-Genesis 1, The Bible
Table of Contents
Video showing Volvox dancing in a water solution. Adapted from Youtube
Etymology and General Description
Volvox carteri is a species of multicellular green flagellated algae, and was originally described by F. Stain in 1873. Volvox is a group of multicellular green flagellated algae and was originally described by Linnaeus (1758), with the type species V. globator (Ehrenberg 1838, Farr and Zijlstra 2012). Its original discovery dates back to 1700s when Antonie van Leeuwenhoek, inventor of light microscope, first reported observations of these dancing creatures. The word “volvox” originates from Latin “volvere” which means to roll. As its name suggests, individual colonies freely rotates and moves toward sunlight through the synchronized movement of flagella. The tiny little creatures that are rolling peacefully in the water tank shown in the video above are Volvox cateri (F. Stein, 1873), dramatis personae of this web page.
Morphological Notes
Cellular Structure
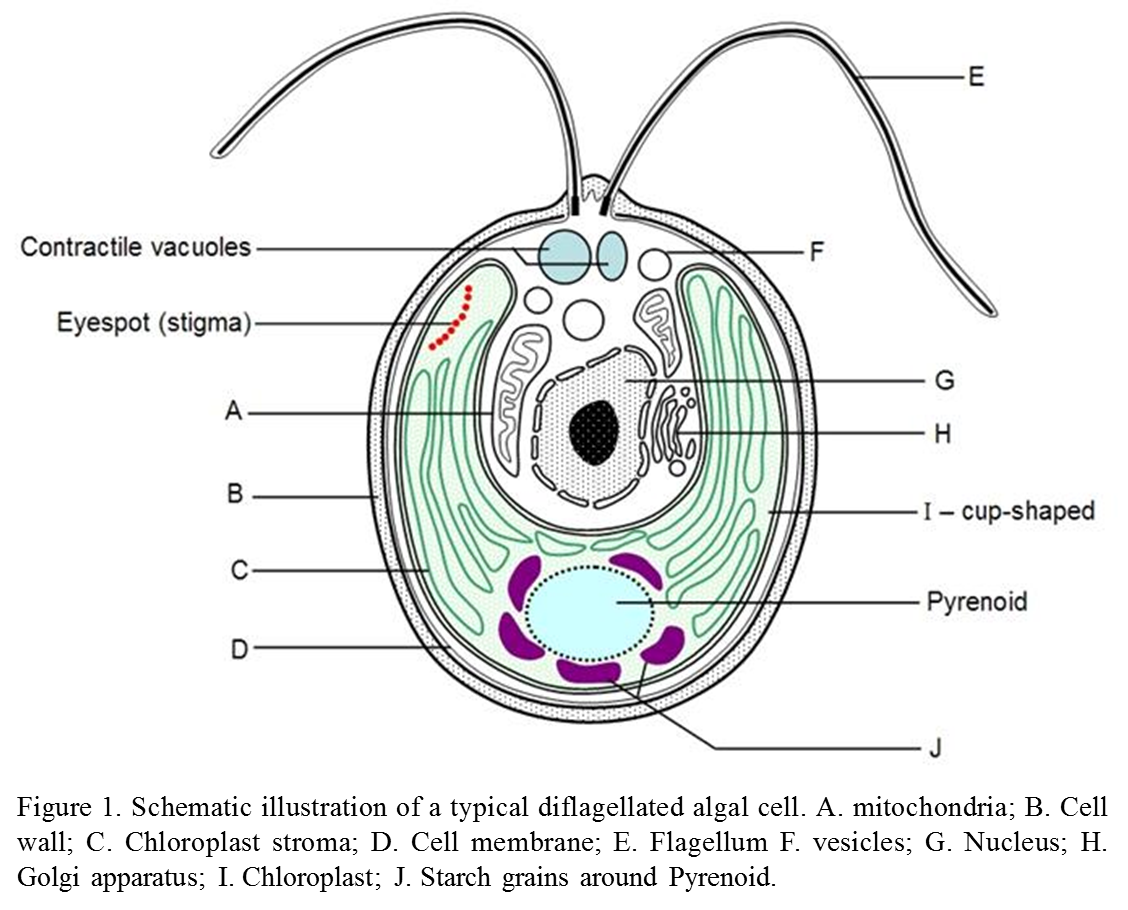
Individual cells of Volvox carteri has the general morphological characters and subcellular organisation of an algal cell (Figure 1).Typically the cell contains two flagellates which help to move the cell colony around in the solution. Morphology of the chloroplast (the factory of photosynthesis) is an important diagnostic tool for algae identification. Inside the chloroplast, a major pyrenoid (an important cell organ in the process of carbon fixation) and several satellite pyrenoids are usually present. Contractile vacuoles are organs that regulates osmolarity, the location and number of which are also a diagnosable characters for species identification.
Individual cells are organised in a spherical hollow colony engulfed in gelatinous shell. As Volvox carteri can reproduce both sexually and asexually, two types colonies are present in nature---vegetative asexual colony and sexual reproductive colony.
Vegetative Colony
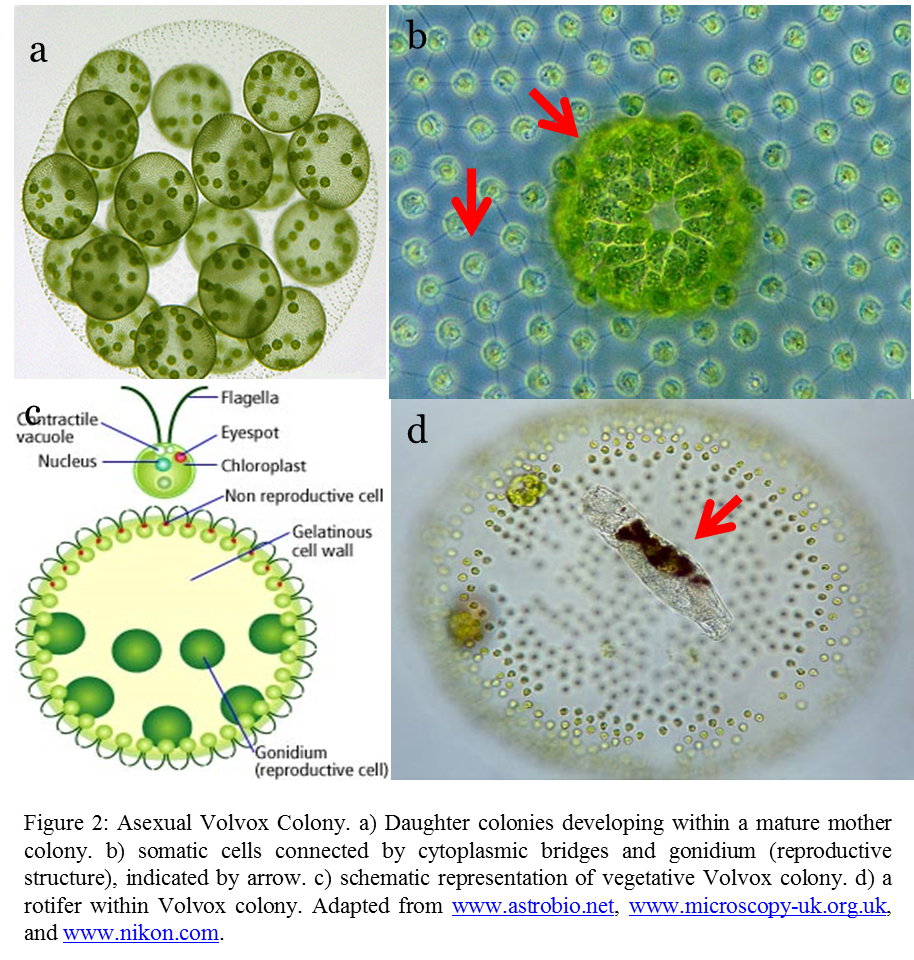
Mature Volvox colony is composed of numerous flagellated algal cells (Figure 2a), up to 50,000 in total. There are several types of cells in the colony. The asexual colony is mainly made up of somatic cells which are small and confer the ability to swim with distinct anterior and posterior poles. Volvox are phototactic (the ability to move toward light).Eyespots of the anterior cells are more developed, hence allowing them to swim towards light.
Larger cells involved in reproduction called gonidia are also present inside the colony(Figure 2b), typically 8 to 16 of them (Nozaki and Coleman, 2011).Usually, one could observed daughter colonies developing inside the mother colony(Figure 2a).
Inhabitants within the colony are quite common. A small rotifer, called Proales parasita lives inside Volvox and feeds on the cells. The rotifer is able to detect when the colony becomes immobile and quickly escape.
Sexual Colony
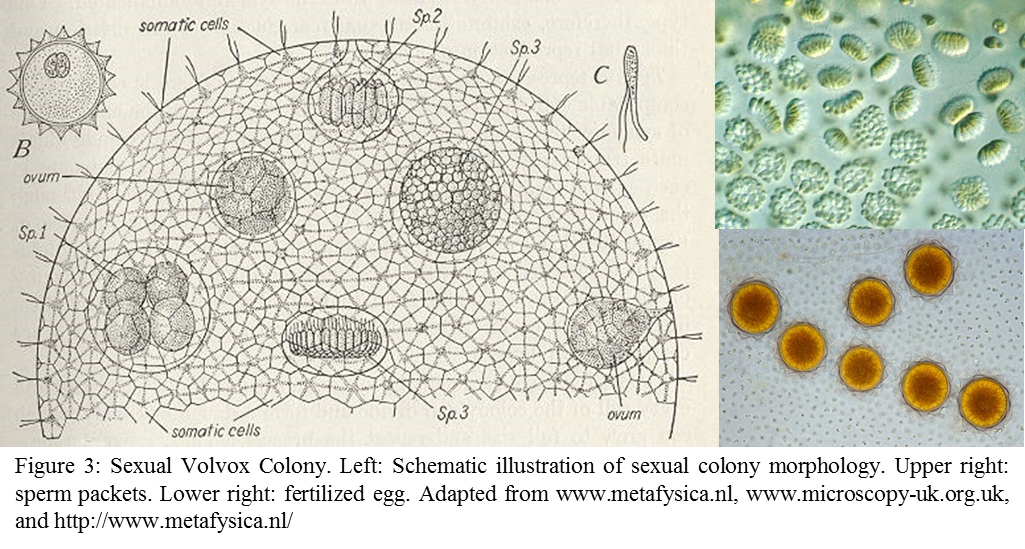
In sexual colonies, sperm packs and female ovum could be observed on the colony (Figure 3). Some Volvox species are monoecious (producing both male and female reproductive organs) others are diecious (produce either male or female reproductive organ). Fertilized eggs have a typical spherical shape with spikes facing outward (Figure 3).Journey Toward Multicellularity
Volvox carteri is among the most simplest multicellular organisms. Volvox and related organisms are usually used as model organisms to study the evolutionary path of multicellularity. To understand this process which took place at least 200 million years ago (Herron et al., 2009), we need to have a good knowledge of the morphological characteristics in terms of cellular organisation of Volvox and related organisms and their evolution relationship.
Taxonomy and Phylogeny
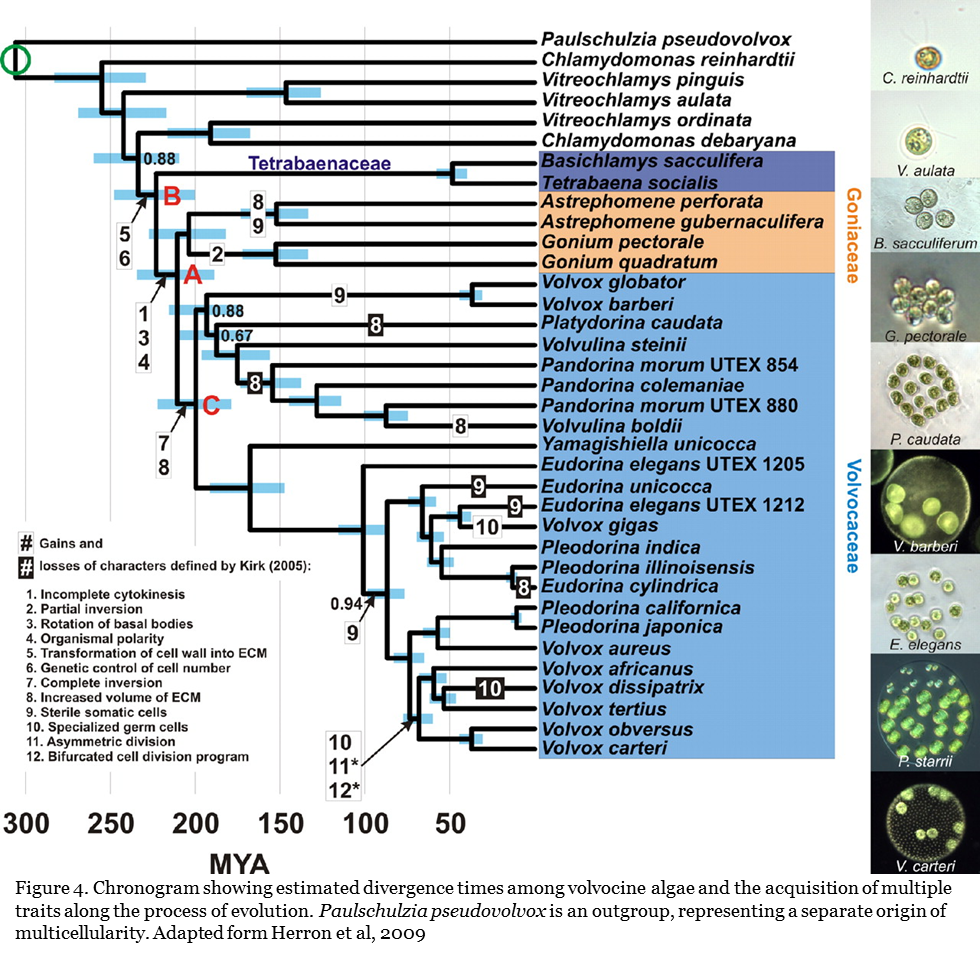
The group Volvox includes species of multiple evolutionary origins. Volvox and its relative taxa show similarity in cellular structure and morphology. These taxa includes Clamydomonas which are single cellular organisms, Tetrabaena which contain four cells, Gonium which contain eight cells, and Eudorina which contain 16 to 32 cells in a single colony. Related taxon such as Pleodorina contain multiple cells and exhibit cellular differentiation. Together they form an evolution time clock revealing the journey from a single algae cell to highly complex cell colonies as in Volvox.
Molecular phylogenetic analysis has shown that Volvox are paraphyletic (Larson et al. 1992). The evolution of multicellularity has occurred multiple times independently. Different species of Volvox have evolved from ancestors of Platydorina, Pleodorina, and Eudorina respectively.
Multicellular Evolution
The transition from unicellular to multicellular life form is the paradigm case of the integration of lower-level individuals (cells) into a new higher-level individual—the multicellular organism. This transition has occurred multiple times independently in algae, land plants, and animals (Bonner, 1998) ). The origin of multicellularity in the green alga Volvox and its relatives (the volvocine algae) is one of the best studied evolution model for developmental genetics of cellular differentiation and multicellular organization regulation. (Kirk, 2001and Prochnik et al, 2010). Members of Volvocacine algae span a range of sizes and levels of complexity from unicells to macroscopic multicellular organisms with cellular differentiation (Figure 4). This offers the opportunity for scientists to have a close examination of the several intermediate stages of multicellular development (gradations that have been fortunately preserved in related taxa through natural selection)—from single cells to fully differentiated multicellular individuals such as Volvox.
Based on molecular phylogenetic analysis and the developmental basis of V. carteri, scientists have reconstructed the evolutionary history of this group of algae (Kirk, 2005 and Herron et al, 2009). It was found that ancestors of Volvox transitioned from single cells to form multicellular colonies at least 200 million years ago, during the Triassic period (Herron et al., 2009). An estimate using DNA sequences from about 45 different species of Volvox and related species suggests that the transition from single cells to undifferentiated multicellular colonies took about 35 million years (Herron et al., 2009).
Evolution of Male and Female Sexuality
 |
| “The Creation of Adam” by Michelangelo. God imparts life to Adam through the tip of his finger. © 2009 Bridgeman Art Library/PPS |
Reproduction and Life Cycle
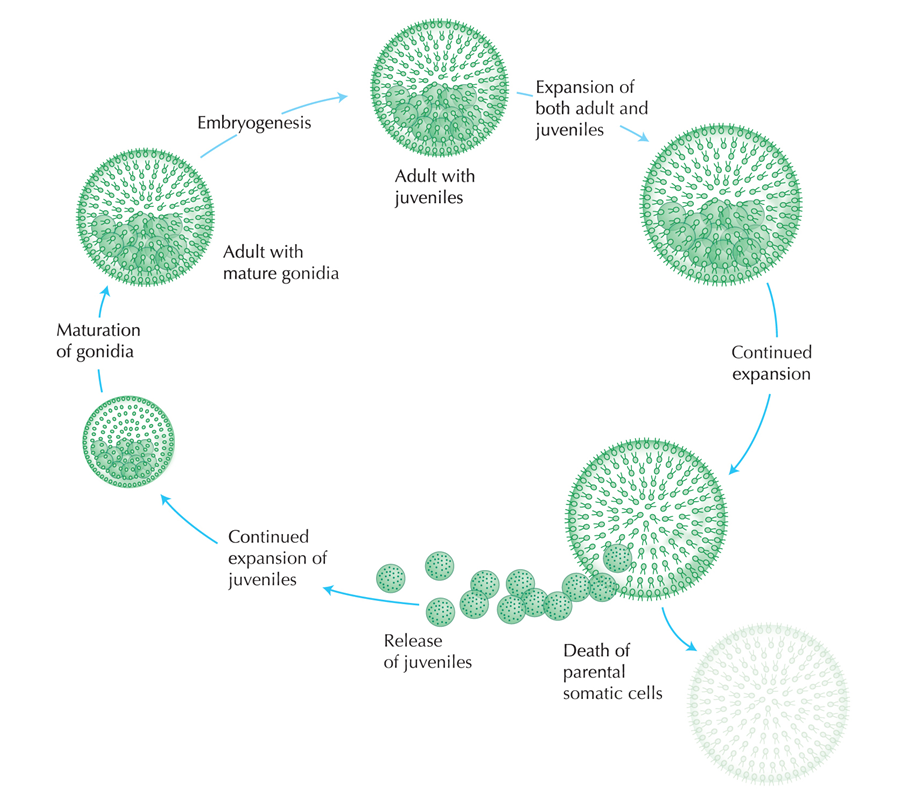 |
| Asexual cycle of Volvox carteri. Copyright: Cold Spring Harbor Laboratory |
Asexual Reproduction
Volvox carteri can reproduce both sexually and asexually. During asexual reproduction, individual gonidium divides through mitosis and develop into a daughter colony within the mother colony. The daughter colonies have flagella facing inward originally and eventually facing out through a process called inversion. Following which, the daughter colonies are freed from constraints of the mother colony upon colony breakage. (Click to see video on Volvox asexual reproduction)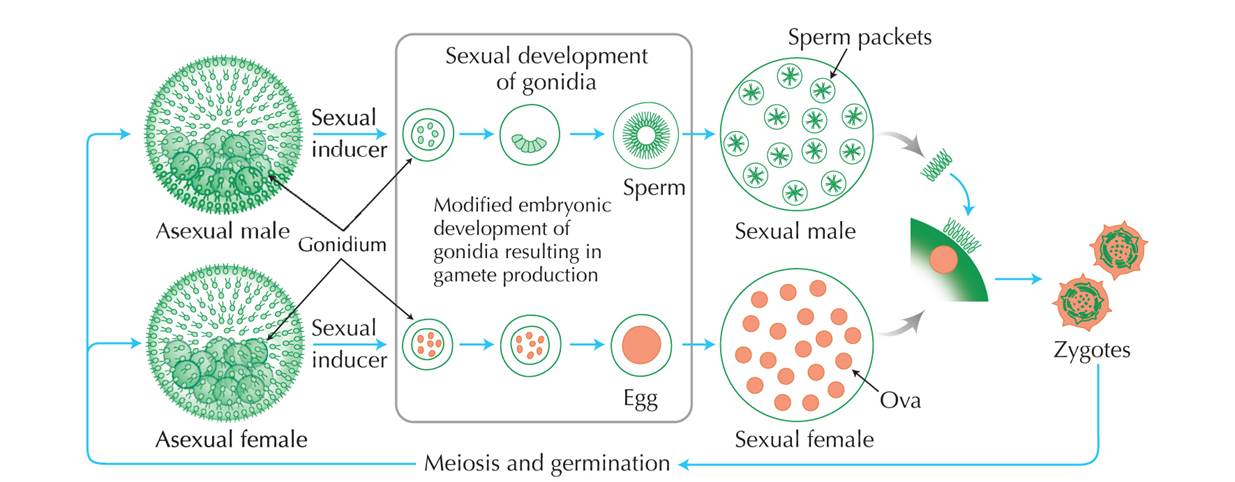
Sexual cycle of Volvox carteri. Copyright: Cold Spring Harbor Laboratory

Sexual Reproduction
During sexual production, the sperm packets dissociates from the male colonies and actively find female colonies. The sperms penetrates the extracellular matrix of female colonies and fertilize the eggs located in the oogonia. After fertilization, a zygote with a hard protective layer is formed which helps to withstand harsh conditions and survive through the winter. (Click to see video on Volvox asexual reproduction)The Male Gene
The evolutionary path from single-celled Chlamydomonas to Volvox is clear (as described in the previous sections). In this group, all members reproduce sexually. However, in simpler members such as Chlamydomonas, reproduction is isogamous (identical gametes--reproductive cells--are formed). In more complex colonial algae Eudorina, reproduction is anisogamous (producing distinct gametes differing in size alone). While in Pleodorina and Volvox, reproduction is oogamous (producing sperm and egg--female gamete that significantly larger than the male gamete and is non-motile; Figure 5).
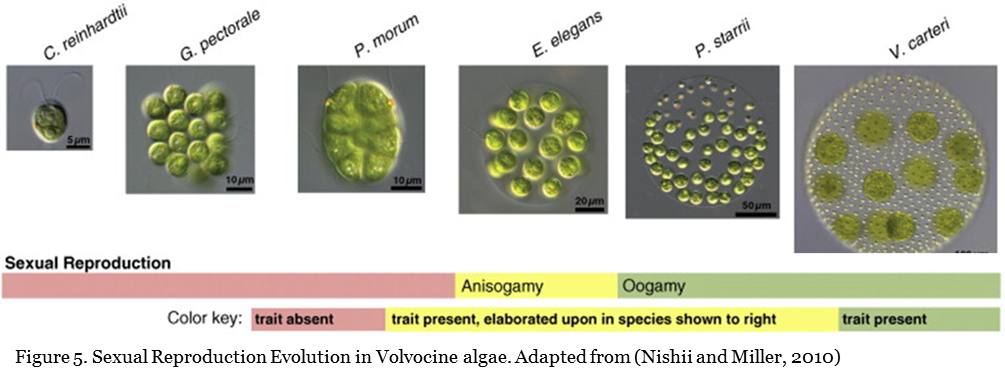
In Chlamydomonas, male and female cannot be differentiated, but in Pleodorina and Volvox, male and female are distinguishable. These closely related organisms with similar genetic configurations are suitable subjects for the study of the differentiation between male and female. By studying the differences between organisms in which male and female have the same appearance and organisms in which the male and female are distinct, scientists have discovered genes that determine male and female. (Refer to Figure 6 for differences in sexual reproduction in Chlamydomonas and Volvox).
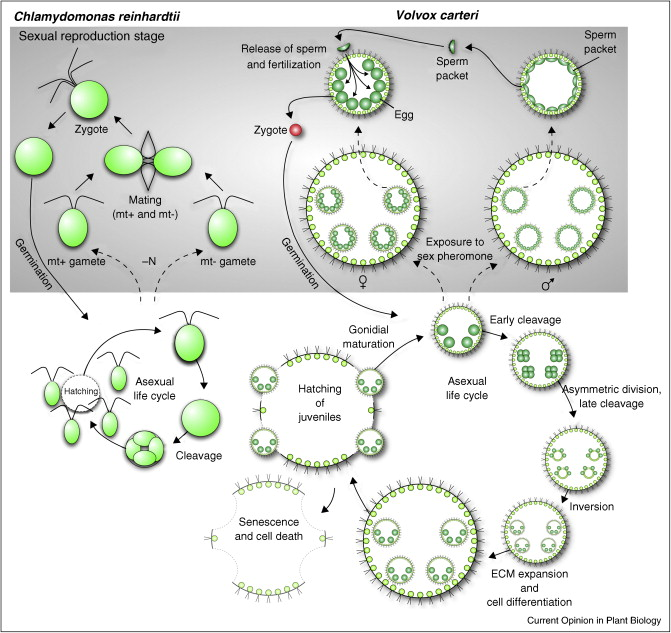 |
| Diagram comparing life cycle of Chlamydomonas and Volvox. Copyright: Current Opinion in Plant Biology |
In isogamous Chlamydomonas, gametes are of identical shape. For descriptive purposes, one is termed the “plus” and the other, which joins with the plus gamete to produce offspring, is termed the “minus”. A sex-determining gene MID (minus dominance), which is only present in the minus gamete, was discovered by Ferris and Goodenough (1997). However at that time is was not know whether male or female gene is evolved from MID like gene.
Later in 2008, a group in Japan discovered the male gene which they called “otokogi”—denoting an inclination towards helping the weak (Nozaki, 2008). This gene was known to operate within the nucleus of Pleodorina sperm and to be characteristic of maleness. This represented the discovery of the gene that undoubtedly determines the maleness of an organism. Molecular phylogenetic analysis show that the otokogi gene and the MID gene evolved from a common progenitor. The results support the view that isogamous minus gamete evolved into male and the plus gamete into female.
Previous studies have shown that in isogamous Chlamydomonas, loss of function mutation of MID gene in a minus gamete converted it into a plus gamete (Ferris and Goodenough, 1997). However, it is impossible to change a plus gamete to a minus gamete. The manipulation of genes can change a male (minus) to a female (plus), but not a female to a male, suggesting that the “original sex” is female, from which male derives. Subsequently, in 2008, using species related to Volvox, a German team discovered the female-specific gene (Hallmann, 2009). The journey of understanding sex determination has just started.
Habitats and Occurrence in Singapore
Volvox are freshwater algae and are usually found in freshwater swamp, marsh, ponds forest stream, ditches and even shallow puddles. In Singapore, they could be found in Changi, Pulau Ubin, East Coast Park, Botanic Gardens etc (Wee, 1987).
Ecological Value
The propagation of Volvox depends on healthy freshwater environment with a certain pH, temperature, salinity and nutritional content. Pollutions of freshwater system may cause Volvox population fluctuation due to changes of the physical parameters, making it possible to use Volvox as planktonic indicators for freshwater quality assessment (Thakur et al. 2013).
Reference
Bonner, J. T. (1998). The origins of multicellularity. Integrative Biology: Issues, News, and Reviews, 1(1), 27-36.
Desnitskii, A. G. (2009). [Volvox (Chlorophyta, Volvocales) as a model organism in developmental biology]. Ontogenez, 40(4), 301-304.
Ferris, P. J. and Goodenough, U. W., 1997. Mating Type in Chlamydomonas Is Specified by mid, the Minus-Dominance Gene. Genetics. 1997 July; 146(3): 859–869.
Hallmann, A. (2009). Retinoblastoma-related proteins in lower eukaryotes. Commun. Integr. Biol. 2, 538-544.
Herron, M. D., Hackett, J. D., Aylward, F. O., & Michod, R. E. (2009). Triassic origin and early radiation of multicellular volvocine algae. Proceedings of the
National Academy of Sciences, 106(9), 3254-3258.
Jiang, J.-G. (2006). Development of a new biotic index to assess freshwater pollution. Environmental Pollution, 139(2), 306-317.
Kirk, D. L. (2001). Germ–Soma Differentiation in Volvox. Developmental Biology, 238(2), 213-223.
Larson, A., Kirk, M. M., & Kirk, D. L. (1992). Molecular phylogeny of the volvocine flagellates. Mol Biol Evol, 9(1), 85-105.
Nishii, I., & Miller, S. M. (2010). Volvox: Simple steps to developmental complexity? Current Opinion in Plant Biology, 13(6), 646-653.
Nozaki, H. (2008). A new male-specific gene “OTOKOGI” in Pleodorina starrii (Volvocaceae, Chlorophyta) unveils the origin of male and female. Biologia, 63(6), 772-777.
Prochnik, S. E., Umen, J., Nedelcu, A. M., Hallmann, A., Miller, S. M., Nishii, I., Rokhsar, D. S. (2010). Genomic Analysis of Organismal Complexity in the
Multicellular Green Alga Volvox carteri. Science, 329(5988), 223-226.
Schmitt, R. (2003). Differentiation of germinal and somatic cells in Volvox carteri. Curr Opin Microbiol, 6(6), 608-613.
Thakur, R. K., Jindal, R., Singh, U., & Ahluwalia, A. S. (2013). Plankton diversity and water quality assessment of three freshwater lakes of Mandi (Himachal Pradesh, India) with special reference to planktonic indicators. Environmental Monitoring and Assessment, 185(10), 8355-8373.