhaps Porcia's ability to move undetected among her male counterparts while plotting a scheme that gives Portia its name (19).(8) Taxonavigation
| Taxonomic Hierachy |
|
| Kingdom |
Animalia |
| Phylum |
Arthropoda |
| Subphylum |
Chelicerata |
| Class |
Arachnida |
| Order |
Araneae |
| Family |
Salticidae Blackwall, 1841 |
| Subfamily |
Spartaeinae |
| Genus |
Portia Karsch, 1878 |
| Species |
Portia labiata (Thorell, 1887) |
(9) Synonyms
Portia labiata is derived from a new combination of genus name and species epithet (18).Sinus fimbriatus Doleschall; Hasselt, 1882 [Misidentification]
Linus labiatus Thorell,1887; Simon, 1901, 1903.
Linus dentipalpis Thorell, 1890, 1892; Simon, 1901.
Erasinus dentipalpis Thorell, 1892.
Erasinus labiatus Simon 1903; Roewer, 1954; Bonnet, 1956; Prószyński, 1971.
(10) Type Information
The holotype for Portia labiata is stored in Rijksmuseum van Natuurlijke Historie (RNH, Leiden, no. 5428).The type locality for Portia labiata is Sumatra, Boven Rawas.
(11) Phylogeny
It was suggested that acute vision might have been essential for the evolution of the complex predatory behaviours of Salticidae (Jackson, 1992; Jackson & Pollard, 1996).The data support the monophyly of Salticidae.
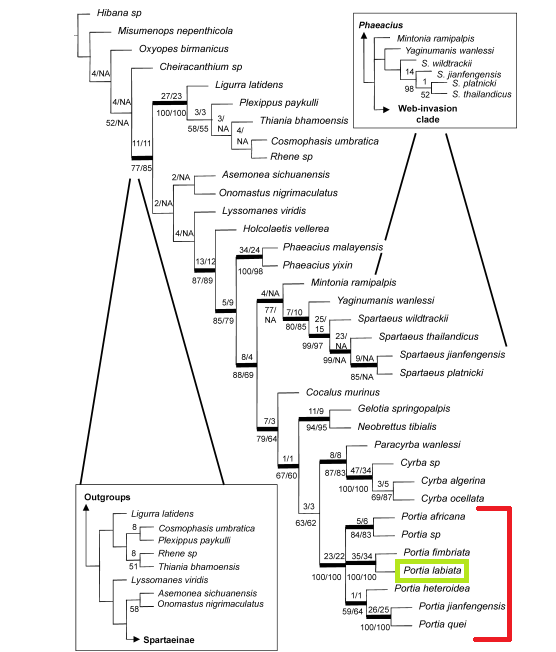
Figure 9: Strict consensus tree of the MPT (most parsimonious trees) from the analysis using gaps as 5th character state. Numbers above each node refer to Bremer support values. Bold lines indicate nodes supported with 100 posterior probability in the Bayesian analysis (Diagram from Su et al., 2007)
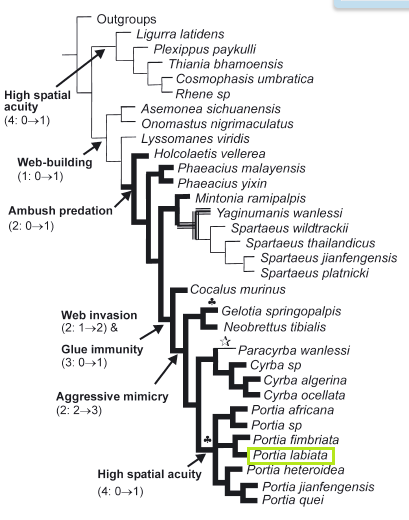
Figure 10: Evolution of predatory behaviours in Salticidae (bold black lines = araneophagy present, thin lines = araneophagy absent, shaded lines = feeding behaviour unknown). (Star symbol) indicates Loss of web invasion, aggressive mimicry, and araneophagy. (Clover symbol) indicates building of large space webs. Numbers in brackets refer to character and states. From Su et al. 2007.
There is strong divergent evolution of predatory behaviour in the Salticidae, where the behaviours range from cursorial hunting and building prey-catching webs to araneophagy involving web invasion and aggressive mimicry (Figure 10).
Araneophagy evolved once at the base of Spartaeinae and was lost twice (20).This suggests that the ancestral Spartaeine was araneophagic (20).
Web invasion and aggressive mimicry evolved later within the Spartaeinae (20). Aggressive mimicry might have evolved from simple web invasion (20). One adaptation that is needed for web invasion and that is seen in all spartaeine web invaders is glue immunity. The majority of jumping spiders have difficulty walking across sticky webs, but some spartaeines have evolved glue immunity such as Portia labiata (21, 22).
Hence, glue immunity to sticky webs is an autapomorphy supporting the web-invasion clade, whereas the ability to enter nonsticky webs evolved earlier in spartaeines incapable of web invasion (20).
(12) Literature and References
1. Forster, L. M., 1982. Visual communication in jumping spiders (Araneae: Salticidae). In: Spider communication: mechanisms and ecological significance. Witt, P. N.; Rovner, J. S. ed., Princeton, New Jersey, Princeton University Press. pp. 161-212.2. Land, M., 1985. The morphology and optics of spider eyes. In: Neurobiology of Arachnids (Ed. F. Barth), pp. 53-78. New York: Springer-Verlag.
3. Hallas, S., 1987. The life cycle of three species of Portia. Thesis: University of Canterbury, pp. 1-132.
4.Jackson, R. R. and Hallas, S., 1986. Comparative biology of Portia africana, P. albimana, P. fimbriata, P. labiata, and P. shultzi, araneophagic, web-building jumping spiders (Araneae: Salticidae): Utilisation of webs, predatory versatility, and intraspecific interactions. New Zealand Journal of Zoology, 13(4), 423-489.
5. Wilcox, R. S., Jackson, R. R. and Gentile, K., 1996. Spiderweb smokescreen: spider trickster uses background noise to mask stalking movements. Animal behaviour, 51, 313-326.
6. Shaller, G., 1972. The Serengeti Lion. Chicago: University of Chicago Press.
7. Jackson, R. R. and Wilcox, R. S., 1993. Observations in nature of detouring behaviour by Portia fimbriata, a web-invading aggressive mimic jumping spider from Queensland. Journal of zoology, London, 230, 135-139.
8. Tarsitano, M. S. and Jackson, R. R., 1994. Jumping spiders make predatory detours requiring movement away from prey. Behaviour, 131, 65-73.
9. Land, M. F., 1974. A comparison of the visual behaviour of a predatory arthropod with that of a mammal. In: Invertebrate Neurons and Behaviour (Ed. by C. A. G. Wiersma), pp. 410–418. Cambridge, Massachussetts: MIT Press.
10. Clark, D. L. and Uetz, G. W., 1990. Video image recognition by the jumping spider, Maevia inclemens (Araneae: Salticidae). Animal Behaviour, 40, 884–890.
11. Land, M. F. & Fernald, R. D., 1992. The evolution of eyes. A. Rev. Neurosci., 15, 1–29.
12. Tarsitano, M. S. and Jackson, R. R., 1997. Araneophagic jumping spiders discriminate between detour routes that do and do not lead to prey. Animal behaviour, 52, 257-266.
13. Tarsitano, M. S. and Jackson, R. R., 1992. Influence of prey movement on the performance of simple detours by jumping spiders. Behaviour, 123(1-2), 106-120.
14. Tarsitano, M. S., 2006. Route selection by a jumping spider (Portia labiata) during the locomotory phase of a detour. Animal behaviour, 72, 1437-1442.
15. Wilcox, S. and Jackson, R. R., 2002. Jumping Spider Tricksters. In The Cognitive Animal: Empirical and Theoretical Perspectives on Animal Cognition (Eds. Bekoff, M., Allen, C., and Burghardt, G.M.) pp. 27–34. Cambridge, Massachussetts: MIT Press.
16. Babu, K.S. (1985). Patterns of arrangement and connectivity in the central nervous system of arachnids. In Neurobiology of arachnids (Ed. Barth, F. G.), pp. 3–19. Berlin: Springer- Verlag.
17. Jackson, R. R. and Wilcox, R. S., 1993. Spider Flexibly Chooses Aggressive Mimicry Signals for Different Prey by Trial and Error. Behaviour, 127(1-2), 21-36.
18. Wanless, F. R.,1978. A revision of the spider genus Portia (Araneae: Salticidae). Bulletin of the British Museum of Natural History (Zool.), 34, 83-124.
19. Behind the name, n.d. Portia. Accessed http://www.behindthename.com/name/portia on 10 November 2015
20. Su, K. F., Meier, R., Jackson, R. R., Harland, D. P. & Li, D., 2007. Convergent evolution of eye ultrastructure and divergent evolution of vision-mediated predatory behaviour in jumping spiders. Journal of Evolutionary Biology. 20(4),1478-1489.
21. Jackson, R. R., 1992. Eight-legged tricksters. BioScience, 42, 590–598.
22. Jackson, R. R. and Pollard, S. D., 1996. Predatory behaviour of jumping spiders. Annu. Rev. Entomol., 41, 287–308.
Additional Notes
Portia labiata can be distinguished from P. assamensis sp. n. by the more slender tibial apophysis in males and undivided epigynal oriface in females. (Wanless)Detailed Description

Figure 11: A detailed description of morphology of a male collected from Malaya (Wanless, 1978). Image from the Biodiversity Heritage Library. Digitized by Smithsonian Libraries. www.biodiversitylibrary.org/

Figure 13: A detailed description of the morphology of a female collected from Malaya
Wanless, 1978). Image from the Biodiversity Heritage Library. Digitized by Smithsonian Libraries. www.biodiversitylibrary.org/