
Table of Contents
Photo by Dennis Farrell
Binomial name: Pseudagrion microcephalum
Common names: Blue sprite, Blue grass dartlet, Blue river damsel, small-headed sprite
OVERVIEW
Pseudagrion microcephalum is a pretty damselfly commonly found in parks and water bodies around Singapore. Do not be fooled by these delicate insects because they are in fact fierce predators both in the larval and adult stages. These fascinating creatures possess intriguing mating behaviours which are often deemed to be rough sex by many. Pseudagrion microcephalum is an Odonate Zygopteran--Odonates are one of the oldest insect taxons in the world -- some species of this prehistoric taxon even existed before the dinosaurs! So next time you spot this creature, think about its way of life and evolutionary history and appreciate the beauty that is Pseudagrion microcephalum.
BIOLOGY
Life Cycle and Ecology
| The oviposited eggs hatch after about a week and larvae emerge. Pseudagrion microcephalum larvae are slender with three caudal gills that increase greatly in size as the larvae develops (Corbet, 1962). This increases the surface area of the gills for respiratory purposes. Larvae are generalised carnivores that feed on any organism smaller than itself. This may include tadpoles, small fish, mosquito larvae and other Odonate larvae. When a larvae spots its prey, it extends its labial mask rapidly and grasps the prey. Then, it brings it close to its mandibles to chew and ingest the prey. |
Use of labial mask in prey capture and mastication by unidentified true dragonfly* larvae. *Note that this is not a damselfly |
| After a series of 10-20 molts which occur over a several weeks, the final instar larvae crawls out of the water and finds a spot in the vegetation to rest on. Next, the larval cuticle splits along the dorsal side and the adult damselfly emerges. This process may take close to an hour and the damselfly is extremely vulnerable to predation at this stage. After emergence, the adult remains motionless as haemolymph is pumped through the body, expanding its wings and hardening its body (Corbet, 1962). Pseudagrion microcephalum may emerge in the earlier parts of the day in sheltered areas for protection against wind and predators (Corbet, 1962). Adults are carnivorous, visual predators and feed on smaller insects such as mosquitoes. |
Pseudagrion microcephalum emerging from last larval instar |

Natural Enemies
| 1) Predators Birds are one of the main predators of Pseudagrion microcephalum because they are fast enough to catch these quick prey. Also, Pseudagrion microcephalum may fall prey to other dragonflies and damselflies which are larger than itself. Frogs and fish may also leap out to capture adults that are hovering above the water surface (Berger and Hansen, 2004). Spiders also feast on Pseudagrion microcephalum. The larvae of Pseudagrion microcephalum are preyed upon by fish and other Odonate larvae. |
 |
| 2) Parasitoids and parasites In general, little is known about parasitoids of Odonate eggs partly owing to the fact that Odonate eggs are often well concealed amongst the vegetation or found submerged in water. However, certain wasp species do oviposit in Odonate eggs, which the wasp larvae feeds on after it hatches (Santolamazza et al. 2011). Some wasps capture Odonates, kill them, and deposit their eggs in the dead insect (Berger and Hansen, 2004). Water mites are common parasites of Odonates. The female mite lays its eggs in the water and as soon as they hatch, the young mites search for an Odonate larvae that is just about to emerge. As the adult Odonate emerges from the last larval skin, the mites attach onto the soft body of the new adult via its piercing mouthparts. The mites feed on the adult's body fluids and return to the water after obtaining sufficient food. If there are too many mites on an Odonate adult, they may weight the insect down preventing proper flight. This directly interferes with the reproductive success of the Odonate. For males, they are unable to partake in courtship flight or defend their territory from other males (Berger and Hansen, 2004). |
 Mites on a damselflyPhoto by Rob Blanken |
| 3) Bacteria Wolbachia bacteria has been found to infect damselfly eggs and hence manifest themselves in the adults. The bacteria has a potential to impact reproduction adversely such as causing post zygotic cytoplasmic incompatibility (Thipaksorn, 2003). |

Mating and Reproduction
Pseudagrion microcephalum partakes in ROUGH SEX!| 1) Display of territoriality Pseudagrion microcephalummales exhibit territorial behaviour and will aggressively defend their territories from both conspecific and heterospecific males (Babu and Sharma, 2012). After a male establishes his territory, which may extend from 0.75m to 1.5m in radius, he will cautiously observe other males for intrusion. If he senses encroachment, he will aggressively chase the rival away. Sexual activity for Pseudagrion microcephalum peaks in the afternoon (Tang et al., 2010). |
A territorial display by two damselflies |
| 2) Tandem position When a female enters a male’s territory and is receptive to his advances, the male will use the clasping anal appendages on his abdomen to hold the female by her prothorax. Tandem position, where two damselflies are joined in midair, is thus achieved when the male successfully holds onto the female. |
 |
| 3) Self insemination Before actual copulation, males have to transfer sperm from their primary genitalia, located under abdominal segment 9, to the secondary genitalia in segment 2. The primary genitalia produces sperm whereas the secondary genitalia functions as a penis. Self insemination is achieved by the male curling his abdomen so that his genitalia touch the opening of the sperm reservoir. This can be completed while in tandem. |
|
| 4) Wheel position The female stores sperm from several males in her oviduct in preparation for fertilisation and oviposition. The male penis has special structures to remove stored sperm. This improves the fitness of the male in question because it maximizes his chance of spreading his genes to the greatest proportion of eggs in the female. Thus, before ejaculation, the male uses his penis to remove previously deposited sperm. The deposition of sperm by males into females requires the female to bend her abdomen such that her genitalia are in contact with the male’s secondary genitalia. This is called the wheel position. Pseudagrion microcephalum males have been observed to vibrate their wings vigorously to fend off intruders while in the wheel position (Babu and Sharma, 2012). |
 Photo by Tang Hung Bun |
| 5) Endophytic oviposition After copulation, the female searches for an optimal location to oviposition. The male may remain in tandem with the female or hover around her to protect her from rival males. Pseudagrion microcephalum females oviposit endophytically. Using their ovipositors, females creates holes in plant material, lay their eggs then seal the slit (Babu and Sharma, 2012). |
Endophytic oviposition by a female damselfly. Note that she is still attached to the male in tandem. |

Paternity Wars
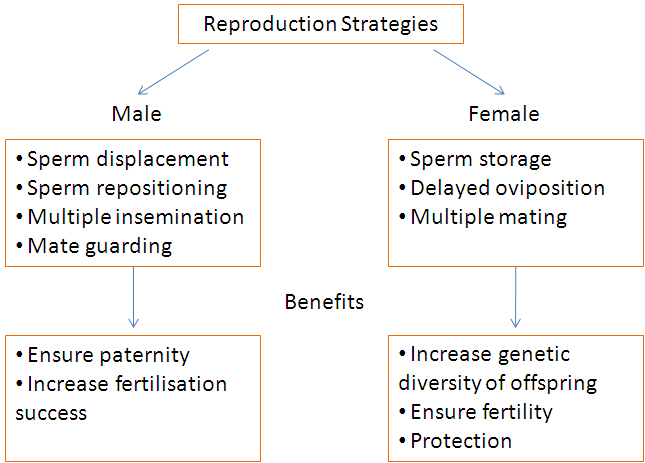
male and female Odonates and their associated benefits.
Image by Shu Min
Odonate mating and reproduction is highly fascinating and intriguing. Female Odonates are capable of storing sperm in specialised sperm storage organs, only fertilising the eggs just prior to oviposition. Their tendency to mate multiple times with different males creates sexual conflict because males have to contend with other males in order to achieve maximum fertilisation success. This has led to the evolution of strategies on the part of the male Odonate to maximise his fertilisation success and ensure paternity over the highest proportion of the female's eggs (Cordoba-Aguilar et al., 2003).
| Male strategies and benefits |
Female strategies and benefits |
1) Sperm displacement
2) Sperm repositioning
3) Multiple insemination
4) Mate guarding
|
1) Sperm storage
3) Multiple mating
4) Protection
|
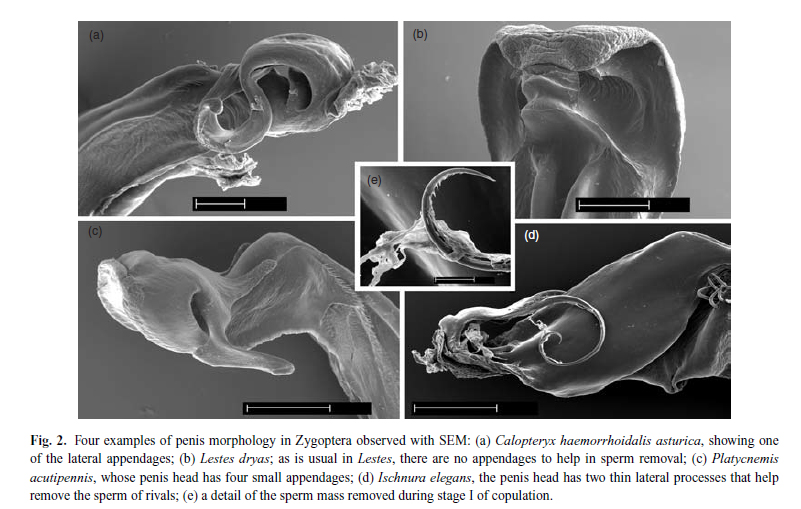
Odonate penises are complex structures which vary largely between species. Some species possess specialised structures to scrape out previously deposited sperm, some do not. Some penises even have spines to aid in the removal of sperm (Codero and Miller, 1992). Interesting, the specialised sperm storage organs in females show increasing complexity in correspondence with the complexity of the male penis in certain species (Waage, 1986). Odonate reproduction represents an arms race--where males and females continually coevolve against each other to maximise benefits for themselves.
In addition to the strategies described above, andromorph females may also exist in a population. These females look like males superficially and this prevents their harrassment during mating and oviposition by other males. However, they run a risk of not obtaining mates when the number of males are low.
Distribution
In Singapore, a study carried out in 2010 by Ngiam and Davison observed that Pseudagrion microcephalum can be found in 14 out of 19 parks surveyed.The parks are Admiralty Park, Ang Mo Kio Town Garden West, Bishan Park, Bukit Batok Nature Park, Bukit Batok Town Park, East Coast Park, HortPark, Kampong Java Park, Kent Ridge Park, Punggol Park, Singapore Botanic Gardens, Tiong Bahru Park, Toa Payoh Town Park, and West Coast Park. The prevalence of Pseudagrion microcephalum across Singapore reflects a healthy and thriving population. Pseudagrion microcephalum has also been found in the Central Catchment area (Norma-Rashid et al., 2008).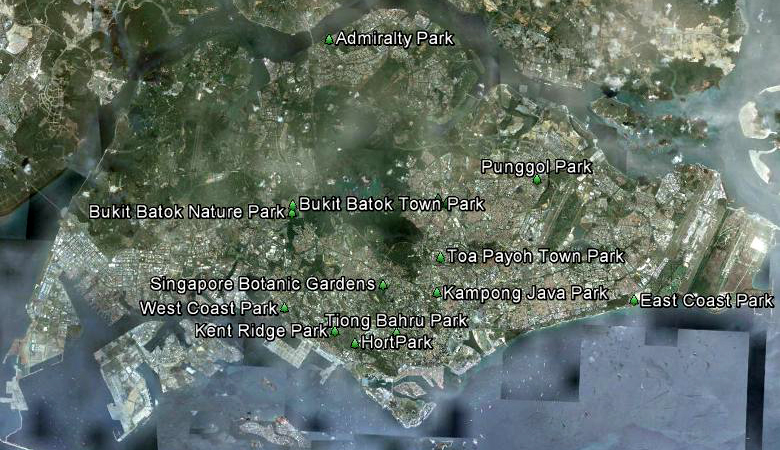
Globally, Pseudagrion microcephalum occupies a range spanning from India to Australia, and the Solomon Islands.
BIOINDICATION AND MONITORING

Adult Odonates are conspicuous, easily captured using a net and thus are highly accessible indicators of water quality. A healthy population of adult Odonates at a site often suggests a thriving larval population in the associated water body, indicating good water quality. In addition, there is much taxonomic information regarding Odonates, easing the process of species identification.The beauty and grace of Odonates also makes them charismatic organisms--this can elicit public empathy and eventually political support for environmental causes (Cordoba-Aguilar, 2008).
Pseudagrion microcephalum is a more tolerant species (Orr, 2005) and its presence may serve to show that the water body in question has achieved the minimal water quality to support an Odonate species.
DESCRIPTION

Adult Morphology
Pseudagrion microcephalum can reach 30-34mm in length.1) Head
2) Thorax
3) Abdomen
|
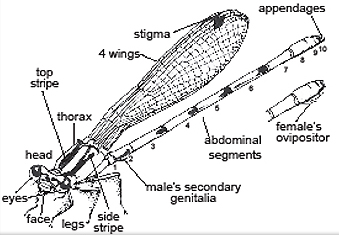 General morphology of a damselflyArt by Tim Manolis |
Sexual Dimorphism in Adults
Pseudagrion microcephalum exhibits sexual dimorphism, which means that males and females look different. Males and females can be distinguished by looking at their colour and examining their genitalia.Females:
|
 |
Males:
|
 |

Larval Morphology
 Pseudagrion microcephalum larvaePhoto by Darren Yeo Pseudagrion microcephalum larvaePhoto by Darren Yeo |
|
1) Head
2) Labial mask The labium, or lip, of the larvae is modified into an extensible structure which is used in prey capture. This is referred to as the labial mask.
3) Thorax
4) Abdomen
|
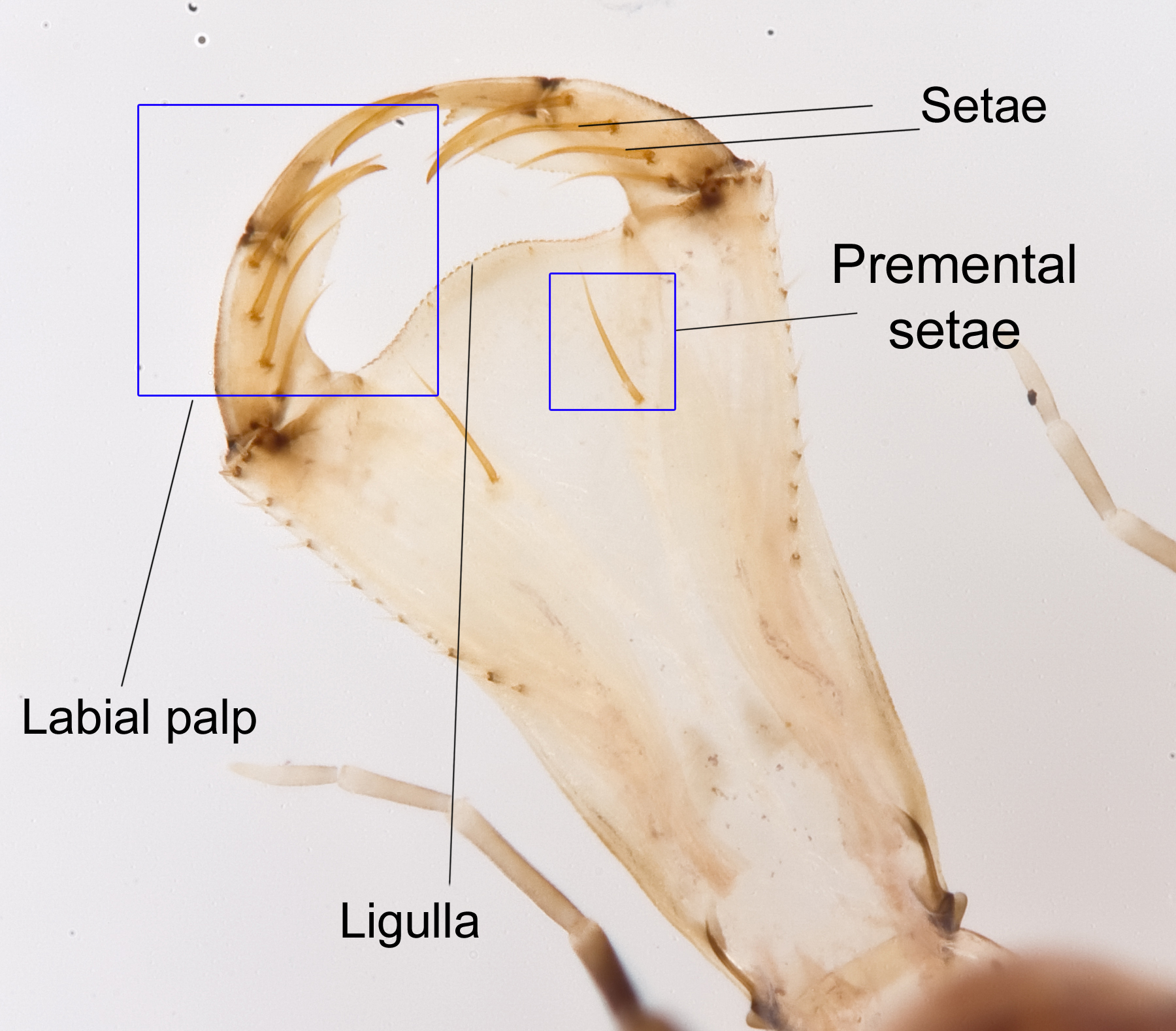 Labial mask Photo by Darren Yeo |
| 5) Caudal gills The caudal gills are highly vascularised and used in gaseous exchange in the aquatic environment.
|
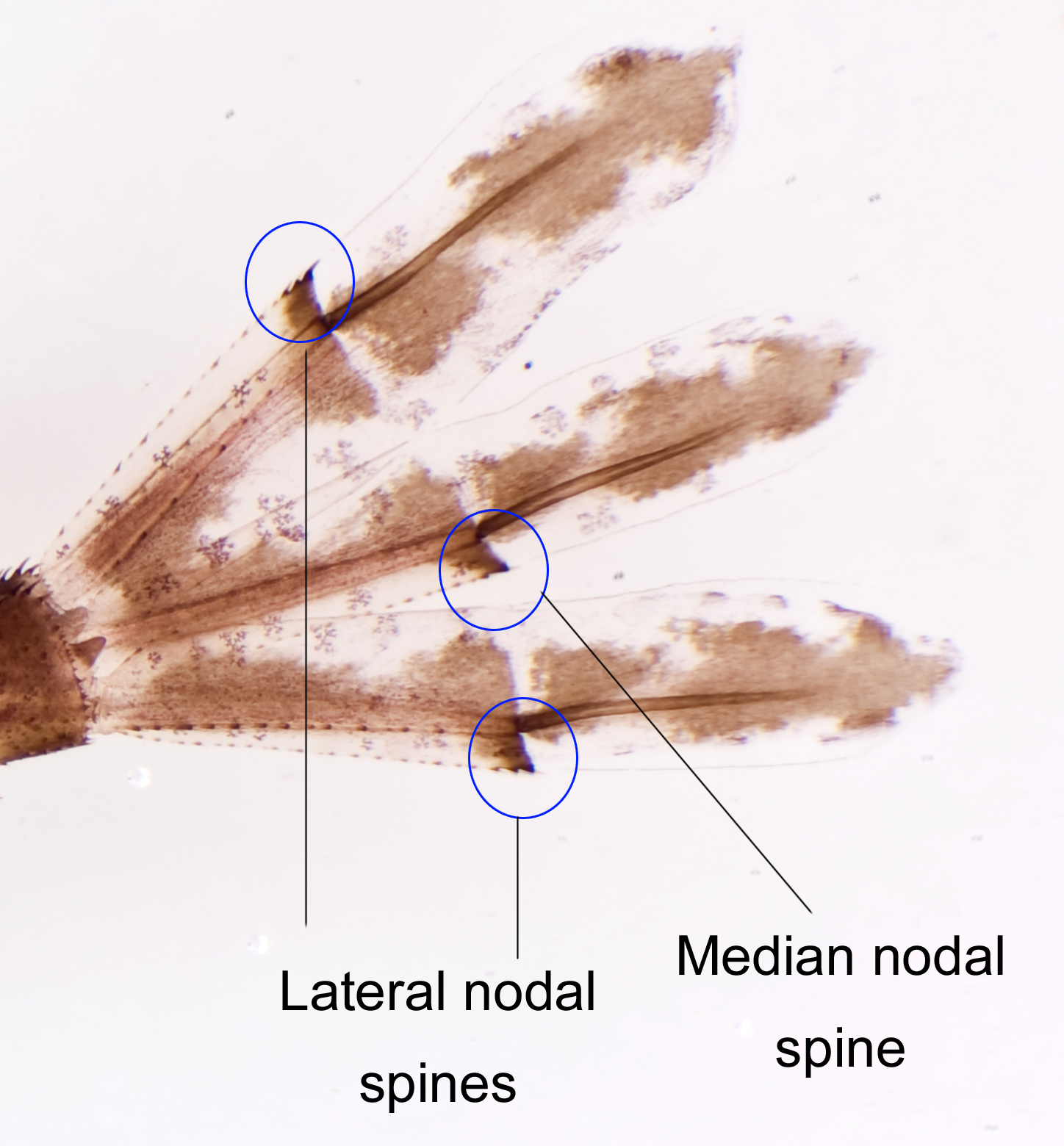 |
TAXONOMY

Taxonavigation
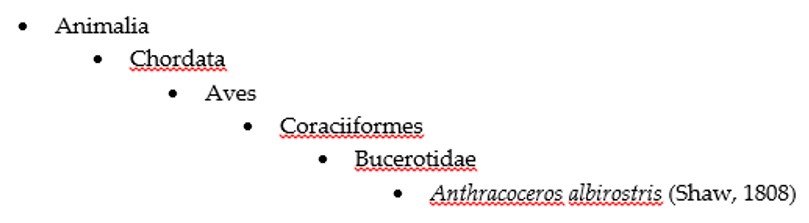
There are about 1,100 described species in Coenagrionidae, out of which 21 can be found in Singapore (RMBR, 2012). Pseudagrion microcephalum is one of them.

Taxonomic History
Pseudagrion microcephalum was originally described in Bombay, India and named Agrion microcephalum by Rambur in 1842 (Rambur, 1842). Lieftinck described its larvae in 1962. One of the synonymns, Pseudagrion australasiae, may be due to a confusion of Pseudagrion microcephalumwith another similar looking damselfly.Pseudagrion microcephalum is the oldest Odonate specimen from Singapore housed in the National Museum of Natural History in Leiden. It was collected on the 22nd of December, 1938 (Norma-Rashid et al., 2008).
Refer to the Diagnosis section below to find out how to distinguish between the two!
Etymology:
|
Synonyms:
|

Diagnosis
Is it a damselfly or a dragonfly?The first step in diagnosing Pseudagrion microcephalum is to first distinguish it from a true dragonfly. Pseudagrion microcephalum is a damselfly, a zygopteran, and thus bears several general morphological differences from a true dragonfly, an anisopteran (Tang et al., 2010; Ngiam, 2011)
Zygoptera (damselflies)
Anisoptera (true dragonflies)
|
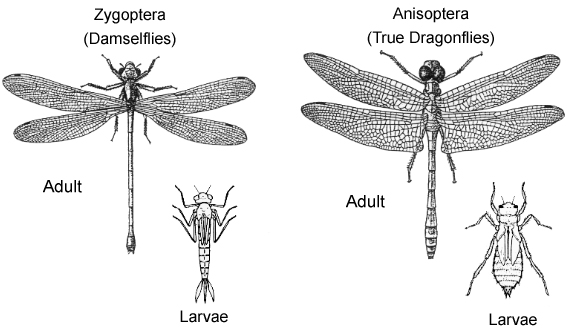 Image adapted from Meyer, 2009 |
What colour is it? Does it have any patterns?
Species identification for Pseudagrion microcephalum, as with all Odonates, relies heavily on colour. However, this can pose several problems due to:
| 1) Sexual dimorphism in colouration Males are blue whereas females are olive coloured. However, this can be overcome by observing mating pairs of Pseudagrion microcephalum. |
 |
| 2) Differences in colour in mature and juvenile adult stages Juvenile adults that have just emerged from the final larval stage are typically dull coloured, taking a few days to acquire the colouration and patterns possessed by mature adults. |
 Photo by Dennis Farrell |
| 3) Interspecific similarity Pseudagrion microcephalum can be easily confused similar looking species such as Pseudagrion australasiae. Close observation is required to distinguish between the two. There are generally 4 morphological characters that can be used to differentiate both species.
 Photo by CY Choong
Pseudagrion microcephalum has longer anal appendages than Pseudagrion australasiae. These differences in the structures of anal appendages could be key to maintaining Pseudagrion microcephalum and Pseudagrion australasiae as two separate species. Anal appendages are used by males to grasp conspecific females (or females of the same species) for mating. If a male mistakenly grasp a female of another species, the structure of the anal appendages makes it unable to hold onto the female properly, leading to the subsequent release of the female before the transfer of sperm can occur. |
 Photo by Tang Hung Bun 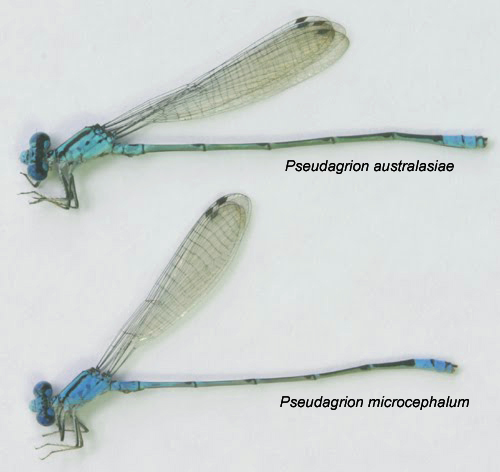 Pseudagrion microcephalum is smaller than Pseudagrion australasiae. The width of the black band is wider on Pseudagrion microcephalum. Photo by CY Choong  Pseudagrion microcephalum has longer anal appendages than Pseudagrion australasiae. Photo by CY Choong. |
How do I identify the larvae and match them to the adult?
The larvae of Pseudagrion microcephalum can be identified and matched to the adult via DNA barcoding. This method is gradually being adopted due to the difficulties in larval identification via the use of morphological characters. Alternatively, Odonate larvae can be captured and reared to emergence to find out which species they are. However, this is time consuming because some larvae are long-lived and emerge under conditions which are not known.
Can I use other features?
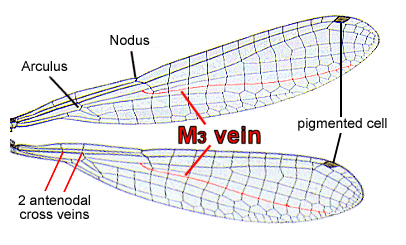
Pseudagrion microcephalum can be identified as a Coenagrionid by examining features of its wing venation because these are shared by other Coenagrionid damselflies.
There are two antenodal cross veins and the M3 vein originates closer to the nodus than the arculus.
Image by Meyer, 2009, NCSU General Entomology
Phylogenetics
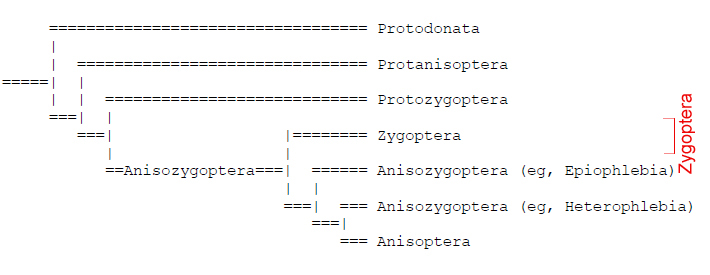
Traditionally, Odonate phylogenetics have been constructed using hypotheses based on interpretations of wing venation. However, there has been a shift towards molecular methods to determine Odonata phyologeny and evolution. Odonata comprises three taxons: Zygoptera, Anisoptera and Anisozygoptera. There were previously two dominant, competing phylogenies which disputed the monophyly of Zygoptera in which Pseudagrion microcephalum is found (Trueman and Rowe, 2009). Whichever phylogeny is used, it is generally agreed upon that the current use of Zygoptera to refer to all damselflies is an artificial, paraphyletic grouping which does not include all descendants of the last common ancestor.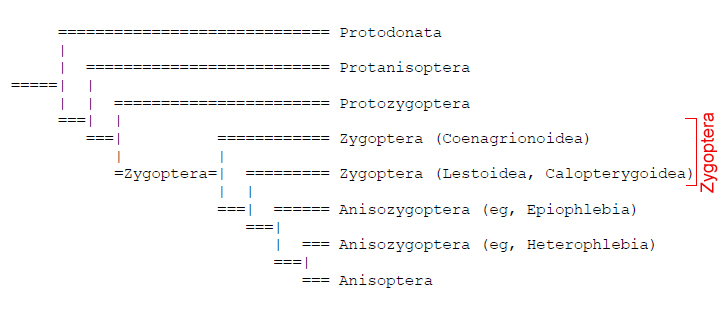 |
 |
| Tree showing a basal monophyletic Zygoptera. Anisozygoptera is derived from within Zygoptera and Anisoptera from within Anisozygoptera. The group Zygoptera in reference to damselflies is thus a paraphyletic grouping which does not include all descendants of the last common ancestor. (Tillyard, 1928; Fraser, 1957) Diagram from Trueman and Rowe, 2009 |
Tree showing a basal monophyletic Anisozygoptera from which Zygoptera is derived. Anisoptera is derived from within Anisozygoptera.(Handlirsch, 1906-1908) Diagram from Trueman and Rowe, 2009 |
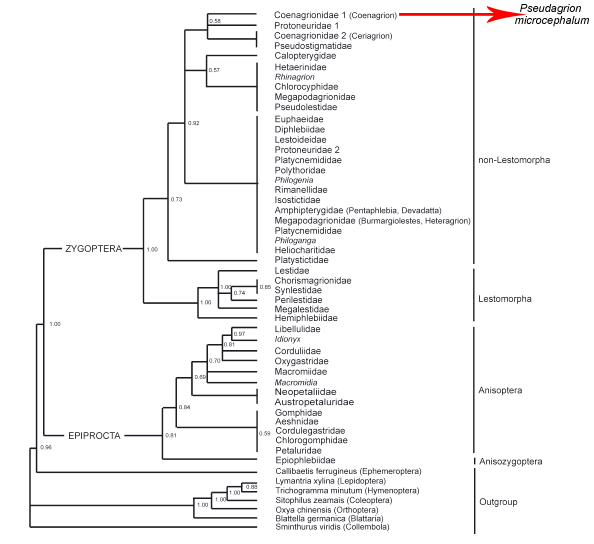
Dumont et al., 2010
CULTURAL SIGNIFICANCE
In Singapore, there are several references made to Odonates, mainly due to their beauty. In Gardens By the Bay, one can find the Dragonfly Lake, an artificial lake with aquatic plants which serve as habitats for Odonate larvae and adults.

To commemorate the opening of Dragonfly Lake at Gardens By the Bay, SingPost has released a stamp with a beautiful Odonate on it.

Another set of stamps featuring Odonates has also been released by the Singapore Post in 2011. Aptly titled "Pond Life", the two Odonate species featured are Rhodothemis rufa (Common Redbolt), an Anisopteran and Ceriagrion cerinorubellum (Ornate coraltail). These two species were chosen as representatives of the Odonata because they were key predators of mosquitoes and other insects, beautiful and commonly found (SingPost, 2011).
Specimen image from SingPost
Hence in Singapore, Odonates widely recognised and appreciated as ecologically useful and aesthetically pleasing organisms which can be used for economic purposes. Ultimately, people enjoy looking at Odonates as their beauty appeals to people of all ages. The a fascination with Odonates is likely to inspire more plans to incorporate Odonates in landscapes and parks. This is no doubt a wonderful and optimistic future for Odonates in Singapore.
FEEDBACK
Any feedback or comments about the page can be directed to Shu Min at shumin_leo@hotmail.com.
REFERENCES

Books
Berger, C. & Hansen, A (2004). Wild Guide: Dragonflies. Stackpole Books, Pennsylvania. 124pp. Online at: http://books.google.com.sg/books?id=oxRD1gAkVVsC&pg=PA36&lpg=PA36&dq=damselfly+self+insemination&source=bl&ots=MKfnrNx3Nk&sig=9Ke56UsoYuaOPcZ05i2Ldla0EY0&hl=en&sa=X&ei=D2KqUIbDFYPirAefnoHwCQ&ved=0CC0Q6AEwAA#v=onepage&q&f=false. [Accessed 20 Nov 2012]
Corbet, P.S. (1962). A Biology of Dragonflies. H.F. & G.Witherby Ltd, London. 247pp.
Cordoba-Aguilar, A. (2008) Dragonflies and Damselflies: Model Organisms for Ecological and Evolutionary Research. Oxford Scholarship Online. DOI:10.1093/acprof:oso/9780199230693.003.0007.
Orr, A. G. (2005). Dragonflies of Peninsular Malaysia and Singapore. Natural History Publications (Borneo), Kota Kinabalu. 127 pp.
Tang, H. B., L. K. Wang & M. Hämäläinen (2010). A Photographic Guide to the Dragonflies of Singapore. Raffles Museum of Biodiversity Research, National University of Singapore, Singapore. 222 pp.
Images
Damselflies and Dragonflies of Singapore by Anthony Quek
Dragonflies and Damselflies of Thailand by Dennis Farrell
Dragonflies and Damselflies of Singapore by Tang Hung Bun
Bird Ecology Study Group
Darren Yeo, Evolutionary Biology Laboratory, National University of Singapore
NCSU, General Entomology by Dr. John Meyer, 2009
Odonata of Peninsular Malaysia by CY Choong
Raffles Museum of Biodiversity Research, National University of Singapore
Rob Blanken
Dragonflies of North America: A Colour and Learn Book by Tim Manolis
Journal Articles
Babu, B.S. & Sharma, G. (2012). On some aspects of territoriality and reproduction of Pseudagrion microcephalum(Rambur) (Insecta: Odonata: Zygoptera: Coenagrionoidea). Biological Forum: An International Journal, Spl. Iss. 4:1: 25-31pp.
Cordero, A. & Miller, P. L. (1992). Sperm transfer, displacement and precedence in Ischnura graellsii (Odonata: Coenagrionidae).
Behavioural Ecology and Sociobiology. 30: 261–267pp.
Cordero, A., Santolamazza Carbone, S. & Utzeri, C. (1995). Male disturbance, repeated insemination and sperm competition in the damselfly Coenagrion scitulum (Zygoptera: Coenagrionidae). Animal Behaviour. 49: 437-449pp.
Dumont, H.J., Vierstraete, A., & Vanfleteren, J.R. (2009). A molecular phylogeny of the Odonata (Insecta). Systematic Entomology. 35:1: 6-18pp.
Fraser, F. C., & Tillyard, R. J., 1957. A reclassification of the order Odonata. Royal Zoological Society of New South Wales.
Handlirsch, A., 1906-08. Die fossilen Insekten und die Phylogenie der rezenten Formen. Ein Handbuch fur Palaontologen und Zoologen. Leipzig. Engelmann
Lieftinck, M.A. (1962) Odonata. Insects Micronesia (Bernice P Bishop Museum) 5 (1); 1-95, figs 1-30, 1 map excl.
Michiels, N. K. (1989). Morphology of male and female genitalia in Sympetrum danae (Sulzer), with special reference to the mechanism of sperm removal during copulation (Anisoptera: Libellulidae). Odonatologica 18: 21–31pp.
Miller, P. L. (1991). The structure and function of the genitalia in the Libellulidae (Odonata). Zoological Journal of the Linnaean Society. 102: 43–73pp.
Norma-Rashid, Y., Cheong L. F., Lua H. K. & Murphy, D. H. (2008), “The Dragonflies (Odonata) of Singapore: Current status of Records and Collections of the Raffles Museum of Biodiversity Research”, Online, Raffles Museum of Biodiversity Research, Singapore, 24 pp. Available from: http://rmbr.nus.edu.sg/raffles_museum_pub/Dragonfly_of_Singapore.pdf. [Accessed 06 Nov 2012]
Rambur, P. (1842) Histoire Naturelle des Insectes. Neuroptères. Insectes Neuroptères. Paris: Librairie Encyclopédique de Roret xvii 534 pp. 12 pls
Santolamazza, S., Baquero, E., & Cordero-Rivera, A. (2011) Incidence of Anagrus obscurus (Hymenoptera: Mymaridae) egg parasitism on Calopteryx haemorrhoidalis and Platycnemis pennipes (Odonata: Calopterygidae: Platycnemididae) in Italy. Entomological Science. 14:3: 366-369pp.
Thipaksorn, A., Jamnongluk, W., & Kittayapong, P. (2003). Molecular evidence of Wolbachia infection in natural populations of tropical odonates. Current microbiology, 47(4), 314-318pp.
Tillyard, R. J., 1928. The evolution of the order Odonata. Part 1. Introduction and early history of the order. Records of the Indian Museum 30:151-172pp.
Tregenza, T. & Wedell, N. (2002). Polyandrous females avoid costs of inbreeding. Nature 415: 71–73pp.
Waage, J. K. (1986a). Evidence for widespread sperm displacement ability among Zygoptera (Odonata) and the means for predicting its presence. Biological Journal of the Linnaean Society. 28: 285–300pp.
Websites
Maine Department of Environmental Protection, Water. (2011). Dragonfly and Damselfly Larvae (Odonata). http://www.maine.gov/dep/water/monitoring/biomonitoring/sampling/bugs/dragonsanddamsels.htm. [Accessed 20 Nov 2012]
Meyer, J. (2009) NCSU, General Entomology. http://www.cals.ncsu.edu/course/ent425/library/spotid/odonata/families/coenagrion.html. [Accessed 10 Oct 2012]
Odonate Nymphs of Singapore: A Digital Reference Collection. http://sepsidnet-rmbr.nus.edu.sg/Odonata/Pseudagrion_microcephalum.html. National University of Singapore, Evolutionary Biology Laboratory. [Accessed 5 Nov 2012]
Singapore Post (2011). Press release: Discover the ecological diversity in a pond in the new low value definitive stamp.
http://www.singpost.com/downloads/media/press_release/11/PR20110412.pdf [Accessed 22 Nov 2012]
The Odonata - Dragonflies and Damselflies (Beta Version). http://medusa.jcu.edu.au/Dragonflies/home.php. [Accessed 15 Oct 2012]
Trueman, John W. H. & Richard J. Rowe (2009). Odonata. Dragonflies and damselflies. Version 16 October 2009. http://tolweb.org/Odonata/8266/2009.10.16 in The Tree of Life Web Project, http://tolweb.org/. [Accessed 12 Oct 2012]
