Name
Binomial: Pycnoporus sanguineus ( L.) Murrill, 1904Vernacular: Tropical Cinnabar Bracket fungus
Table of Contents
 |
| Fruiting bodies of Pycnoporus sanguineus growing on a log at Dairy Farm park |
Description
Macrofungi generally composes of two main parts, the mycelium which is hidden in the substrate where the fungi is found and the fruiting body which forms when conditions are favourable for the fungus to disperse its spores. For identifications, the fruiting bodies are generally used instead of the mycelium due to identifiable characteristics and ease of access. The growth habits of Pycnoporus sanguineus may vary from solitary to clustered, and individuals may even appear to have fused together. When fresh, the fruiting body resembles leather in texture, but feels more flexible when dried.
The fruiting body of Pycnoporus sanguineus, comprising of a cap (pilus) and stem, is a brilliant reddish-orange in colour, characteristic to all members of the Pycnoporus genus. Caps can range from 3 –14cm in diameter, and up to 5mm in thickness at the margins. Stems when present may also be 2–7cm long. The underside of the cap reveals a reddish-orange surface that contains numerous tiny circular pores (5-6 per mm)[1] .
 |
| Top view of the pycnoporus sanguineus cap. |
 |
| Underside of Pycnoporus sanguineus revealing a dense porous underside |
Spore prints of Pycnoporus sanguineus are white. The spores, which require a microscope to be observed are approximately cylindrical, smooth and translucent, ranging from 5–6um in length and 2–2.5um in width[2] .
Diagnosis
Pycnoporus sanguineus differs from other polyporaceae (bracket fungi) due to their vibrant red-orange colouration. The vivid reddish-orange colouration of fruiting bodies is a key characteristic of all Pycnoporus members, although the vibrancy of the colours may fade over time. Caps of Pycnoporus members also turn reddish-purple to black upon contact with Potassium Hydroxide (KOH). Pores on the underside of Pycnoporus members are also bright reddish-orange in colour, which does not fade. In the Pycnoporus genus, there are currently 4 known species, of which P. coccineus and P. sanguineus looks identical.
 |
| Members of the Pycnoporus genus |
Pycnoporus sanguineus can be recognised from its thickness of the caps. Cap margins of Pycnoporus sanguineus generally grows up to about 5mm thick. The cap of pycnoporus sanguineus is also slightly more rigid than other members of the Pycnoporus genus, and its colour is more resistant to fading with time. Pycnoporus sanguineus is also more common towards the tropics[3] .
A summary of the differences between members of the Pycnoporus genus is summarized in the table below[4] :
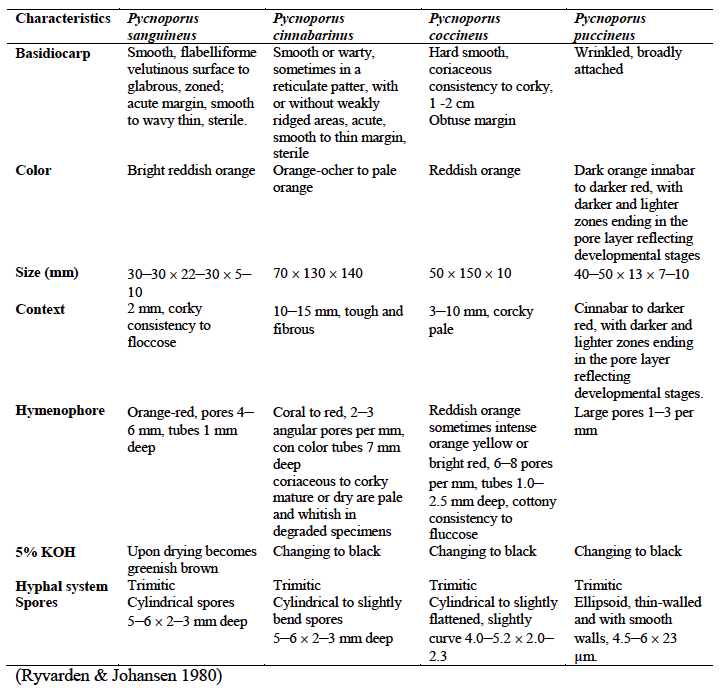 |
| Other differences between members of the Pycnoporus genus. Ryvarden & Johansen, 1980 |
Biology
Life cycle and Reproduction
Fungi have different modes of reproduction, namely through mycelial fragmentation, asexual or sexual spores and through budding. Pycnoporus sanguineus, as a member of the basidiomycota phylum generally undergoes sexual reproduction, where the spores are produced on cells called basidia.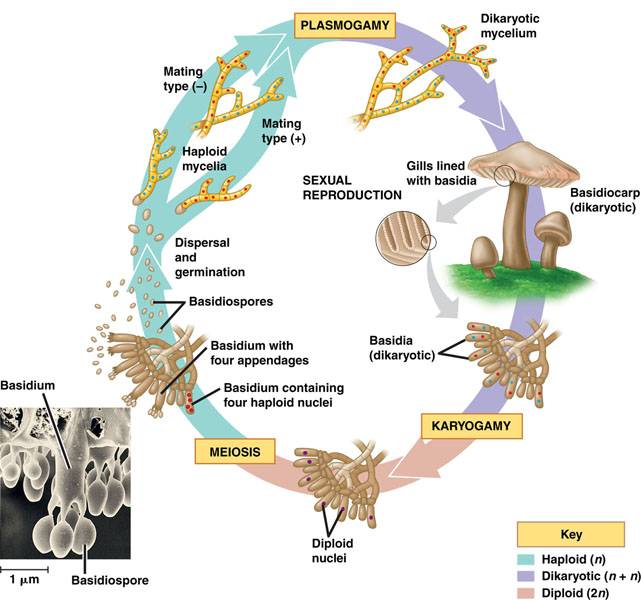 |
| Basidiomycete lifecycle. Image taken from Campbell and reece et al. 2009 |
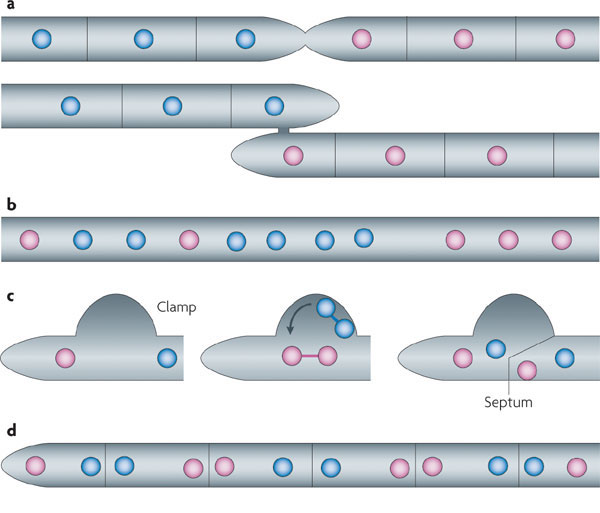 |
| Steps illustrating how a dikaryotic mycelium is formed using a clamp structure (Gladfelter & Berman, 2009) |
separate until they undergo Karyogamy, when the two nuclei fuse to become diploid. The nuclei then undergo meiosis such that each spore on each of the four appendages on the basidium contains a haploid nucleus. Spores produced on a basidium is also called a basidiospore. The spores are released and carried by the wind, restarting the entire cycle again when it lands on a favourable location[6] .
Nutrition
Most Fungi are generally saprotrophic in nature, meaning that they obtain their nutrition through decomposition and recycling of minerals. As a heterotroph, Pycnoporus sanguineus is unable to produce its own food, and thus acquires its nutrition from the breakdown of wood and the environment. The nutrition of Fungi generally consists of organic carbon, nitrogen and phosphorus.Organic carbon is acquired through the breakdown of lignin, hemicellulose and cellulose in wood. To aid the breakdown of wood, Pycnoporus sanguineus contain a number of enzymes specially catered to breakdown cellulose and lignin. Pycnoporus sanguineus is a white rot fungus, and hence bleaches the wood it grows on as it breaks down the lignin in wood in order to obtain its nutrition from hemicellulose and cellulose.
Pycnoporus sanguineus can commonly be found on dead hardwood, though it may also parasitise on tree hosts especially on plane (Planatus sp.) and mango trees (Mangifera indica). Nitrogen and Phosphorus are also scavanged, stored and recycled from the environment.
Distribution
Global distribution
In addition to morphological features used to differentiate members of the pycnoporus genus, distributions of the particular species can also help to identify the species, especially between Pycnoporus sanguineus and Pycnoporus coccineus, which look very similar in appearances.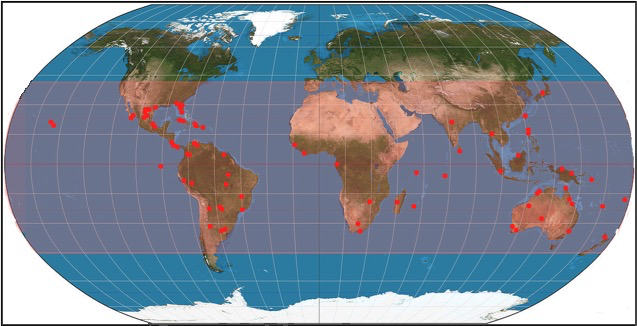 |
| Known distributions of Pycnoporus sanguineus around the world |
Local distribution in Singapore
Fungi are generally poorly studied in the tropics, including in Singapore. As a result,the overall distribution of Pycnoporus sanguineus in Singapore is unknown due to the lack of fungal knowledge and consensus studies. As of date, only Pycnoporus sanguineus has been sighted in Singapore. Although the true distribution throughout Singapore is unknown, Pycnoporus sanguineus has been observed in various nature parks with dead logs including Dairy Farm Nature Park, Coney Island Park.Significance
Ecological significance
Fungi play important roles in ecosystems due to their nutritional needs as saprotrophs. Fungi including Pycnoporus sanguineus are largely responsible for the decomposition of wood, allowing for carbon and other minerals to be recycled through the break down of lignin in a process called mineralization. The release of carbon in Hemicellulose and cellulose and the breakdown of lignin is important in the carbon cycle.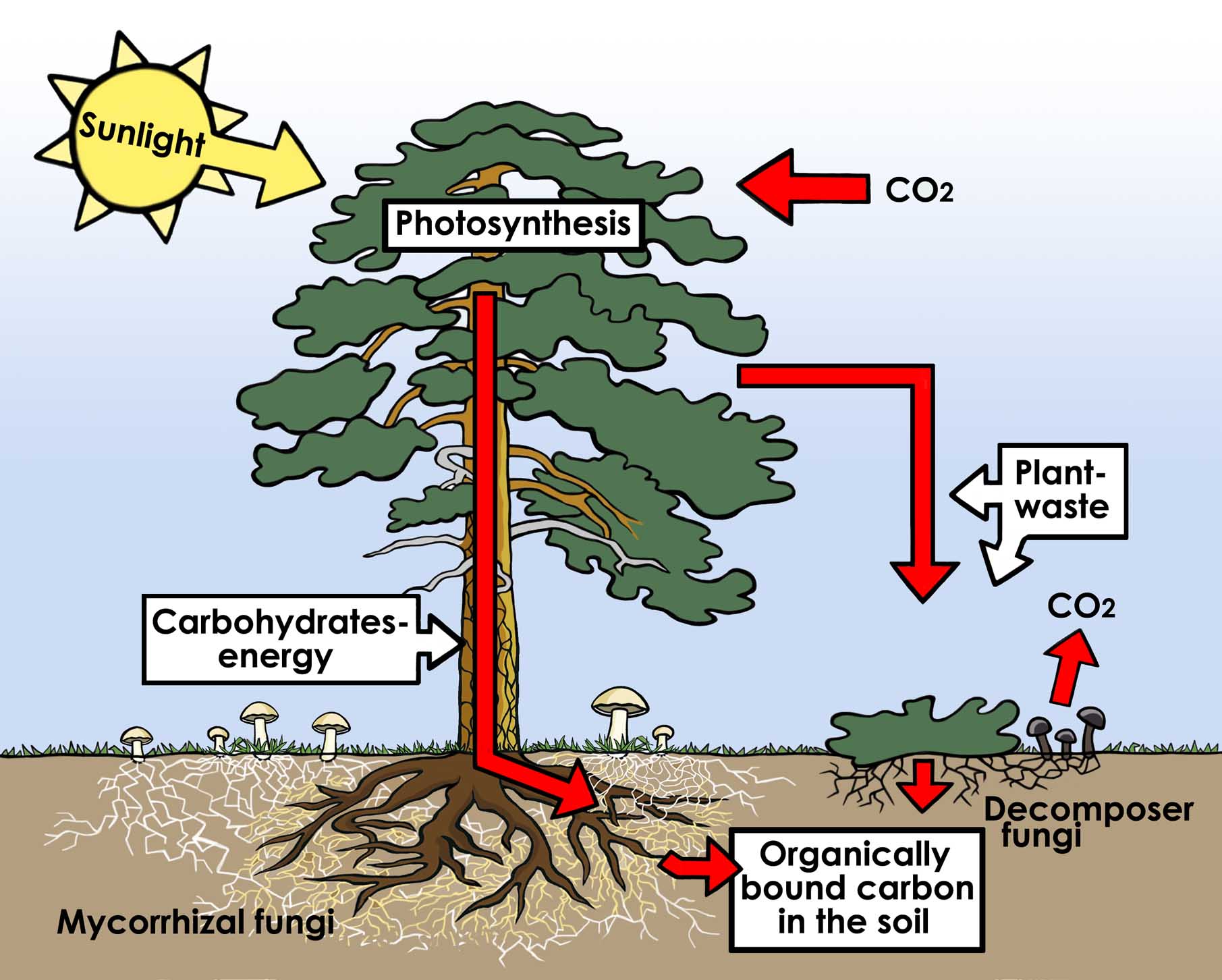 |
| Role of Fungi in the carbon cycle. Illustrated by Lisa Larsson |
The removal of lignin by Pycnoporus sanguineus also allows the cellulose in wood to be consumed by other organisms that lack the ability to break down lignin. Researchers have also speculated that fungal decay of wood may have led to diversification of invertebrates during the early years, as a greater range of food sources became avaliable[8] [9] .
Industrial Uses
Pycnoporus sanguineus also serves as an important source of enzymes that play vital roles in various industries, especially in the paper and pulp, textile, timber, medicine and bioremediation industries Cellulase, a major enzyme used in textile, paper, detergent, food, pulp and biofuel industries can be found in Pycnoporus sanguineus[10] . In textile industries, Cinnabarin, a pigment found in the caps of Pycnoporus sanguineus is commonly used in decolourizing particular dyes including the toxic indigo dye which has been a cause of environmental concern[11] .In addition to being a source of industrial enzymes and compounds, Pycnoporus sanguineus also holds a potential in the bioremediation of waste water, crude oil spills and environments polluted by heavy metals including copper (II) ions. In Pycnoporus sanguineus and other saprobic fungus, the enzymes used in the breakdown of lignin in wood has been found to be able to perform other complex decompositions, converting sources such as plastic and oil into nutrients[12] . Bioremediation of polluted environments using fungi has been termed as Mycoremediation. In Veracruz, Mexico, researchers had discovered a strain of Pycnoporus sanguineus which could not only tolerate higher temperatures and salt levels from a plot of oil polluted site, but had also developed different isoforms of laccase enzymes to breakdown crude oil[13] . Pycnoporus sanguineus has also been discovered to be efficient in absorbing heavy metal ions from aqueous environments through ion transport[14] , giving it much potential in waste water treatment. Through the use of enzymes and compounds present in Pycnoporus sanguineus to treat toxic chemical wastes before release into the environment, further harmful cascading effects on the ecosystems can be prevented, benefiting the ecosystem.
Medicinal Uses Past and Present
Although inedible, Pycnoporus Sanguineus, like many other fungal species has been used in traditional medicine and is crucial to the pharmaceutical industry today. In the past, it was used as a form of traditional medicine by the aborigines of Australia and the natives of Guana Island to treat a number of ailments ranging from sore throat, ulcurs and oral thrush, arthritis, gout, styptic, tooth aches, fevers and haemorrhages . However, it is generally not advised to eat fungi found in the wild as they may have absorbed toxin and pollutants from the environment, or may look identical to toxic species.Present research has also shown that Pycnoporus sanguineus contains numerous antibacterial compounds, effective against Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella typhi, Staphylococcus aureus and Streptococcus sp[15] .
Cultural significance
Due to its vivid colouration, Pycnoporus sanguineus has become an iconic bracket fungus, easily identifiable in the field and distinguishable from fungi of other genus. This has led to use of Pycnoporus sanguineus in many stamps in featuring the fungi present. Since 1936, Australia has used images of her flora and fauna on stamps. In 2001, Australia released stamps for Christmas Island that showcased two of her more prominent fungal species as part of the Australian flora and fauna stamps series[16] . Other countries that also featured Pycnoporus sanguineus included the Solomon Islands, Brazil and Dominican Republic. |
| Image taken from Australian National Botanical Gardens. |
 |
| Stamp from Solomon Islands featuring Pycnoporus sanguineus |
 |
| Brazil stamp featuring Pycnoporus sanguineus |
 |
| Dominican Republic Stamp featuring Pycnoporus sanguineus |
Etymology
The name Pycnoporus sanguineus comes from the unique appearance of its fruiting bodies. Pycnoporus is made up of the latin prefix 'pycno-' which means dense and porus which describes a surface as having small holes. 'Pycnoporus' illustrates the underside of the fruiting bodies, which contain many closely packed pores from which spores are released. The species name, 'sanguineus' is derived from a latin term that refers to blood. 'Sanguineus' was used to describe the bright red appearance of the fungal species.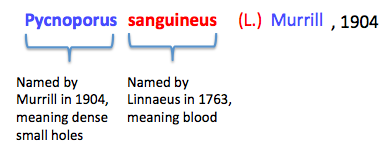 |
| Summary of Pycnoporus sanguineus name origins |
The fungal species was not always referred to as Pycnoporus sanguineus. It was initially named as Boletus sanguineus after being discovered by Linnaeus in 1763[17] .
The presence of a parenthesis indicates that there had been a change in genus from the original it was described and named with. In 1904, Murrill placed the fungal species under the Pycnoporus genus, resulting in its current name.
The fungal species had undergone various name changes, resulting in a wide number of synonyms as shown below :Synonyms of Pycnoporus sanguineus in chronological order
- Boletus sanguineus L. (1763)
- Boletus nitens Batsch (1783)
- Boletus ruber Lam. (1783)
- Polyporus sanguineus (L.) G.Mey. (1818)
- Polyporus cristula Klotzsch ex Berk (1839)
- Polystictus sanguineus (L.) Fr. (1851)
- Microporus sanguineus (L.) Kuntze (1898)
- Trametes Sanguinea (L.) Lloyd (1924)
- Trametes cinnabarina var. sanguinea (L.) Pilat (1940)
- Trametes sanguinea (L.) Imazeki (1943)
- Coriolus sanguineus (L.) G. Cunn. (1949)
- Fabisporus sanguineus (L.) Zmitr (2001)
Taxonavigation
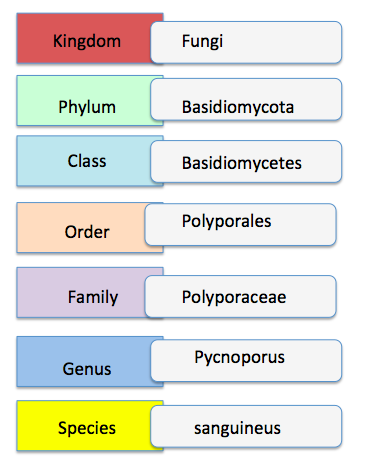 |
| Taxonavigation of Pycnoporus sanguineus |
Type Information
The type specimen according to Mycobank[18] was collected in the Netherlands by Visscher in March 1985, and was identified by Stalpers. The specimen has since been kept in Suriname, South America, under herbarium records of CBS-H17581 .Phylogenetic relationships
For the longest time, identification of Pycnoporus species had been due to similarity of morphological traits (cap shape and pore size) to type specimens and distributions, resulting in four species (Pycnoporus cinnabarius, Pycnoporus puniceus, Pycnoporus sanguineus, Pycnoporus coccineus) being identified. The high variability in both macroscopic and microscopic morphological traits has led to difficulty in species delineation amongst the Pycnoporus members. Moreover, the high similarity in morphological traits amongst the four species has contributed to the difficulty in identifying species of the genus Pycnoporus. As molecular data analysis tools developed, a precise method for assessing identification arose. These included using molecular markers such as the internal transcribed spacer (ITS) region of the nuclear ribosomal DNA, beta-tubulin, and laccase isoenzyme lac I.The ITS region of the nuclear ribosomal DNA (ITS1-5.8S-ITS2) is commonly used to infer the relationships between wood saprotrophic basidiomycota species of particular genra including Phanerochaete, although it fails to resolve the phylogenetic relationships between the species. Beta-tubulin genes were often used to resolve phylogenetic relationships in ascomycete genera that were indistinguishable using traditional morphological methods. Laccase isoenzyme lac I genes are used to identify between the Pycnoporus diversity, since members of Pycnoporus genus have been described to overproduce Laccase enzymes. In 2011, Lesage-Meessen et al., constructed three different maximum likelihood trees had been generated using three different genetic markers, the internal transcribed spacer (ITS) region of nuclear ribosomal DNA, beta-tubulin and laccase isoenzyme lac I.[19]
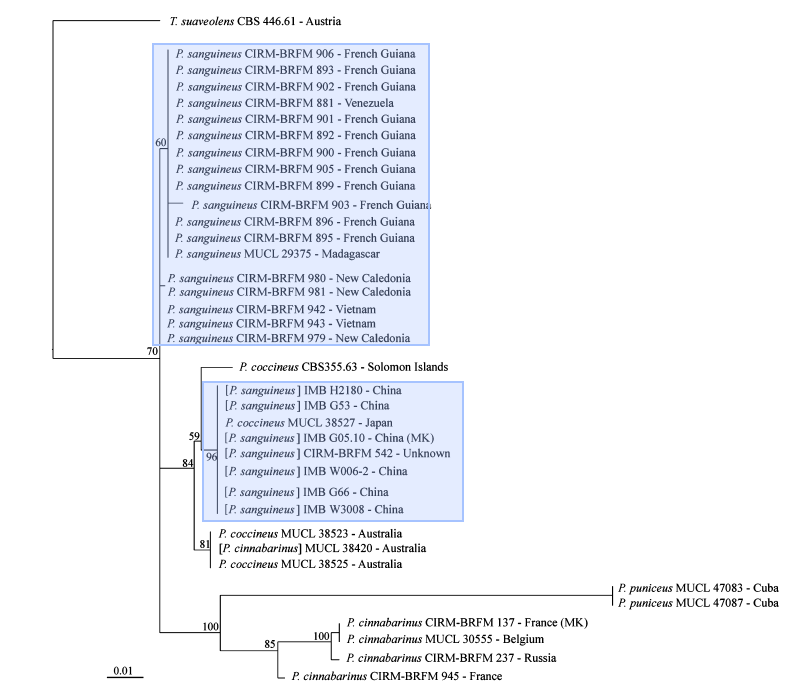 |
| Maximum likelihood tree based on ITS1-5.8S-ITS2 |
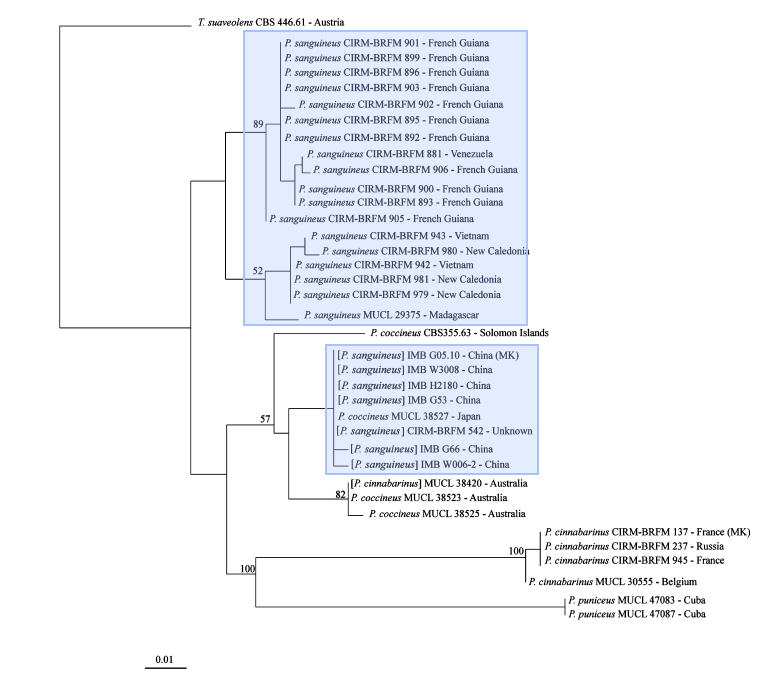 |
| Maximum Likelihood tree based on partial regions of beta-tubulin gene sequence |
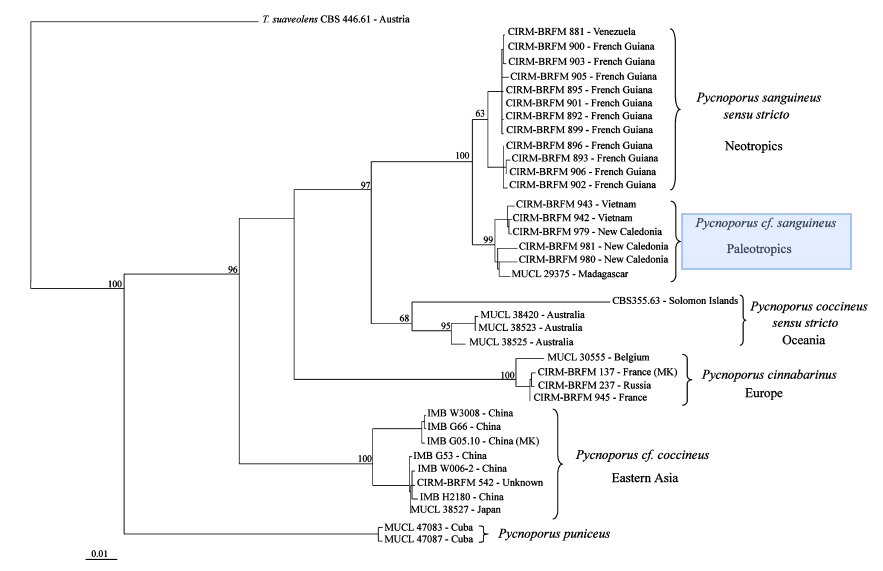 |
| Maximum likelihood tree using F2-R8 partial laccase gene sequences |
Data analysis using the internal transcribed spacer (ITS) region of nuclear ribosomal DNA and beta-tubulin molecular markers also show that there was a misidentification of Pycnoporus sanguineus in Japan as Pycnoporus coccineus, showing how sole dependency on morphological features may lead to errors in identifications.
The Maximum likelihood trees show strong support for Pycnoporus sanguineus as a separate species from other members of the pycnoporus genus. However, the maximum likelihood tree using the laccase isoenzyme lac I as a molecular marker reveals support that Pycnoporus sanguineus may be divided into two further species based on locality in the Neotropics and the Paleotropics.
Glossary
| Terms used |
Definitions |
|---|---|
| Basidium (Basidia) |
Microscopic club-shaped spore-bearing structure (s) found in Basidiomycota fungi |
| Basidiocarp / Fruiting Bodies |
The multicellular structure made of hyphae that contain spore bearing surfaces |
| Dikaryotic |
Reproductive phase where by more than one genetically different nuclei are present in the same cell |
| Diploid |
Two sets of chromosomes present in each nucleus |
| Haploid |
one set of chromosomes present in each nucleus |
| Heterotroph |
An organism that requires to consume or absorb organic carbon in order to acquire energy |
| Karyogamy |
Reproductive phase where by the genetically different nuclei fuse |
| Macrofungi |
Fungi that have a fruiting body visible to the naked eye |
| Mycelium |
Vegetative part of a fungus consisting of a mass of branching, thread-like hyphae |
| Plasmogamy |
Reproductive phase where the cytoplasm of two parent cells fuses together without the fusion of nuclei |
| saprotrophic |
Extracellular digestion of nutrients through decay and decomposition |
References
Useful links :Mycobank records of //Pycnoporus sanguineus//
Field identification of Pycnoporus sanguineus
Image uses and permissions:
| Image 1: Photo of Pycnoporus sanguineus in Dairy Farm Nature Park |
Photograph by Amelia Lim |
| Image 2: Photo of Pycnoporus sanguineus cap |
Photograph by Amelia Lim |
| Image 3: Underside of Pycnoporus sanguineus |
Photograph by Amelia Lim |
| Image 4: Members of Pycnoporus sanguineus |
Image created by Amelia Lim |
| Image 5: Table of differences between Pycnoporus genus members |
Image taken from Ryvarden & Johansen, 1980 Permissions: Fair use |
| Image 6: Life cycle of Basidiomycota |
Image taken from Campbell and reece et al. 2009 Permissions: Fair use |
| Image 7: Clamp connections |
Image taken from Gladfelter & Berman, 2009 Permissions: Fair use |
| Image 8: Known distribution of Pycnoporus sanguineus |
Image created by Amelia Lim based on GBIF maps |
| Image 9: Role of fungi as decomposers in the ecosystem |
Image and permissions by Lisa Larsson |
| Image 10: Christmas Island Australian stamp |
Image taken from Australian National Botanic Gardens permissions: Fair use |
| Image 11: Solomon Island Stamp featuring Pycnoporus sanguineus |
Creative commons |
| Image 12: Brazil Stamp featuring Pycnoporus sanguineus |
Creative commons |
| Image 13: Dominican Republic stamp featuring Pycnoporus sanguineus |
Creative commons |
| Image 14: Summary of Pycnoporus sanguineus name |
Created by Amelia Lim |
| Image 15: Maximum likelihood tree based on ITS1-5.8S-ITS2 |
Image taken from Lesage-Meessen et al., 2011 Permissions: Fair use |
| Image 16: Maximum likelihood tree based on Beta-tubulin |
Image taken from Lesage-Meessen et al., 2011 Permissions: Fair use |
| Image 17: Maximum likelihood tree based on Laccase isoenzyme Lac I |
Image taken from Lesage-Meessen et al., 2011 Permissions: Fair use |
- ^ Zoberi M.H. (1972) Tropical macrofungi some common species. Macmillian Press Ltd, London. pp. 50
- ^ Bessette A.R., Bessette A.E. (2001) The Rainbow Beneath My Feet: A Mushroom Dyer's Field Guide. Syracuse University Press. Syracuse, New York. pp.112
- ^ Mushroom the Journal (n.d.) Pycnoporus sanguineus. http://www.mushroomthejournal.com/greatlakesdata/Taxa/Pycnosangu298.html (Last accessed 15 Nov 2017)
- ^
Ryvarden L., Johansen I. (1980) A Preliminary polypore flora of East Africa. Sypnosis fungorum 5. fungiflora, Oslo
Gladfelter A.S., Berman J.(2009) Dancing genomes: fungal nuclear positioning. Nature Reviews Microbiology 7:875–886
Campbell, N.A., Reece J.B., Urry L.A., Cain M.L., Wasserman S.A., Minorsky P.V., Jackson R.B. (2009) Biology. San Francisco: Pearson, 8 Pp.542.
Pycnoporus sanguineus (L.) Murrill, 1904 in The Catalogue of Life Partnership (2017). Catalogue of Life. Checklist Dataset https://doi.org/10.15468/rffz4x (Last accessed via GBIF.org on 6 Nov 2017)
Watkinson S.C., Money N., Boddy L. (2016) The Fungi (Third Ed). Elsevier Ltd.
Muhammad A.A.M.A., Jalil R., Kalil M.S.(2016) Production of Cellulase from Pycnoporus sanguineus. Indian Journal of Science and Technology 9(21):974–5645
Viswanath B., Subhosh Chandra M., Pallavi H., Rajasekhar Reddy B.(2008) Screening and assessment of laccase producing fungi isolated from different environmental samples,” African Journal of Biotechnology 7(8): 1129–1133
Smania A., Delle Monache F., Smania E.F.A., Gil M.L., Benchetrit L.C., Cruz F.S. (1995) Antibacterial activity of a substance produced by the fungus Pycnoporus sanguineus (Fr.) Murr. Journal of Ethnopharmacology 45(3):177–181
Australian National Botanical Gardens (n.d.) Australian plants on stamps. Pycnoporus sanguineus- Christmas Island Stamps 2001.
https://www.anbg.gov.au/stamps/christmas-island/stamp-xmas-pycnoporus-sanguineus-2001.html (Last accessed: 6 November 2017)
Robert V., Stegehuis G., Stalpers J.(2005) The Mycobank engine and related databases. http://www.mycobank.org(Last accessed 7 Nov 2017)
Lesage-Meessen L., Haon M., Uzan E., Levasseur A., Piumi F., Navarro D., Taussac S., Favel A., Lomascolo A. (2011) Phylogeographic relationships in the polypore fungus
Pycnoporus inferred from molecular data. FEMS Microbiology Letters 325:37–48