 |
| Figure 1. Malayan Flying Fox, Pteropus vampyrus. Photo credit: Andrea Janda (www.flickr.com/photos/littleredelf/215375990/) |
Malayan Flying Fox; Large Flying FoxPteropus vampyrus (Linnaeus 1758)
Table of Contents
Introduction
Diagnosis
Pteropus vampyrus is one of the largest bat species in the world, with a noticeably dog- or foxlike face, characteristic to all flying foxes [12]. P. vampyrus has no tail, and it has long and pointed ears [12]. P. vampyrus possesses blackish underparts, similar to dark colour of their dorsal surface [12]. It possesses a black mantle, however one colour morph has a light-coloured mantle [12]. A yellow-gold patch of fur on its head, if present, usually extends posteriorly to a sharp border on the dorsal surface [12].
Ecology
Feeding and Diet
The Malayan flying fox feeds mainly on fruits (they are frugivorous), including figs, rambutans, mangoes, bananas, and langsat [12] [13]. When this bat feeds on fruit, it typically slices open the rind and extracts the pulp [12]. Figs are an important component of the diet of flying foxes, being an available food source all year round [13].
They also opportunistically visit flowers for their nectar, licking nectar within the flowers with their long tongue and not damage the flowers, and pollinate them in the process [12] [13]. As it inserts its tongue into the flower to obtain the nectar, pollen would stick to its face, and get transferred to the next flower it visits [13]. They are known to visit flowers of durian trees (Durio zibethinus) [12]. Each bat visits several different flowers at least twice each night, up to over 22 flowers, and defends the tree from conspecifics [12].
 |
| Figure 2. Malayan Flying Foxes leaving their roost. Image by: Lary Reeves |
Colony members of P. vampyrus leave roost sites near sunset in a silent and loose stream that may continue past nightfall, flying up to 50 km nightly to reach its feeding grounds, often following a set route each night [12]. Individuals may shift feeding sites in response to changes in food availability [12].
Upon arriving in feeding areas, large flocks may separate into family or feeding groups numbering from a few to 50 or more individuals. They may circle a fruit tree before landing on the tips of tree branches in a head-up position and then fall into the head-down position from which they feed [12]. Feeding aggregations of P. vampyrus are very noisy [12].
The Malayan flying fox has a preference for foraging in undisturbed forests, and for fruiting and flowering trees. However, it still frequents agricultural areas for food in places with human development [25].
Pollination and Seed Dispersal
Flying foxes play important roles in tropical habitats as pollinators and seed dispersers [13]. This relationship between the plants and the bats are a mutualistic interaction where both parties benefit: the plant providing a nutritional reward (nectar, pollen, and fruit pulp) for the beneficial service of pollination and seed dispersal from the bat [13]. An 800-g Malayan flying fox can carry a food load up to 200 g [12]. Being large, the flying foxes are able to travel further and spread the seeds further, providing important mobility and spread for plant genes and propagules [13].Durians and petai are reliant on bats for their pollination, and a decrease in bat population would result in a decline in durian fruiting [13].
There is a misconception that flying foxes are destructive to fruit trees such as the durian, and are killed as pests [30]. They are however in fact pollinators of durian flowers while they feed on the nectar of the flowers [30]. It has also been noted that morphologically, durian trees are evolved to be pollinated by bats, making them the main and most effective pollinators [30]. A drop in the bat population due to hunting and habitat loss could result in a drop in the durian harvest and increase the prices of the “king of fruit” [30].
Disease
Bats are disease reservoirs, including several severe acute respiratory viruses such as SARS, Ebola, Marburg, Hendra, and Nipah [29]. Flying foxes are known reservoirs of the Hendra and Nipah paramyxoviruses that infect humans [29].Viruses can spread within the flying fox population when there is contact between the body fluids of individuals during courtship and mating, or when they are fighting [22]. Male bats are likely to have greater potential to spread viruses due to their greater contact rates and movement during the daytime [22]. Due to the high volume of transboundary movement of the large flying fox, there is high introduction of infectious disease between populations [18].
Due to bats, in evolutionary terms, being “ancient” mammals, viruses which evolved in bats use highly conserved cellular receptors (think of it like a multi-adapted power plug) which enhances its ability to transmit viruses to other mammals, including humans and livestock [29].
Viruses from P. vampyrus can be introduced into livestock such as pig farms through fruit bat secretions [20]. Such pathogens transmitted by bats threaten livestock and human health, having significant health and economic impacts [18]. Due to increasing bat-human contact due to urbanisation in bat-occupied habitats, there is increased transmission of such diseases [29].
The Nipah virus, also called novel paramyxovirus Nipah virus (NiV), in particular is known to be found in the Malayan flying fox Pteropus vampyrus [18] [21]. The genus Pteropus is the natural host and reservoir of the virus [19]. Recent findings in 2009 realised that the NiV strain found in Malaysian P. vampyrus is a new strain compared to previous strains, and a new strain would mean a change in viral infectivity, persistence, and recurrence [19].
Biology
RoostingThey form colonies ranging in size from a few individuals to thousands of bats at the roost tree [12] [28]. They roost in trees with low canopy cover, and that had horizontal branching patterns and fissured barks to have the most space available [28]. Flying foxes were not observed roosting on other big trees with high canopy cover and ascending branching patterns [28].
In Mindanao (Philippines), the Malayan flying fox is often found sharing roosts with Acerodon jubatus, forming large mixed colonies in the canopies of emergent trees [12].
The return around dawn after a night of feeding is characterized by raucous vocalizations and considerable movement [12]. Restlessness of roosting bats continues until midmorning, where some locomotor activity occurs later during the day, often involving short flights around the roost [12]. A bat will use its thumb claws to move to a suitable roosting site after landing, often fighting others as it moves along the branches [12]. P. vampyrus commonly roosts with its head downward and its wings wrapped around its body; but during warm hours of the day individuals often cool themselves by fanning with partly outstretched wings [12]. In captivity individuals suspend themselves from their thumbs when they defecate [12].
| Figure 3. Roosting colony of Malayan Flying foxes[1]. |
 |
| Figure 4. Mixed colony of Pteropus vampyrus and Acerodon jubatus at Mambukal, near Murcia, Negros Occidental, Philippines. Image by: Lary Reeves |
Hygiene
Flying foxes spend most of their day sleeping [28]. The high amount of time they spent sleeping at night is likely a result of the high energy expenditure from flying around for food [28]. Sleeping positions were driven by environmental conditions like weather, temperature, and sunflecks [28]. They sleep by hanging with their claws on twigs while their wing membranes, or patagium, wraps them covering their head and ventral parts of the body, but sometimes seen with open wings [28].Flying foxes may be nocturnal animals, but they are still active during the daytime hours, performing both solitary general maintenance and social behaviours besides sleeping [22].
While awake at their roost, the bats performed general maintenance behaviours such as grooming, locomotion (adjusting positions on branches), urinating and defecating, flying (to other branches), and fanning themselves to cool down [28].
Self-grooming is one of their most common behaviours during the daytime besides sleeping, mostly occurring in the early morning after returning from their night of flying and feeding [22].
Flying foxes use their hind claws, foreclaws, mouth, tongue, and teeth for grooming, either by nibbling or liking their fur, inner and outer wing membranes, licking their genitals and mouth, scratching their body, cleaning their ears, and nibbling their claws. They do most of their grooming after perching on twigs [28].
They move from one branch to another on roost trees by “walking” upside down on the branch or climbing up the twigs of the tree [28].
Flying foxes urinate and defecate either by flipping their position on the branch and holding the branch with their thumbs rather than their feet with the body dropped down to expel the urine and waste so that it does not come into contact with their body, or sometimes they simply urinate while hanging by their feet and allowing the slow stream of urine to cover their ventral side (stomach) [28].
They also fan themselves with their wings in the late morning during fair weather conditions [28]. The large flying fox lacks sweat glands that help to cool its skin surface and reduce its body temperature, and thus flaps is wings in its roost during the day, especially at noon and in the early afternoon where the temperature is the highest, in order to cool themselves down [22].
 |
| Figure 5. Malayan Flying Fox grooming itself. Image by: Arctic Wolf Pictures, Flickr. |
| Figure 6. Drawn representation of urination positions of Pteropus vampyrus. Image adapted from Cayunda et. al. (2004). |
Social Behaviour
When P. vampyrus returns to its day roost at dawn, it may be met by hostile behavior from conspecifics [12]. Agonistic behaviors such as sparring with wrists and thumbs, biting, and the production of loud vocalizations seem to promote spacing, which maintains individuals just beyond the reach of one another [12].
They showed aggressive behaviour to neighbours by loud squeaking towards the neighbouring bat, before approaching or chasing the neighbour, raising its claws to strike the neighbour or biting the other individual [28].
Territorial behaviour in P. vampyrus seems to be promoted by presence of flowers on trees, manifesting as bats spread their wings and swing their bodies in a 45° arc while growling at conspecifics in order to discourage them from landing in the defended tree [12]. In a study in Malaysia, two or three bats may occupy one large durian tree when feeding, but only single individuals seem to occupy smaller trees [12]. Two distinct feeding periods occur at 1930-2130 h and 2230-2330 h, between which times bats seem to rest in trees [12].
Courting was done by males who would first approach a female, extend its head towards the female’s head, lower body, and genitals and licking them, and if the female accepts the male and is not aggressive towards him then initiating copulation with the female [28]. There is both frontal and dorsal copulation, although dorsal copulation is more common [28].
Courtship largely occurred in the early morning after the early morning after the flying foxes returned from a night of feeding [22]. The male would approach a female and lick her genital area, which often leads to her rejection, and he would subsequently approach other females and initiate contact until he mates with a female [22]. When rejected by a female the male would sometimes masturbate by licking his penis, resulting in the erection of the penis [22].
Courtship, Reproduction, and Parental Care
Female Malayan flying foxes are only able to reproduce after one to two years of age, and only produce one infant a year which are nursed for up to a year [12] [26]. Gestation period of the Malayan flying fox is approximately four months, however they have a mechanism to delay embryo implantation or interrupt embryo development in order to coordinate with the period of maximum food availability and to create optimal conditions for raising the young bats [22].Typically, the mother gives birth to a single pup (baby bat), which is usually carried for the first few days, but later left in the roosts while she forages [12]. At birth, the body mass and forearm length of the pup averages at 133 g and 79.5 mm respectively, which is approximately 11.7% and 35.4% of the maternal body mass and length of forearm (meaning the pup’s size compared to its mother) [12].
The mating and birthing season of Pteropus vampyrus varies geographically and seasonally [22]. The flying foxes living in Peninsular Malaysia have peak pregnancy from November to January; in Thailand pregnancies occur at around the same time with infants born during March and early April. In the Philippines, young are born in April and May, indicating the peak of mating season for Philippines to be during November and January [22] [28]. The breeding season of the large flying fox in Indonesia is around the period of May [22].
Vision and Echolocation
Pteropus vampyrus, as part of the Old World fruit bats, does not echo-locate and instead relies on its good vision and sense of smell to find food [3]. It has a reflective layer at the back of its retina called the tapetum lucidum, which allows it to see under low light conditions (similar to cat eyes) [3].The family Pteropodidae, or Megabats, are the only family of bats in the order Chiroptera that is not capable of echolocation. Echolocation developed early in the chiropteran lineage, and was later lost in Pteropodids due to the uncoupling of flight and echolocation, as their larger body size made it energetically expensive to echolocate [3].
Echolocation in bats is linked to their breathing; each time they breathe out as they flap their wings upwards, and emit their echolocation pulse at together with their expiration [3]. Because larger bats have slower wingbeat frequencies, there is an upper size limit for echolocation in bats as too low a pulse emission rate would be ineffective for echolocation [3]. This might explain the loss of echolocation in Megabats.
Flight and Migration
Flight of P. vampyrus is strong and deliberate and occurs without accompanying vocalisations [12].Flying foxes move in response to variable food availability, due to differing fruiting seasons in different plants [18].
The migratory nature (short term short distance) of flying foxes [20].
The large flying fox does not roost at the same spot, but switches short-term between roosts, mostly to escape from large populations of ectoparasites and reduce infestations [27].
The large flying fox faces little natural predation, largely from raptors such as the white-bellied sea eagle and brahminy kite [27].
Description
 |
| Figure 8. Young Malayan Flying Fox at Columbus Zoo. Image Credits: OZinOH, Some rights reserved. |
Fur
P. vampyrus has short and stiff hairs on its upper back, coarse hairs on the belly, and longer woolly hair on the lower back, mantle, underparts, and sides [12]. The colour and texture of fur also varies according to age and sex, where the males tend to have stiffer and thicker fur than females [12]. Males have also been noted to have neck tufts of stiffer fur [12].
The colour of the fur on the head ranges from mahogany-red and orange-ochreous to blackish, while the underparts vary from blackish to brown, with some occasional chocolate, gray, or sliver colours sprinkling the fur [12].
Immatures are often uniformly dull gray-brown, and in young males, their dark-coloured mantle may become lighter in colour upon maturity [12].
Wings
P. vampyrus has short, slightly rounded wing tips, which produce a slow, manoeuvrable flight [12]. The wing membranes are naked and strengthened by longitudinal ribs of thickened skin [12]. The wings are distinctly furred near the body and along the trailing edge of the wing membrane [12]. There is a heavy growth of fur on the forearm membrane and the sides of the forearm extending about four-fifths along its length [12]. Wing membranes are attached along the back, 28- 30 mm apart [12]. The wings have and aspect ratio of 8.4, a tip to length ratio of 1.30, a tip to area ratio of 0.72, and a tip to shape index of 1.24 [12].Legs
 |
| Figure 9. Foot of the Malayan Flying Fox. Image by: Phil Marion (Permission Pending). All Rights Reserved. |
The Malayan flying fox lacks a bony patella (knee cap), and instead has a fibrocartilage and hyaline cartilage within the quadriceps tendon [12]. A pad of hyaline cartilage is found on the deep surface of the tendon, adjoining a layer of "herringbone" fibrocartilage, in which large bundles of collagen fibers are located at acute angles to each other [12]. Distally, the fibrocartilaginous layer of connective tissue eventually blends with the normal, dense connective tissue, which forms the bulk of the tendon [12].
Baculum
 |
| Figure 10. Malayan Flying Fox with clear baculum exposure. Photo Credit: Angus Choo |
The baculum, or penis, of the Malayan flying fox is saddle-shaped when viewed laterally, but it lacks an apical prominence [12]. Horns (proximal prongs) on the baculum in P. vampyrus are separate instead of fused [12]. The group that includes P. vampyrus has a caput penis with a lateroventral groove at least as long as one-half the length of the spinous area and no transverse, subapical groove [12]. Longitudinal and transverse spinosa areas of baculum have 22 and 11 spines, respectively [12]. Baculum ranges from 4.5 to 8.2 mm long and from 5.5 to 10.0 mm wide [12]. Ratio of length to width ranges from 0.80 to 0.90, indicating that the baculum is wider than long [12]. Dorsal length and maximum width of caput penis (head of penis) range from 9.7 to 10.6 and 7.7 to 10.1 mm, respectively [12].
Skull and Dental Structure
| Figure 11. Pteropus vampyrus skull view from top, bottom, and side (from left to right). Image adapted from Kunz and Jones (2000) |
Distribution and Habitat
The Malayan flying fox is found from southern Burma and Thailand to the Philippines, and Sumatra, Java, Borneo, and Timor [12]. There is also a reported single population in inland China [24]. It is common in coastal regions in Peninsular Malaysia and Borneo, but also occurs inland at altitudes up to 1,370 m [12]. Insular distribution may coincide with the fruiting season of trees [12]. In Singapore there is no longer a native population as a result of habitat loss from development and urbanisation, however there are occasional visitors on Pulau Ubin [1]. There is, however, a local "resident" population of Large flying foxes at the Singapore Zoo.The Malayan flying fox occupies various habitats, including primary forests, mangrove forests, coconut (Cocus nucifera) groves, and mixed fruit orchards [12]. Trees in mangrove forests and coconut groves are common day roosts [12]. In Malaysia, this bat is found most often in lowland habitats below 365 m [12]. In Borneo, the flying fox is common in lowland coastal areas, but may invade nearby islands during the fruiting season [12]. Roosts of flying foxes are in tops of trees on leafless branches and are more often in level lowland forests than in hilly, forested regions [12].
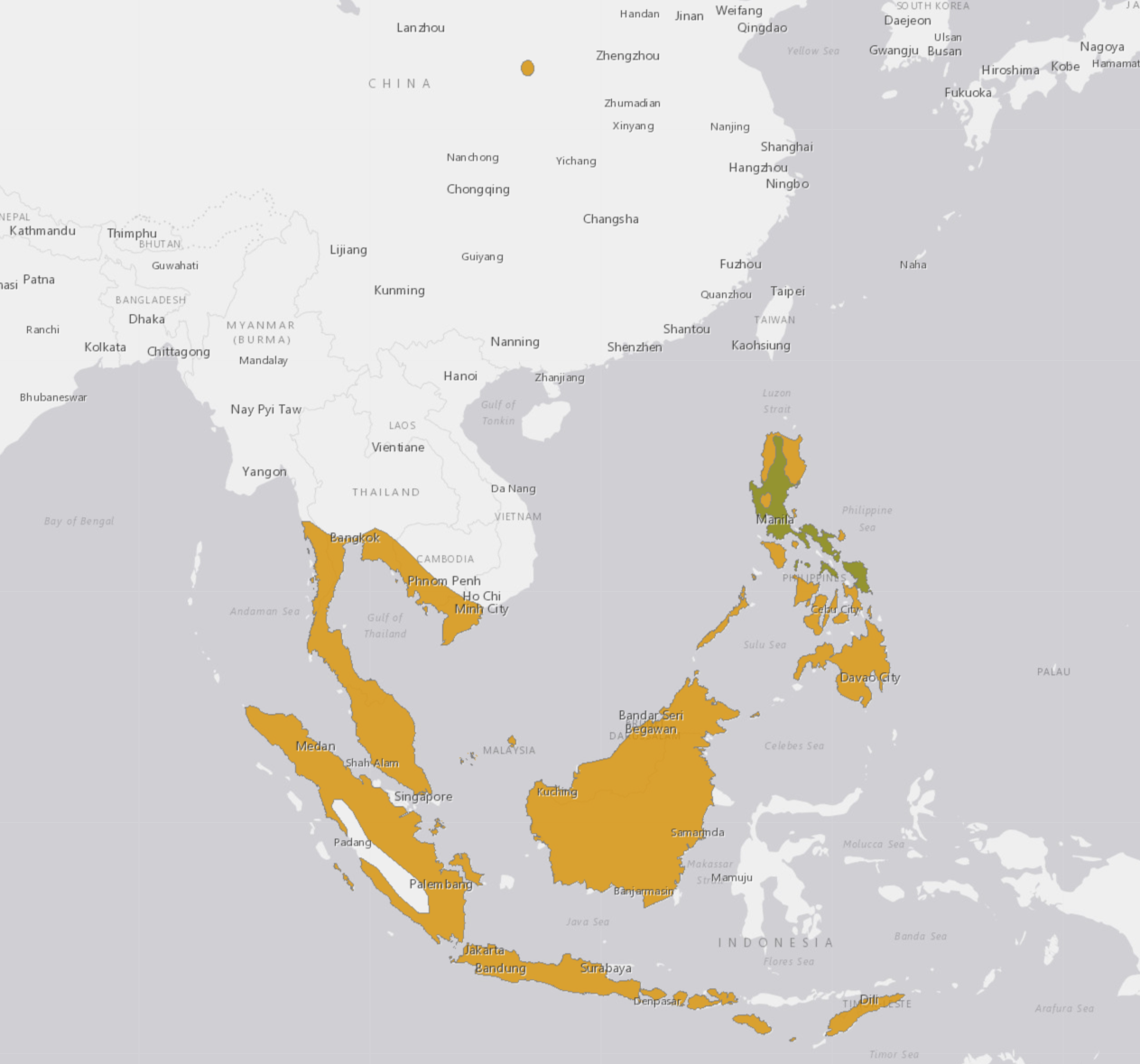 |
| Figure 12. Distribution of Malayan Flying Fox. GIS map adapted from geographical distribution data from IUCN website. |
Threats and Conservation
The Malayan flying fox is listed in the IUCN Red List as “Near Threatened” as of 2008, when not a decade ago in 1996 it was listed as “Least Concern”, due to a significant decline in the species due to habitat degradation and over-harvesting for food [24]. Its status is close to being vulnerable [24].
Flying foxes, including the Malayan flying fox, are keystone species in Southwest Pacific ecosystems as pollinators and seed dispersers [26]. The Malayan flying fox is the most threatened of the Old World fruit bats in Southeast Asia, having suffered significant population decline throughout much of their inhabited range due to over-hunting exacerbated by typhoons and introduced predators, deforestation resulting in habitat loss, and a low level of local support and and formal government commitment for conservation [25] [26].
The flying foxes are threatened by habitat loss to agriculture, and without their roost sites vulnerable populations would decrease [27]. They are also threatened by hunting at their roosts, which may reduce the size of the colony and lead to the abandonment of traditional maternity roost sites, affecting the reproduction of the flying fox [27]. Habitat loss in Sarawak from the proposed conversion of lowland peat swamp forests into agricultural and forest plantations threatens maternity colonies found in those areas [27]. Roosting sites in mangroves and lowland forests in Malaysia also are threatened by deforestation [12].
Malayan flying foxes are important for conservation as they are keystone species in an ecosystem [25]. In Sarawak, the Large flying fox was once widespread throughout coastal lowland areas [27]. However, the local population is threatened due to overhunting for wildlife trade (wild meat and traditional Chinese medicine) and sport hunting [27]. In Philippines, at Subic Bay, the Pteropus vampyrus and Acerodon jubatus colony is important to the area ecologically as pollinators and seed dispersers, and also to the local community as an indigenous food source and in eco-tourism [25].
Flying foxes have few natural predators, primarily raptors and snakes, however they are vulnerable to unnatural predation and catastrophic hazards due to their limited reproductive capacity [26].
It has been captured for food in Philippines and Thailand [24]. Flying foxes are being hunted for food and because they are believed to be orchard pests in Philippines [28]. These activities have been suspended as of 2004 in some parts of the Philippines such as Davao City by the city government and Regional Office of the Department of Environment and Natural Resources, however such protection is not available in all areas and they are still susceptible to hunting in other non-protected areas [28]. (2004 tho find something more updated?) Because humans eat large flying foxes, illegal poachers and international trade continue to find a market for P. vampyrus as a prized food item [12]. Several shipments of bats, originating in the Philippines and confiscated in Guam, contained the carcasses of 20 P. vampyrus [12]. P. vampyrus is considered a nuisance to farmers, in whose orchards individuals sometimes feed, and tactics such as flapping and whirling devices and lights have been used to discourage this and other bat species from feeding on fruit crops [12]. P. vampyrus is a frequent target of hunters because it is noisy and conspicuous [12].
There should be rehabilitation of the habitat and replanting of fruit trees that flying foxes feed on as a management priority [28].
P. vampyrus has been a candidate for captive breeding as a conservation approach in Singapore [1]. It is a seasonal visitor to Singapore with no permanent roosts as hunting and habitat loss depleted native populations during Singapore’s urban development [1]. Its maximum life span in captivity is about 15 years [12].
Parasites
A number of parasites infect P. vampyrus. At least two types of ectoparasites are found on the large flying fox: flies and mites [27].
There is a high level of gene flow in the blood-feeding parasite (Cyclopodia horsfieldi) which is found on flying foxes, suggesting there is frequent contact among Southeast Asian flying fox species [22]. Contact between the bat hosts could occur when Pteropus species forage in the same habitat or when roosting in dense mixed species colonies, thus enabling ectoparasite movements between hosts [22].
Taxonomy and Phylogeny
Etymology
The generic name "Pteropus" means "wing-footed", originating from the Greek words pteron meaning "wing, feather, or fin" and pous meaning "foot". The name vampyrus is derived from the Slavic word "wampir" which means "blood sucking ghost or demon: vampire", as this species was originally considered a blood-sucking bat [12].Classification
RankingKingdom: Animalia
Phylum: Chordata
Class: Mammalia
Order: Chiroptera
Family: Pteropodidae
Genus: Pteropus
Species: Pteropus vampyrus
All bats are part of a monophyletic clade, the order Chiroptera. Chiroptera are a sister group to flying lemurs and tree shrews, and also part of the superordinal group Achonta, which includes primates, tree shrews, and flying lemurs [10, 14].
Recent molecular studies has put Icaronycteris at the base of the tree of all bats, and Pteropodidae nested within the Microbats [10] [17].
Megabats (Pteropodidae) diversified from other bats approximately 58.15 ± 2.85 MYA, and further diversified between Pteropus and Cynopterus bats approximately 32.61 ± 3.72 MYA and 31.74 ± 3.90 MYA [5]. Among extant Chiroptera, morphological data provides strong bootstrap support monophyly of megachiropterans (Old World fruit bats) and microchiropterans [14, 15], unlike past hypotheses.
| Figure 13. Parsimony tree of Chiroptera phylogeny from Springer et. al. (2000). |
A study of six species of Pteropus, four of which were from the Philippines, was conducted to infer their evolutionary relationship using the cytochrome b gene sequence [5]. The DNA sequences were analysed using neighbour-joining, parsimony, and maximum likelihood [5].
Among the six Pteropus species, the first evolutionary event occurred approximately 13.90 ± 1.49 MYA, with a subsequent diversification a short time after, forming two clusters in the topology [5]. Pteropus vampyrus and Pteropus dasymallus formed a cluster together, with large genetic distances between this group and the other cluster [5].
| Figure 14. Phylogenetic tree depicting evolutionary relationships of six Pteropus species. From Bastian Jr et. al. (2001) |
Evolution of Echolocation
Laryngeal echolocation evolved in the common ancestor of fossil and extant bats, and was subsequently lost in megabats, including Pteropus [10]. The short clicks used by Rousettus, a megabat with echolocation-like abilities, independently evolved, and is unlike laryngeal echolocation found in microbats [3] [15].
The family Pteropodidae, or Megabats, are the only family of bats in the order Chiroptera that is not capable of echolocation. Echolocation developed early in the chiropteran lineage, and was later lost in Pteropodids due to the uncoupling of flight and echolocation, as their larger body size made it energetically expensive to echolocate [3].
Echolocation in bats is linked to their breathing; each time they breathe out as they flap their wings upwards, and emit their echolocation pulse at together with their expiration [3]. Because larger bats have slower wingbeat frequencies, there is an upper size limit for echolocation in bats as too low a pulse emission rate would be ineffective for echolocation [3]. This might explain the loss of echolocation in Megabats.
Other Hypotheses
Pteropodidae was originally thought to be earlier diverged from fossil and crown group bats [10] [17]. Fossil bats make up a paraphyletic assemblage at the base of the Chiropteran tree [10]. Megabats (Pteropodidae) diversified from crown-group and fossil bats approximately 58.15 ± 2.85 MYA [5], following which Crown-group bats last shared a common ancestor 52 to 54 MYA [10].While there is strong support for the node of divergence between Pteropodidae and the fossil bats, the overall tree is weak and has low support at its lower branches?
| Figure 15. Parsimony tree of Chiroptera phylogeny from Simmons and Geisler (1998). |
Type information
The type specimen is an adult male Pteropus vampyrus, part of the Division of Mammals Collections stored at the Smithsonian Institute in the United States National Museum of Natural History [2]. It was collected on 7 September 1903 from Mindanao, Philippines, at the lower Pantar camp near Lake Lanao in the Lanao del Sur Province [2].Original Description and Translation
|
Rough translation from google: VESPERTILIO (bat) Teeth: Alert, sharp, 4 incisors (?). Hand: palmate, skin between the digits. [11] Vampyrus Simple membrane divided between the thighs No tail Large flying dog. Lives in Asia. Nocturnal, sleeps during the day (?) [11] |
DNA Barcode
The complete mitochondrial genome of the Malayan flying fox, Pteropus vampyrus (Pteropus, Pteropodidae) [23]SPAdes-3.0.0 was used with the next generation sequencing to determine the complete mitochondrial genome sequence of P. vampyrus (GenBank accession no. KP214033) [23].
The complete mitochondrial genome sequence of P. vampyrus (16,554 bp in length) consisted of 13 protein-coding genes, 22 tRNA genes, two rRNA genes, and one putative control region. The order of all genes was identical to other vertebrate species. ND6 gene and eight tRNA genes were encoded on the L-strand; the remaining genes were encoded on the H-strand. The overall base composition was A (33.1%) > C (27.1%) > T (25.4%) > G (14.4%), with an AT bias of 58.5% [23].
Related Literature and References
- Leong TM & Chan KW (2011). Bats in Singapore - Ecological Roles and Conservation Needs. Proceedings of Nature Society, Singapore's Conference on 'Nature Conservation for a Sustainable Singapore', pp 41-64.
- Fisher RD & Ludwig CA (2015). Catalog of type specimens of recent mammals: Orders Didelphimorphia through Chiroptera (excluding Rodentia) in the National Museum of Natural History, Smithsonian Institution. Smithsonian Contributions to Zoology. 644: 1-110.
- Altringham JD (2011). Echolocation and other senses. In Bats: From Evolution to Conservation.
- Jones G & Teeling EC (2006). The evolution of echolocation in bats. Trends in Ecology and Evolution, 21(3):149-156.
- Bastian Jr. ST, Tanaka K, Anunciado RVP, Natural NG, Sumalde AC & Namikawa T (2001). Evolutionary Relationships of Flying Foxes (Genus Pteropus) in the Philippines Inferred From DNA Sequences of Cytochrome b Gene. Biochemical Genetics, 40(3/4).
- Hutcheon JM & Kirsch JAW (2006). A moveable face: deconstructing the Microchiroptera and a new classification of extant bats. Acta Chiropterologica, 8(1):1-10.
- Giannini NP & Simmons NB (2007). The Chiropteran Premaxilla: A Reanalysis of Morphological Variation and Its Phylogenetic Interpretation. American Museum Novitates, 3585.
- Speakman JR & Racey PA (1991). No cost of echolocation for bats in flight. Letters to Nature, 350:421-423.
- SIMMONS AND GEISLER (1998). Relationships of Eocene Bats. Bulletin American Museum of Natural History, 235.
- Springer MS, Teeling EC, Madsen O, Stanhope MJ & de Jong WW (2000). Integrated fossil and molecular data reconstruct bat echolocation. Proceedings of the national Academy of Sciences of the United States of America, 98(11):6241-6246.
- Linnaeus C (1758). Systema Naturae.
- Kunz TH & Jones DP (2000). Pteropus vampyrus. Mammalian Species, 642:1-6.
- Kunz TH, de Torrez EB, Bauer D, Lobova T & Fleming TH (2011). Ecosystem services provided by bats. Annals of the New York Academy of Science, 1223: 1–38.
- Novacek MJ (1992). Mammalian Phylogeny: shaking the tree. Nature, 356:121-125.
- Simmons NB (1998). In: Bat Biology and Conservation. pp. 3–26.
- Gunnell GF & Simmons NB (2005). Fossil Evidence and the Origin of Bats. Journal of Mammalian Evolution, 12(1/2):209-246.
- Teeling EC, Springer MS, Madsen O, Bates P, O'Brien SJ & Murphy WJ (2005). A Molecular Phylogeny for Bats Illuminates Biogeography and the Fossil Record. Science, 307(5709):580-584.
- Breed AC, Field HE, Smith CS, Edmonston J & Meers J (2010). Bats Without Borders: Long-Distance Movements and Implications for Disease Risk Management. EcoHealth, 7:201-212.
- Sharifah SH, Sohayati AR, Maizan M, Hang LY, Sharina M, Syamsiah AK, Latiffah K, Arshad SS, Zaini CM, Humes F, Daszak P & Epstein J (2009). Genetic characterization of a Nipah virus isolated from a Pteropus vampyrus in Malaysia. Neurology Asia, 14:67-69.
- Chua KB, Koh CL, Hooi PS, Wee KF, Khong JH, Chua BH, Chan YP, Lim ME & Lam SK (2002). Isolation of Nipah virus from Malaysian Island flying-foxes. Microbes and Infection, 4(2):145-151.
- Johara MY, Field H, Rashdi AM, Morrissy C, van der Heide B, Rota P, bin Adzhar A, White J, Daniels P, Jamaluddin A & Ksiazek T (2001). Nipah Virus Infection in Bats (Order Chiroptera) in Peninsular Malaysia. Emerging Infectious Diseases, 7(3):439-441.
- Hengjan Y, Pramono D, Takemae H, Kobayashi R, Iida K, Ando T, Kasmono S, Basri C, Fitriana YS, Arifin EMZ, Ohmori Y, Maeda K, Agungpriyono S & Hondo E (2017). Daytime behavior of Pteropus vampyrus in a natural habitat: the driver of viral transmission,The Journal of Veterinary Medical Science, 79(6):1125-1133.
- Lu J, Li XF & Yuan H (2015). The complete mitochondrial genome of large flying fox, Pteropus vampyrus (Pteropus, Pteropodidae). Mitochondrial DNA Part A > DNA Mapping, Sequencing, and Analysis, 27(6).
- Bates, P., Francis, C., Gumal, M., Bumrungsri, S., Walston, J., Heaney, L. & Mildenstein, T. 2008. Pteropus vampyrus. The IUCN Red List of Threatened Species 2008: e.T18766A8593657. Downloaded on 02 December 2017.
- Mildenstein TL, Stier SC, Nuevo-Diego CE & Mills LS (2005). Habitat selection of endangered and endemic large flying-foxes in Subic Bay, Philippines. Biological Conservation, 126(1):93-102.
- Cox PA, Elmqvist T, Pierson ED & Rainey WE (1991). Flying Foxes as Strong Interactors in South Pacific Island Ecosystems: A Conservation Hypothesis. Conservation Biology, 5(4):448-454.
- Gumal MT (2004). Diurnal home range and roosting trees of a maternity colony of Pteropus vampyrus natunae (Chiroptera: Pteropodidae) in Sedilu, Sarawak. Journal of Tropical Ecology, 20:247-258.
- Cayunda IEB, Ibanez JC & Bastian Jr ST (2004). Roosting behaviour and roost site characterisation of Pteropus vampyrus in Malagos Watershed, Davao City. Agham Mindanaw, 2:61-72.
- Luis AD, Hayman DTS, O’Shea TJ, Cryan PM, Gilbert AT, Pulliam JRC, Mills JN, Timonin ME, Willis CKR, Cunningham AA, Fooks AR, Rupprecht CE, Wood JLN & Webb CT (2013). A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proceedings of the Royal Society B, 280(1756).
- http://www.straitstimes.com/asia/se-asia/dwindling-flying-fox-numbers-in-malaysia-threaten-durian-industry
- Stier SC & Mildenstein TL (2005). Dietary Habits of the World's Largest Bats: the Philippine Flying Foxes, Acerodon jubatus and Pteropus vampyrus lanensis. Journal of Mammalogy, 86(4):719-728.
