Varanus salvator (Laurenti, 1768)



Note: Photos by Wong Li Jing (Crystal) taken at Sungei Buloh Wetland Reserve, Singapore, and individuals photographed are of the subspecies Varanus salvator macromaculatus (Malayan Water Monitor or Southeast Asian Water Monitor), unless otherwise stated.
Table of Contents
Introduction
Water Monitors are among the largest lizards in the world with a wide distribution. Varanus salvator are a common sight in Singapore (subspecies macromaculatus) and across other parts of South and South-east Asia. Their coloration range from brown to black with orange, yellow or white spots or ocelli. Their heads are ventrally flattened with an elongated snout. Their tails are long and powerful and are used for swimming, fighting or hunting.General Information
 Distribution and Habitat
Distribution and Habitat
Varanus salvator are typically found near freshwater or marine water sources and have a very wide distribution (Fig 1, Left) ranging from Sri Lanka in the north-west to Philippines in the north-east, all the way down to Java in the south. There are currently four recognized subspecies (2011), and the distributions of the different subspecies do not overlap.They live in tropical evergreen rainforests and sometimes in subtropical forests, but never stray too far from a water source. They are classified as semi-aquatic, spending significant time on land or in water, although they are occasional arboreal (climb trees), particularly to escape predators.
The current status about the distribution of the different subspecies are as follows: the nominal subspecies described as Varanus salvator salvator is endemic (restricted) to Sri Lanka, Varanus salvator adamanensis is endemic to the Andaman Islands, Varanus salvator macromaculatus has the widest distribution ranging from India to Indonesia[1] and Varanus salvator bivittatus can be found in Java (Terra typical), Lombok, Sumbawa, Flores and Wetar[2].
Diagnosis
Due to changes in taxonomic changes (See section below on what this means) and recently discovered great variation within this species, currently no description that encompasses all the variation within this species without including others exists. A key is available in The Natural History of Monitor Lizards by Harold F. De Lisle[3], however, in view of the great variation within the species (see the different sections for each of the subspecies), the key is only useful for deriving the identity of V.s. salvator.Physical Description[3]
Tongues are long, deeply forked, protruding and slender and are used to locate food sources. This is enabled by the Jacobson’s organ.Monitor lizards have multiple pleurodont (attached to the side of the jaw) rows of teeth that are replaceable and are hidden by a fold in the gums.
Size
Up to 2.5 meters (from snout to the tip of the tail)[3],
Head
Narial openings (nostrils) range from round to oval, closer to the snout tip than the eye. It has a broad and elongated head which is twice as long as it is wide. The snout is long, has a rounded tip and a large tympanum (middle ear).
Tail:
Tails are laterally compressed and have a double keeled upper edge. They are up to 1.36 times longer than their body length. This trait is sexually and ontogenetically (age) dimorphic; males have proportionally longer tails and older individuals have shorter tails.
Scales
Scales on the head are large, flat and sooth with four to eight well-differentiated supraoculars (enlarged scales on the crown immediately above the eye) and 48 to 60 scales positioned in a straight line from rictus to rictus (gape) above the head.
Physical characteristics are summarized in Fig 2 above.
Senses[3]
Vision
Their visual field is approximately 240° and consists of monocular as well as a small range of binocular vision (which allows for depth perception). The eye is covered by two eyelids, one which remains open when the animal is swimming.
Smell
They have a highly developed sense of smell that helps them acquire prey with the Jacobson’s organ, as well as keep track of fellow individuals in the area by the means of the vemeronasal organ.
Hearing
It is believed that the primary function of the ears in lizards is for maintaining balance.
Physiology[3]
Being ectotherms (“cold-blooded” creatures), Water Monitors have a low basal metabolic rate and rely heavily on anaerobic respiration for their energetic needs, which is suitable because aerobic respiration is not optimal at low temperatures. Having a low metabolic rate also means that their energetic requirements are lower as they do not have to spend energy on maintaining a constant body temperature as in homeotherms (“warm-blooded” creatures). Their heartbeat rates are proportional to their body temperature and increase with increased body temperature.Monitor lizard hearts have efficient blood buffering activity and high blood acidity tolerance to deal with the fluctuation in pH levels due to the high dependence on anaerobic respiration for their energetic needs. A positive feedback exists to increase breathing rate to remove excess carbon dioxide and increase pH levels when blood acidity is high.
Due to the animal’s ability to respire anaerobically (without oxygen)for long periods of time, they are able to spend relatively long periods of time underwater, which allows them to escape from predators or to catch prey.
Varanids have scales and no sweat glands and so reduce body temperature by water loss through the eyes and mouth. In warmer climates, they may even enter the water to cool off.
Behavior
Being cold-blooded (ectotherms), Monitor lizards spend a good deal of their time basking in the sun (Fig 3, Left).They are also known to spend some time on trees although they lack specialized climbing structures. Juveniles spend a lot of their time in
tree hollows (Fig 4, Right) as cannibalism is common in Water Monitors and they are vulnerable while young.
Water Monitors are good swimmers (see link: http://www.youtube.com/watch?v=jlOfo6eeEbA).
Water Monitors are territorial and males engage in fights to establish the pecking order within a certain area (see left: http://www.youtube.com/watch?v=SN3Wh18xzms).
Reproduction
Male have internal testes and a hemipenis. Females have a cloaca through which reproduction and excretion of bodily wastes occur. Clutch size is typically related to female body size, and in V. salvator, the egg mass produced is approximately 23% of the female’s body mass.Feeding habits
Carnivorous and opportunistic, V. salvator eats a wide variety of foods. Diet may consist of small mammals, carrion, other varanids, crustaceans, insects and even human food waste.Conservation Status
Least concern, although it is heavily exploited in the leather trade[1].Introduction to the more technical sections of this article
Below, additional information regarding the specie’s taxonomic status and more detailed information is discussed.Taxonomy is a branch of science that deals with the organizing and naming of plants and animals, as well as the process of it. This system of classifying plants and animals was introduced by Carolus Linnaeus. All species that are formally recognized have been “described” by a person or a few people. For a description of a species to be valid, it must have a unique name including genus name and species epithet, a written description as well as what is known as a “type”. This type may be the specimen used to describe the species, or may be bones, fossils, drawings or photographs. It must be something that can be used to compare with and identify if another specimen is similar to it or not.
The scientific names of species are usually italicized when typed or underlined when handwritten. The name of a species is important as scientific research is tied to the name of a species as a “file name” of sorts and is vital to information storage and retrieval.
Also highly discussed in this article is the issue of subspecies. A species is the smallest recognized taxonomic unit. There are several competing theories on what defines a species, but while this is clearly a problem, I shall not discuss the issues in this article due to space issues). The most popular theory is that of reproductive isolation, and this is the theory most commonly taught in schools. According to this definition, species are groups of organisms that are able to interbreed to produce viable (fertile) young. Subspecies are members of the same species that are able to interbreed, but appear different. Subspecies differ more than races, but less than different species.
Nomenclature[4, 5]
Vernacular: Water Monitor, Common Water Monitor, Monitor LizardBinomial: Varanus salvator (Laurenti, 1768)
This species was first described as Stellio salvator by Laurenti in 1768 with the type locality stated as “America” which is an obvious error because the species cannot be found in the continent. The subspecies that was described is now known as Varanus salvator salvator and is of a variety that can only be found in Sri Lanka, then known as Ceylon. Hence, Mertens designated Ceylon as the terra typica. The nominal subspecies is Varanus salvator salvator.
Synonyms: Stellio salvator Laurenti 1768, Lacerta monitor (Linnaeus 1768), Tupinambis elegans Daudin 1802, Monitor nigricans Cuvier 1829, Monitor exilis Gray 1831, Monitor bivittatus var. celebensis Schlegel 1844, Hydrosaurus salvator Gray 1845, Monitor salvator Blyth 1846, Hydrosaurus salvator Kelaart 1854, Varanus scutigerulus Barbour 1932, Tupinambis bivittatus Kuhl 1820, Lacerta bivittatus Cuvier 1831, Varanus bivittatus Dumeril & Bibron 1836, Monitor bivittatus var. javanica Schlegel 1844, Varanus crocodilinus Owen 1845,
Subspecies: Varanus salvator salvator (Laurenti 1768), Varanus salvator adamanensis Deraniyagala 1944, Varanus salvator bivittatus (Kuhl 1820), Varanus salvator macromaculatus Deraniyagala 1944[1]
Etymology: The Genus name, Varanus has Arabic origins waral or waran, meaning ‘lizard’[6]. The species epithet salvator has Latin origins meaning ‘savior’.
Species previously included: Varanus salvator marmoratus, Varanus salvator cumingi, Varanus salvator nuchalis, Varanus salvator togianus
Classification[4]
Kingdom: AnimaliaPhylum: Chordata
Class: Reptilia
Order: Squamata
Family: Varanidae
Genus: Varanus
Species: Varanus salvator (Laurenti, 1768)
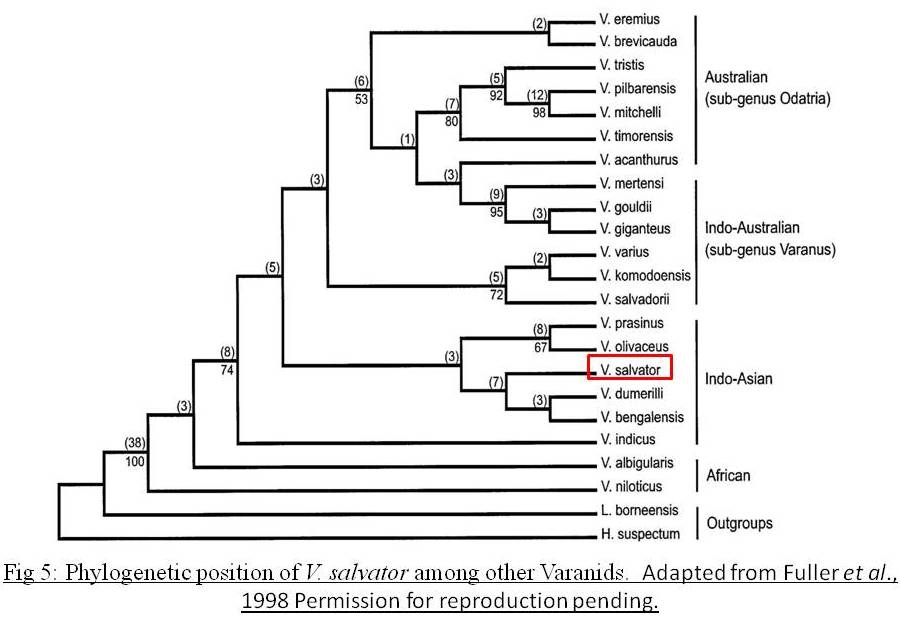 Phylogeny
Phylogeny
According to morphological, chromosomal morphology, MCF tests and sequencing data, the phylogenetic relationship of V. salvator against other varanids is as follows[7]:As seen from Fig 5, Varanus salvator is mostly closely related to Varanus dumerilli and Varanus bengalensis. Although is this the most recent paper presenting the phylogeny of Varanids, this paper was published in 1998. The phylogenetic position is based on a specimen from V. s. macromaculatus; locality Indo-Malaysia. Since then, many species have been elevated to the species level from the V. salvator complex. It is likely that in view of these changes, several species were excluded as they were previously considered under the V. salvator complex.
 Original Description[8]
Original Description[8]
The Description for Stellio salvator was written in a book (Fig 6), page 56, by Josephus Nicolaus Laurenti entitled Specimen medicum, exhibens synopsin reptilium emendatam cum experimentis circa venena et antidota reptilium Austriacorum in 1768 which was published in Vienna in Latin (Fig 6, Left). From what I understand, the description described the animal as black, with white on the underside; feet punctuated and stained; has many ocelli in transverse series on its back, with the sides of its spots toothed. The description included an iconotype- a reference to a drawing byAlbertus Seba’s (1735) “Thesaurus”, Tome II (Fig 7, Left). However, the picture has been lost, so a neotype was selected.
The original description in Latin: “Diag. Atro – fuscus ; rostrum fasciis alternis albis, nigrisve ; pedes punctato – maculati ; dorsum ocellis multiplici serie transversa positis . Latera maculis dentatis. Habitat in America.”
Translation by Google Translate: “Diag. Black - swart; loop alternate white beak, nigrisve; punctato feet - stained; eyes back manifold transverse series the text. With teeth in the sides of the spots. Dwells in America.”
Type information[4]
Neotype: ZFMK 22092, juvenile, collected by B. Schulz, March 1978, donated by B. Schulz, April 1978.Holotype: IM 2176, juvenile [andamanensis]
Lectotype: RMNH 3179 [celebensis, designated by De Lisle 2009] The type belongs to the subspecies Varanus salvator salvator, which is a subspecies endemic to Sri Lanka. The type species was described by Laurenti in 1768 in Sri Lanka
Taxonomic controversy
Although common, many aspects about the species have not been resolved due to their phylogeny. The consequences are such that due to longstanding confusion, some of the information in the past (particularly before 2007) filed under this species name actually belong to taxa that are not included under Varanus salvator.It is said that Varanus salvator is a species complex which comprises of several cryptic species. In previous years, several subspecies have been upgraded to the species level, and much work is still under way to resolve the confusion about its phylogeny.
Although the Water Monitor has been described well over two hundred years ago and their presence greatly felt, the taxonomic status of the species is very uncertain. Upon close inspection it appears that Varanus salvator may actually be a species complex. In the past, may sub-species have been described, which have subsequently been elevated to a species, or species relegated to Varanus salvator. Indeed, numerous shuffling was and is still occurring with regards to the various members of the Varanus genus, which explains the high number of synonyms this species has. Even the statuses of the subspecies are unresolved and have been renamed and regrouped multiple times in the past as well as in the present. All this confusion is amid analyses and comparisons of morphology, biochemistry and even molecular methods[2]. With this, it is advisable to take its unresolved taxonomic status into consideration when reading relevant literature.
Since up till recently (2007 to 2010) much of the literature has lumped numerous subspecies and cryptic species together, it is generally unclear in older literature to which of the distinctly different subspecies the literature refer to. It is possible that some of the information published under the species complex may not apply to all of the subspecies currently included under V. salvator. It is also possible that the research was done on species that were previously considered under V. salvator but are now considered as separate species (marmoratus, nuchalis, cumingi as well as two more from the Palawan region [9]). In view of the species complex and potential future changes in its taxonomic status, where it is possible to narrow down the research subjects to subspecies level, I have presented the information in the appropriate section.
Subspecies
The different subspecies may be recognized and distinguished from each other by the following characteristics:Additional information about the description of each of the Types of the subspecies can be found here: http://www.museumkoenig.de/web/ZFMK_Mitarbeiter/KochAndr/Publikation_neu/Koch_etal_2007_V.//salvator//_Review.pdf
Varanus salvator salvator (Laurenti, 1768)
Distribution
Endemic to Sri Lanka (Fig 8). Nominotypic subspecies.
Physical description
See above section (Table 1; Fig 9).
Behavior
A unique feeding behavior was observed in this subspecies involving tail lashing in a water hole to displace fish for consumption. This behavior may be an adaptation to find food during the dry season when such isolated water holes are common [10].
Synonyms
Lacerta monitor Linnaeus 1758
( Nomen rejectum according to ICZN 1959, Opinion 540), Varanus salvator kabaragoya Deraniyagala 1947
Type Information
Type: Nominotypic subspecies. Neotype: ZFMK 22092; type locality Sri Lanka
Molecular Information
Taxonomy ID: 169831
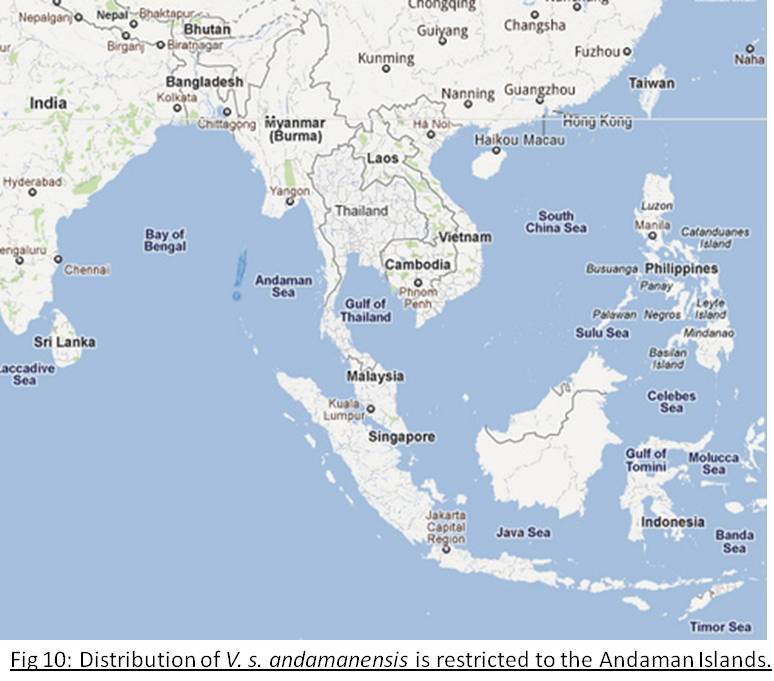 Andaman Islands Water Monitor
Andaman Islands Water Monitor
Varanus salvator andamenensisDistribution and Habitat
Endemic to the Andaman Islands in the Indian Ocean (Fig 10). Found in evergreen rain forests, dried flat wetlands and littoral forests[2].
Physical description
See above section (Table 1; Fig 11).
Physical Dimensions
This form is believed to have a smaller tail length than the nominate. Size of hatchlings: weight 29.5 to 34.0g (mean = 31.4g); Tail length of 30.0 to 33.4cm (mean=30.1cm); SVL 13.4 to 15.0cm (mean=14.2).
Diet
Feeds on sea turtle and crocodile eggs, crustaceans and carrion [2].
Behavior
One recorded incident of it feigning death after caught[2].
Reproduction
Hatchings observed in March and April.
Incubation period of about 300 days under semi-natural conditions[2].
Conservation status
Not hunted for skin. Sustainable hunting by locals [2].
Synonyms
No known synonyms.
Type Information
Holotype: IM 2176, juvenile. Type locality: Port Blair, South Andaman Island, Andaman Islands, Gulf of Bengal;
Malayan Water Monitor/ South-east Asian Water Monitor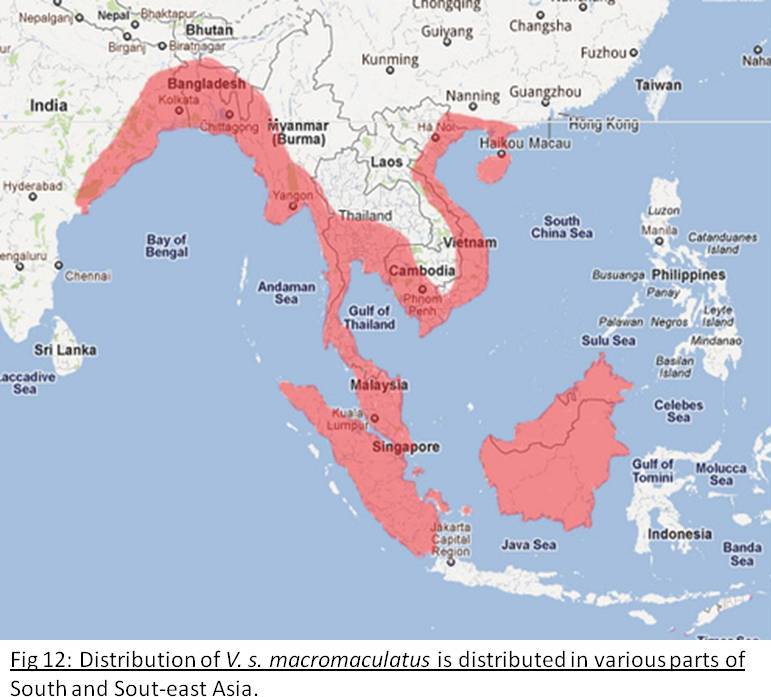
Varanus salvator macromaculatus Deraniyagala, 1944Distribution and Habitat
This subspecies has the widest distribution of all the V. salvator subspecies.[1]. Ranges from East India, South China to Indonesia (Fig 12). Individuals are generally found within 200 meters of a permanent water source except during the rainy season, but mostly following a coastal distribution. Although V.S. macromaculatus has been observed to swim in the open sea, it is not found in large rivers [11].
Physical description
See above section (Table 1; Fig 13).
Physical Dimensions
Sizes reach up to 2.5m, 20kg. Males are larger than females with longer tails than females. Males mature at about 40cm and females at about 50cm[12].
Regional Variation
All information presented[11] ( Source from Cota et al. 2009 unless otherwise stated)are specific to Thailand only. No information is available for variations in other countries.
Variant 1: A completely melanistic form (komaini) has been observed in the 1980s and named “Black Color Morph[2]” or the “Black Dragon”. This version is called V.s. macromaculatus var. komaini or previously known as V.s. komaini. They are found in the mangrove forests of the La-Ngu District of Satun Province and associated offshore islands. Members of the population are completely melanistic, including their tongue[2] and pattern less since hatching. Hemipeneal analysis with the colored variant showed identical morphology[2].
Variant 2: Found in southern peninsular Thailand, similar to those observed in Malaysia and Singapore. Has an overall grey coloration with subdued pattern, which is more vibrant in juveniles.
Variant 3: Found in the mangrove forests of Thailand. Dark colored and subdued patterns to the extent that it is hardly visible. Overlapping range with the common variant but, this variant can only be found in or near mangroves.
Variant 4: Found in Eastern Thailand. Similar to the common form but with smaller ocelli.
Variant 5: Found in the Sanburi Province in an arid environment. Bright yellow or white spots in place of ocelli.
Diet
Carnivore, hunts for crustaceans, small mammals and other varanids[12]. Largely known as a scavenger[13].
Conservation status
Varanus salvator is in the region, particularly in Indonesia, subjected to immense hunting pressure due to international demand for lizard skin. Comparatively, local demands for lizard skin or flesh for medicinal or ceremonial purposes play little impact on the population[12]. However, the species has proved to be resilient due to its ability to adapt to human presence, high reproductive rate, avoidance of hunting of large individuals[13] and even it forming a commensal relationship with humans due to their indiscriminate eating habits[13].
Cultural Issues
In Thailand, the species face great discrimination due to the local belief that it is the “lowest and dirtiest” animal. Due to this cultural belief, it has not been extensively studied in the country until recently[11].
Synonyms
Tupinambis elegans part. Daudin 1802, Tupinambis exilis Gray 1831(Nomen dubium), Varanus vittatus Lesson 1834 (Nomen dubium),Varanus binotatus Blyth 1842 (Lapsus according to Mertens, 1942), Lacertus tupinambis part. Mertens 1942 (non Lacépède, 1788), Varanus salvator nicobariensis Deraniyagala, 1947 (Synonym according to Mertens), Varanus salvator komaini Nutphand 1987(Synonym)
Type Information
Lectotype: MNHN 871 Type locality: “Siam” = Thailand
Molecular Information
Taxonomy ID: 735375 (Varanus salvator komaini or Varanus salvator marmaculatus var. komaini)
Two-banded Monitor Lizard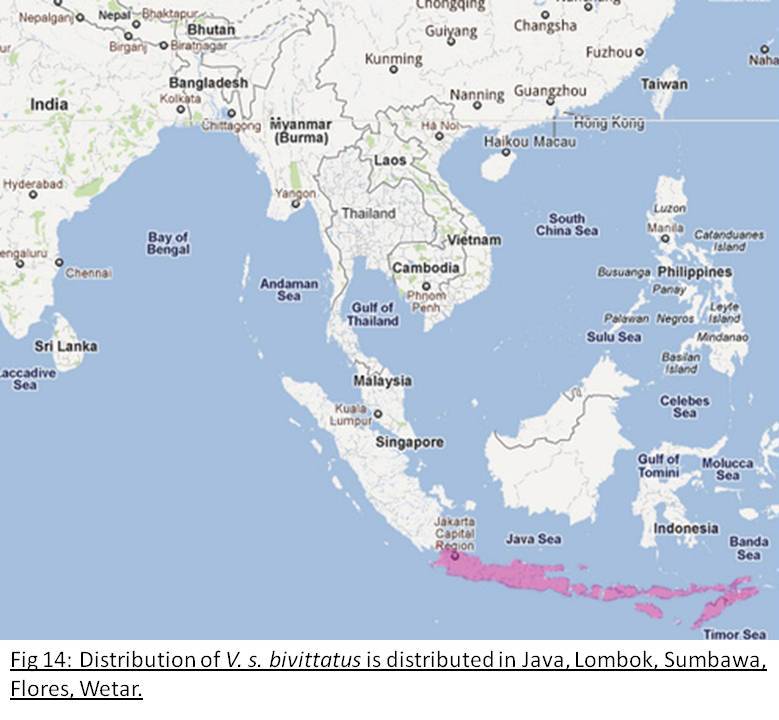
Varanus salvator bivittatusDistribution and Habitat
Found in Java, Lombok, Sumbawa, Flores, Wetar. Inhabits evergreen rain forests[2](Fig 14).
Physical description
See above section (Table 1; Fig 15).
Physical Dimensions
Similar to nominate form.
Diet
Feeds on carcasses, Eggs and fledglings of birds, crustaceans and sea turtles[2].
Reproduction
In captivity, clutch size observed to be 6 (4 infertile) with an incubation period of 205 to 241 days. Hatchlings weigh 31 to 40g[2].
Conservation status
Heavily hunted for the leather trade[2].
Synonyms
Tupinambis bivittatus Kuhl 1820; Monitor nigricans Cunier 1829; Monitor bivittatus var. javanica Schlegel 1844; Varanus crocodilinus Owen 1845; Varanus salvator salvator part. Mertens 1942
Type Information
Iconotype: Albertus Seba’s (1735) “Thesaurus”, Tome II.
References
1. Bennett, D., et al. Varanus salvator. IUCN 2011. IUCN Red List of Threatened Species. [Web Site] 2009 January 2011 28 November 2011]; Available from: http://www.iucnredlist.org/apps/redlist/details/178214/0/print.2. Pianka, E.R., D.R. King, and R.A. King, Varanoids Lizards of the World. 2004, Bloomington: Indiana University Press.
3. De Lisle, H.F., The Natural History of Monitor Lizards. 1996, Malabar, Florida: Krieger Publishing Company.
4. Peter Uetz, J.H., Varanus salvator (Laurenti, 1768).
5. Koch, A., et al., Morphological Studies on the Systematics of South East Asian Water Monitors (Varanus salvator Complex): Nominotypic Populations and Taxonomic Overview. Advances in Monitor Research III, 2007: p. 109-180.
6. Mikaili, P. and J. Shayeg, An Etymological Review of the Lizards of Iran: Families Lacertidae, Scincidae, Uromastycidae, Varanidae. International Journal of Animal and Veterinary Advances, 2011. 3(5): p. 322-329.
7. Fuller, S., P. Baverstock, and D. King, Biogeographic origins of goannas (Varanidae): A molecular perspective. Molecular Phylogenetics and Evolution, 1998. 9(2): p. 294-307.
8. Laurenti, J.N. Josephi Nicolai Laurenti ... Specimen medicum, exhibens synopsin reptilium emendatam cum experimentis circa venena et antidota reptilium Austriacorum. 1768; Available from: http://www.biodiversitylibrary.org/bibliography/5108.
9. Koch, A., M. Gaulke, and W. Bohme, Unravelling the underestimated diversity of Philippine water monitor lizards (Squamata: Varanus salvator complex), with the description of two new species and a new subspecies. Zootaxa, 2010(2446): p. 1-54.
10. Mendia Wickramasinghe, L.J., et al., A Remarkable Feeding Behavior and a New Distribution Record of Varanus salvator salvator (Laurenti, 1768) in Eastern Sri Lanka. Biawak, 2010. 4(3): p. 93-98.
11. Cota, M., T. Chan-ard, and S. Makchai, Geographical Distribution and Regional Variation of Varanus salvator macromaculatus in Thailand. Biawak, 2009. 3(4): p. 134-143.
12. Shine, R., et al., Commercial harvesting of giant lizards: The biology of water monitors Varanus salvator in southern Sumatra. Biological Conservation, 1996. 77(2-3): p. 125-134.
13. Uyeda, L., Garbage Appeal: Relative Abundance of Water Monitor Lizards (Varanus salvator) Correlates with Presence of Human Food Leftovers on Tinjil Island, Indonesia. Biawak, 2009. 3(1): p. 9-17.
Any feedback regarding this page can be sent to Crystal Wong at U0805282@nus.edu.sg