(Skuse, 1894)
 |
| Fig. 1: Aedes albopictus (Skuse 1894), Female. Image by: Theodore Lee |
Table of Contents
Overview
Aedes albopictus is a mosquito (Culicidae) characterized by its stark black and white colouration. Also known as the forest mosquito or the tiger mosquito, it is so named for the black and white stripes on its body and legs, similar to a tiger.
This mosquito species is famous in Singapore for being the dreaded "dengue mosquito". There are many campaigns to limit the spread of dengue, many of which focus on vector control (i.e. limiting the spread of the tiger mosquito). Some of these campaigns include the 5-Step Mozzie Wipeout, as well as providing information about dengue clusters in Singapore or simply increasing awareness of how dengue spreads (via Aedes mosquitoes) and how to recognise the mosquito vector. More information is provided in the section "Campaigns against dengue"
There are a few closely related species that look superficially similar, however; care should be taken to ensure that species are properly distinguished when examining specimens. For example, Figures 2 and 3 show Aedes albopictus and Aedes aegypti respectively. Both mosquitoes look superficially similar without further inspection.
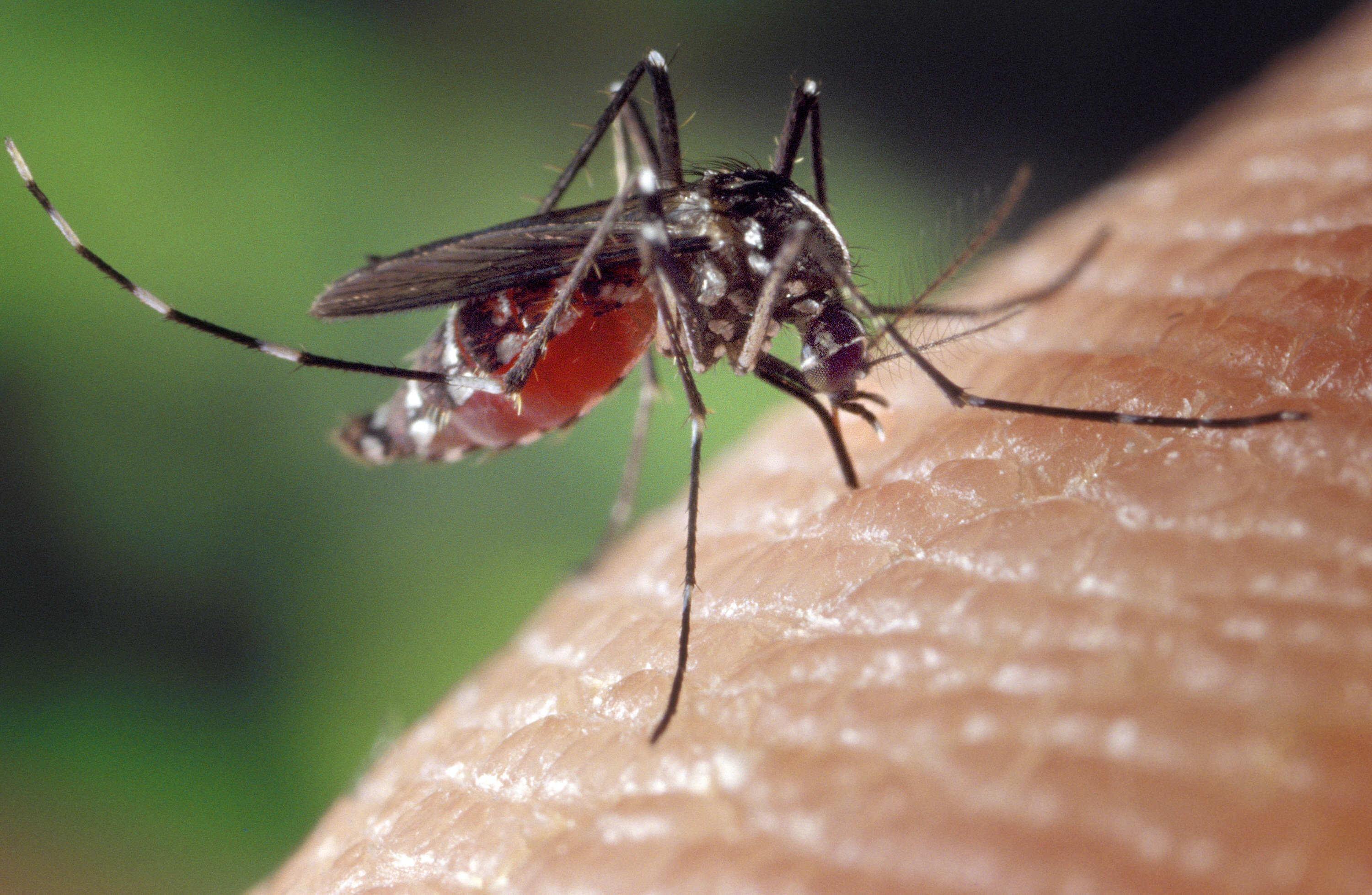 |
| Fig. 2: Aedes albopictus on human skin. Source: James Gathany, CDC. Public domain image |
 |
| Fig. 3: Aedes aegypti on human skin. Source: James Gathany, CDC. Public domain image |
Native to Asia, this mosquito has spread rapidly all over the world in the past few decades (1). The spread was assisted mainly by increased transport of goods across the globe (1), although some evidence exists that it may outcompete native mosquitoes (2) (3) (4).
Mosquitoes are well known for the itchiness that results after they bite; in this regard Ae. albopictus generally bites (and flies) in the daytime, as well as dusk and dawn. This is a nuisance, except that the Asian tiger mosquito is a vector for many pathogens. These include the viruses causing dengue, chikungunya and yellow fever, as well as the filarial nematodes responsible for heartworm (5).
Name and description
In 1894, Frederick A. Askew Skuse scientifically described this mosquito and named it Culex albopictus (latin for "white painted") (Figure 4). It was later reassigned to the genus Aedes in 1920 (6), although recent phylogenetic analyses may result in reclassification as Stegomyia albopicta (7) (8). Systematicists continue to debate the reclassification.
 |
| Fig. 4: The original description of Aedes albopictus, (first named as Culex albopictus) by Skuse in 1894). The banded mosquito of Bengal. Indian Museum Notes 3(5):20. Public domain image |
Distribution
Locality
Aedes albopictus was first described in Bengal, although it was accepted to be present in the general South-East Asian area as well. A highly invasive species, Ae. albopictus has since spread almost worldwide. (9) This was facilitated at least in part by the used tire trade; as a floodwater species, rainwater collecting in the tires provide prime breeding grounds, and eggs laid on tires could easily escape notice (10) (11). Climate change is also predicted to further increase the ability of Ae. albopictus to invade new areas (12) (13).
Habitat
Ae. albopictus can be found in a wide range of habitats. While it is commonly known as a forest mosquito, Ae. albopictus has adapted to occurring in suburban and even dense, urban areas like Rome (14) (15). In fact, compared to rural and suburban areas, urban locations had shorter larval development time, increased density of larval habitats (16). Mosquito adults in urban areas also had a longer lifespan as well as higher emergence rates (16). In a Singaporean context, mosquito breeding sites were mostly artificial, with one study finding 96% of breeding habitats being "domestic containers" (17).
Biology
Diet
Mosquitoes are famous for biting and feeding on human blood. Most mosquito species are obligatory blood feeders, although in these species it is the female who is responsible for seeking a host to feed off (18). Males are generally thought to feed on nectar or plant juices and water before mating and dying.
Ae. albopictus females require a blood meal for eggs to develop. Thus, females have to seek out a viable host to bite. Although blood contains various chemicals and substances, protein is the most important substance in the blood for the mosquito. Ae. aegypti, a closely related species, has been known to survive and successfully breed under laboratory conditions when fed a substitute blood meal consisting of a mixture of albumin proteins, γ-globulins (to induce egg development), hemoglobin (as a visual marker of feeding), ATP and various salts (to induce gorging) (19).
Ae. albopictus is highly anthropophilic; given equal availability of five hosts (calf, dog, chicken, goat and human), females still preferred human blood (20).
Larvae feed on detritus in water bodies; small pieces of algae or leaf litter.
Locating hosts
Females display non-specific searching behaviour until host specific substances are detected, such as carbon dioxide (21), octenol and L-lactic acid (22). The mosquito then targets the host more specifcally, using the host specific cues to locate its next blood meal.
Feeding behaviour
Ae. albopictus is primarily active in the daytime, with peak activity around dusk and dawn (23). Females are mainly exophilic (rest outdoors) and exophagic (feed outdoors) (20). In regions with seasonal changes, Ae. albopictus displays significant seasonal variances in bite rates (20) (23) (24).
Mosquitoes feed by piercing the skin of the host, probing for a suitable capillary and extracting blood from it. The mosquito starts by pushing its proboscis into the skin, anchoring the tip of the feeding fascicle (structures in the proboscis used to feed). Two microsawtoothed maxillas are next used to saw deeper into the skin, with the frequency of oscillations of the maxillas decreasing as depth of penetration decreased (25). This process is usually complete within 10-20 seconds, with the mosquito drawing blood for about 2-3 minutes under laboratory conditions (25). While mosquito saliva is known as the itch-causing agent, it actually plays a few roles in assisting mosquitoes in getting a successful blood meal. Mosquito saliva contains various anticouagulants (26), apyrases and α-glucosidase (27). Mosquito saliva also acts as a way to locate blood vessels; compounds in saliva may promote haematoma formation, and increased amounts of blood in that area may lead to increased blood feeding success (28).
Video 1 shows the probing action of Anopheles gambiae, as it searched for a suitable capillary under the skin to feed from.
Vid. 1: Video showing Anopheles gambiae probing and feeding from a capillary.Obtained from YouTube under fair use. Credit: Choumet et al, PLOS 2012
Ae. albopictus larvae feed on organic detritus, and play a part in leaf litter breakdown and decay (29) (30). They feed by gathering detritus into their mouthparts (Video 2). Ae. albopictus larvae are classified as "shredders" and "collector-gatherers" (31). Pupae do not feed.
Vid. 2: Video showing Ae. albopictus larvae feeding on organic detritus.
Obtained from YouTube under fair use. Credit: Sean McCann
Mating Behaviour and Oviposition
Newly emerged Ae. albopictus mosquitoes are ready to mate after 24 hours (32). Prior to mating, the male chases the female, from behind, lateral or even from the front. Females reject males by kicking or simply flying away. If genital contact is allowed, copulation occurs (33).
If mating is successful (usually if copulation lasts longer than 30s) (34), sperm will be transferred from the male to the female. Sperm is stored in the spermathecae of the female (32); eggs are only fertilized as they are laid (35). Dissection of the spermathecae can be performed to investigate if successful sperm transfer has occured; if so, spermatozoa can be seen in the spermathecae (Figure 5).
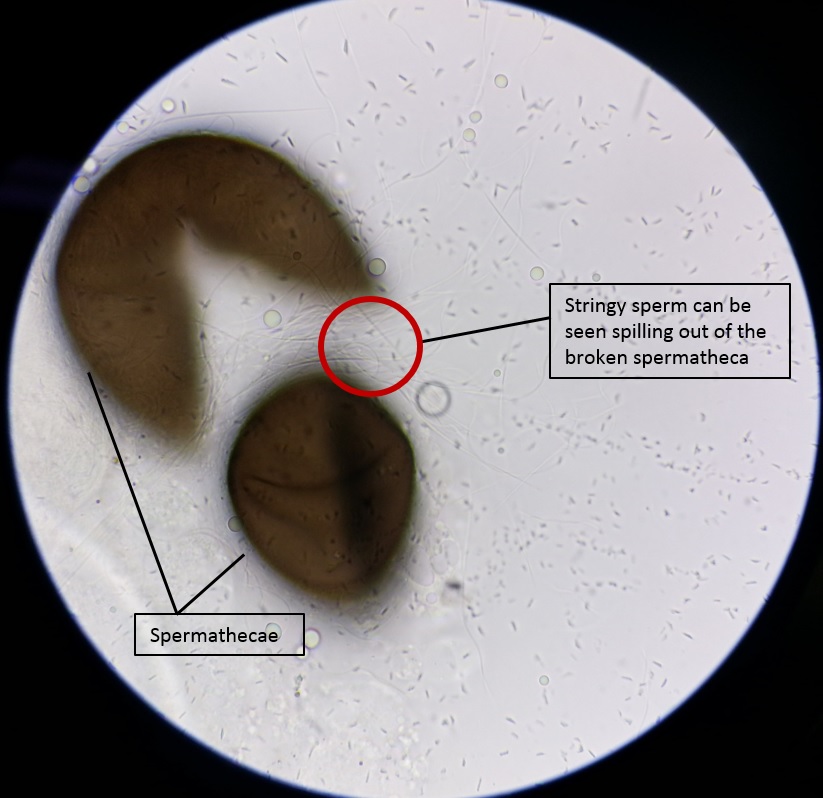 |
| Fig. 5: Long, string-like sperm seen spilling from the broken spermatheca. Image by: Theodore Lee |
Ae. albopictus is a floodwater species; females lay eggs just above the waterline. These eggs, when first laid, are white, but slowly turn black as they dry and harden (Figure 6 & 7). Eggs laid this way can be viable for almost 6 months (36). The eggs stay dormant in this state until the water level rises,usually due to rains or floodwaters covering the eggs. Low oxygen levels are a hatching stimulus for the eggs (35) (36) (37). In some temperate countries, combinations of short light periods and low temperatures may result in Ae. albopictus females laying diapausing eggs. These eggs are able to survive winters and stay viable until spring (38).
Development
The entire life cycle of Ae. albopictus potentially takes as little as 7-9 days: while development is dependent on temperature, larvae usually pupate in about 5-10 days, and the pupal stage lasts about 2 days. The lifespan for adults is about 3 weeks.
About 150 to 250 eggs may be laid per oviposition, with about 1-4 ovipositions for each female. The eggs do not all hatch at the same time; Ae. albopictus eggs display scattered, or installment hatching (36). This phenomenon in Aedes eggs was first described by Gillett in 1955 (39), and it was subsequently shown that bacteria density on the surface of egg affects the egg hatching process. The more the bacteria, the smaller the stimulus required to hatch the egg; similarly, eggs laid in clumps were much more likely to hatch together due to the generally higher bacterial density (40). Even larvae from earlier hatched eggs feeding on bacteria growth of unhatched eggs was enough to delay hatching (40). The results have been recently reproduced (41); unfortunately, the process of how bacteria affect hatching of Aedes eggs is still unclear, and warrants more study.
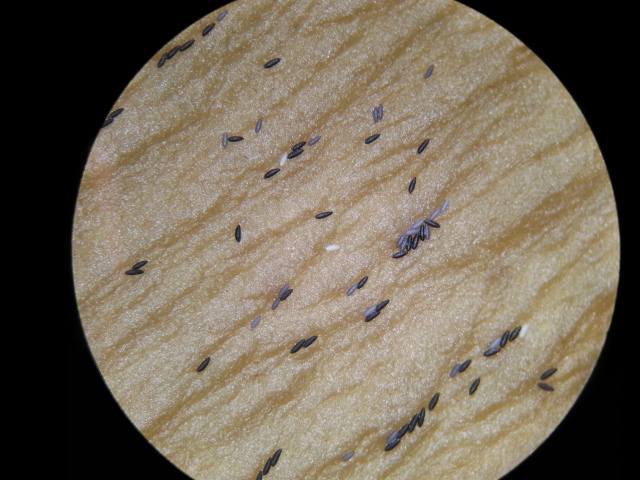 |
| Fig. 6: Aedes albopictus eggs (freshly laid). Image by: Theodore Lee |
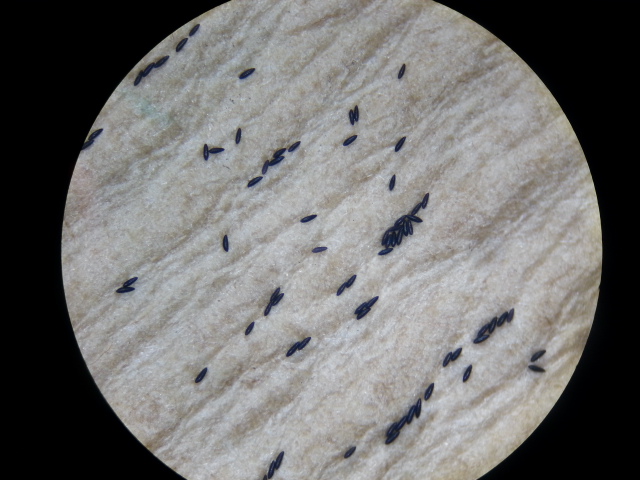 |
| Fig. 7: Aedes albopictus eggs (after 30 minutes). Image by: Theodore Lee |
Predation & Parasitism
The larvae of mosquitoes are commonly predated upon by various fish. In fact, guppies (Poecilia reticulata) have been studied as a potential way to control mosquito populations (42). Copepods have also been studied as potential biological controls (Video 3) (43), as well as other, predatory species of mosquitoes like Toxorhynchites splendens (44) (Figure 8). Odonate nymphs also predate on mosquito larvae. As adults, mosquitoes face predation from insectivorous birds and bats. However, most of these predators are not specific to Ae. albopictus, but to the general type of prey ("wrigglers" or "flies").
 |
| Fig. 8: Toxorhynchites splendens feeding on a captured 4th instar larvae of Ae. albopictus. Image by: Theodore Lee |
Vid 3: Macrocyclops albidus (a copepod) preying on first instar larvaeObtained from YouTube under fair use. Credit: David Whittall and ecotippingpoints.org
Defensive Adaptations
The larvae and pupae of Ae. albopictus thrash downwards, away from the surface of the water when they are disturbed. This behaviour can be seen in Video 4; although the tray is shallow, as a shadow falls over the water the larvae start thrash in an effort to swim away from the surface and lower in the water column.Vid. 4: Video of larvae/pupae swimming down (away from the surface) when disturbed.
Obtained from YouTube under fair use.
Significance
As vectors of disease
Aedes albopictus is a known vector for chikungunya (45) (46), dengue fever (24) (47), yellow fever (16) (38), and West Nile virus (1) (38). These diseases are not only painful, but they carry an economic cost in lost time and medical expenditures. While Singapore has strong epidemiological control and healthcare systems, some countries do not. Studies have been done to estimate the cost of only dengue on the economy, and estimates run as high as USD1.11bn (~SGD 1.58bn) in India (48). Similarly, the economic cost of dengue fever to the Philippines is estimated at USD345 million (USD3.26 per capita) (49). In South-East Asia, costs of dengue in 12 countries were estimated to run close to USD1b (~USD1.65 per capita) (50). While no statistics for the other diseases are presented, it is easy to see why the spread of the tiger mosquito worldwide is a cause for concern.
Such a high economic cost drives many attempts to find a solution; while dengue vaccines are being developed, most countries focus on vector control. This involves studying the biology and physiology of the vector, in this case, Ae. albopictus, in the hopes of understanding ways to control or limit the spread of the vector, and hence, any associated zoonotic diseases. In Singapore, many studies (17) (46) (47) (51) have been funded to understand Ae. albopictus. This increased understanding of the various mosquito vectors in our environment in turn enables better policy-making, to better combat this public health issue. However, this is not a process that is close to completion. New emerging infectious diseases are always on the horizon; zika virus (ZIKV) is one of these potential new arboviruses (52). While zika virus is usually spread by Ae. aegypti, Ae. albopictus has recently been proven to have potential to spread ZIKV (52). Dengue control plans already in place in Singapore may mitigate the threat of these new problems.
All 3 species (Ae. albopictus, Ae. malayensis and Ae. aegypti) can be found in Singapore (52).
Campaigns against dengue
In Singapore, there are several campaigns against dengue. Most of these campaigns focus on vector control of Ae. albopictus and Ae. aegypti, as it is something that can be done by the community and does not require specialized training. Some of the campaigns are as follows:
- A website (run by the National Environment Agency, NEA) providing public information about the dengue virus and the vectors that spread it. (http://www.dengue.gov.sg)
- The website also has latest information about dengue clusters in Singapore. (Dengue clusters in Singapore)
- The 5-Step Mozzie Wipeout campaign to stop mosquito breeding
- Various social media initiatives to remind Singaporeans to perform the 5-Step Mozzie Wipeout regularly.
- TV advertisements and Youtube videos (Video 5 & 6)
- Even home-made videos by comedy personality mrbrown (Video 7)
Vid. 5: Mozzie Wipeout Day campaign. Obtained from YouTube under fair use.
Vid. 6: The 5-Step Mozzie Wipeout advertisement Obtained from YouTube under fair use.
Vid. 7: Mozzie Wipeout song Obtained from YouTube under fair use.
Conservation
As a pest species, Aedes albopictus does not have many supporters. As an invasive species almost globally, much money has been spent eradicating this mosquito, but its reach continues to spread into various countries.
Ae. albopictus has a wide range and is hence unlikely to face global extinction.
Morphology and Identification
General Anatomy and Characteristics
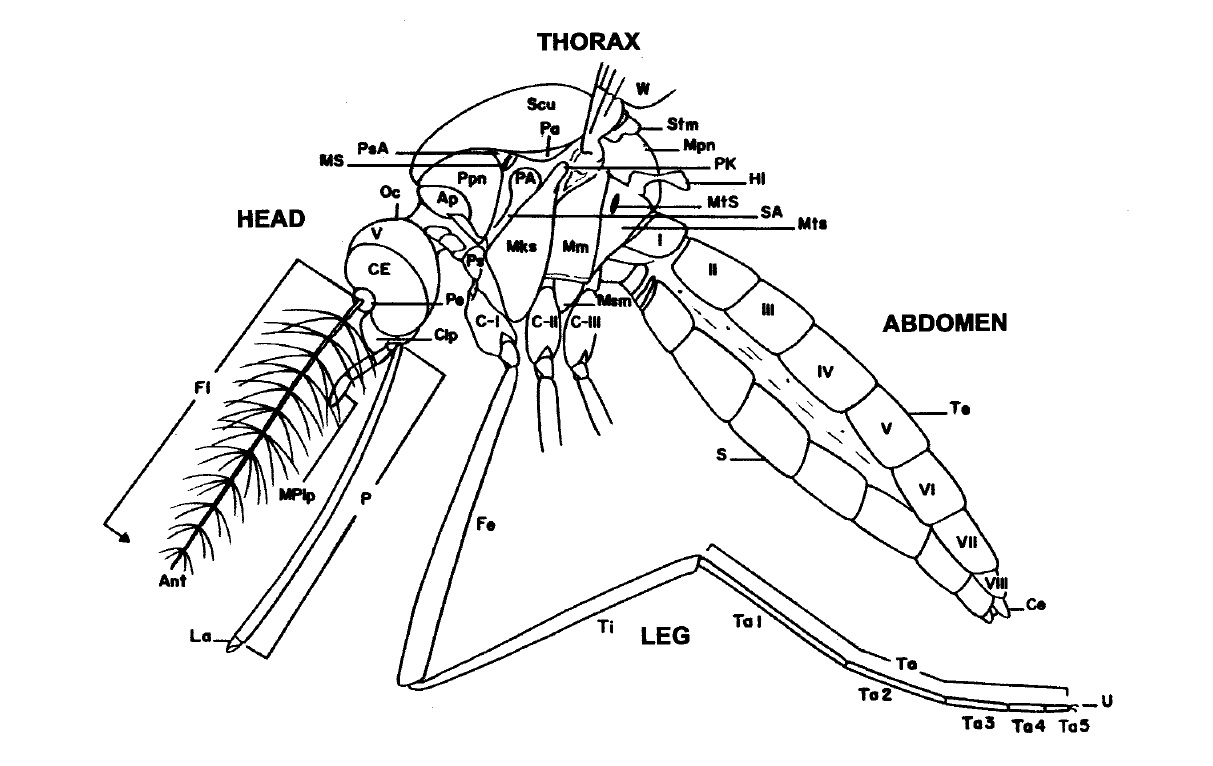 |
| Fig. 9: Generalized diagram of a female mosquito. Image from Rattanarithikul, R. et al. (2005). (53) |
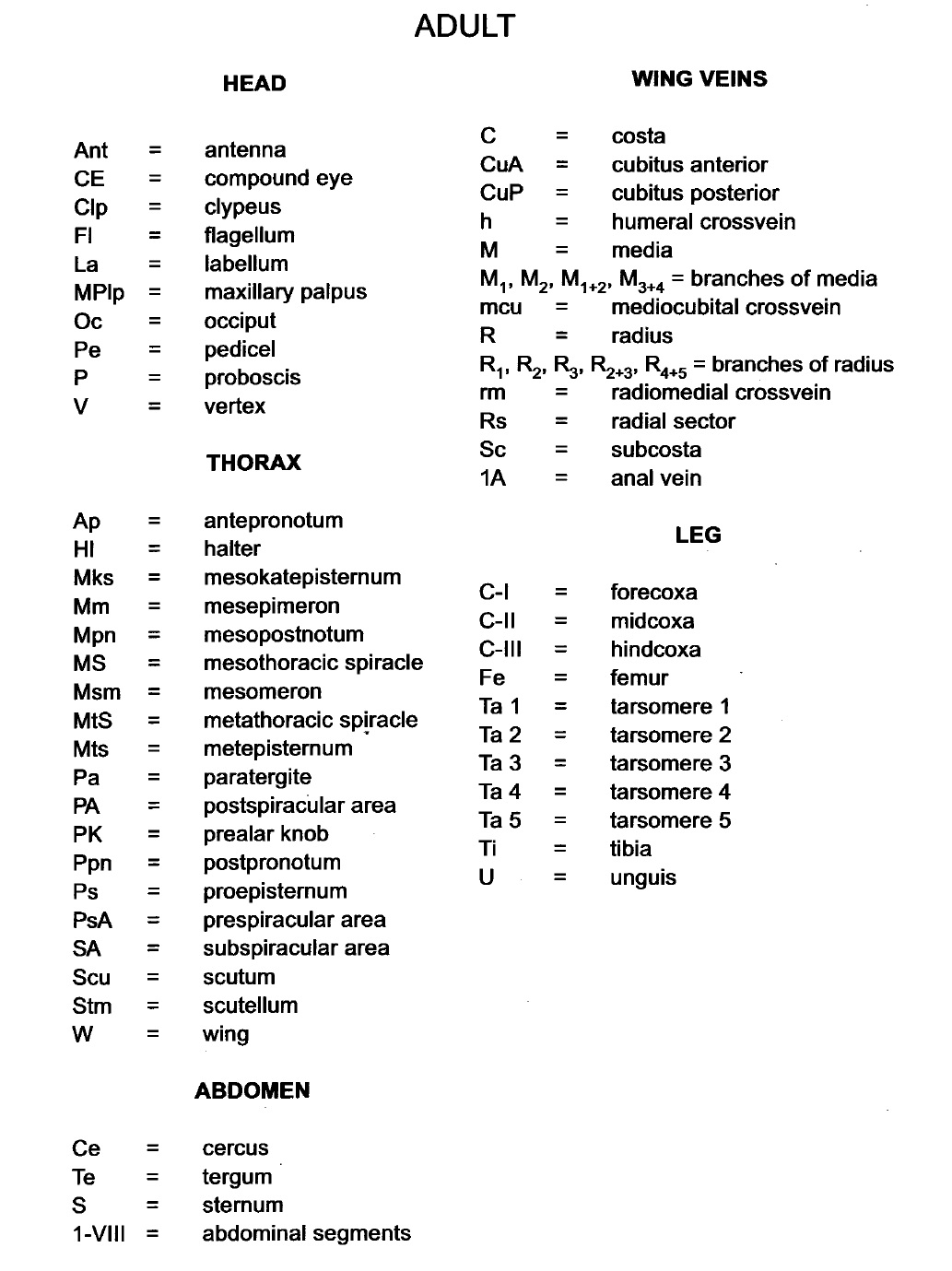 |
| Fig. 10: Abbreviations for features used in Fig. 9. Image from Rattanarithikul, R. et al. (2005). (53) |
Adult Description
The main features of Ae. albopictus are laid out in the table below.
| Feature |
Property |
|---|---|
| Antennae |
|
| Head |
|
| Thorax |
|
| Abdomen |
|
| Legs |
|
Ae. albopictus is often confused with another, very similar species: Ae. malayensis.
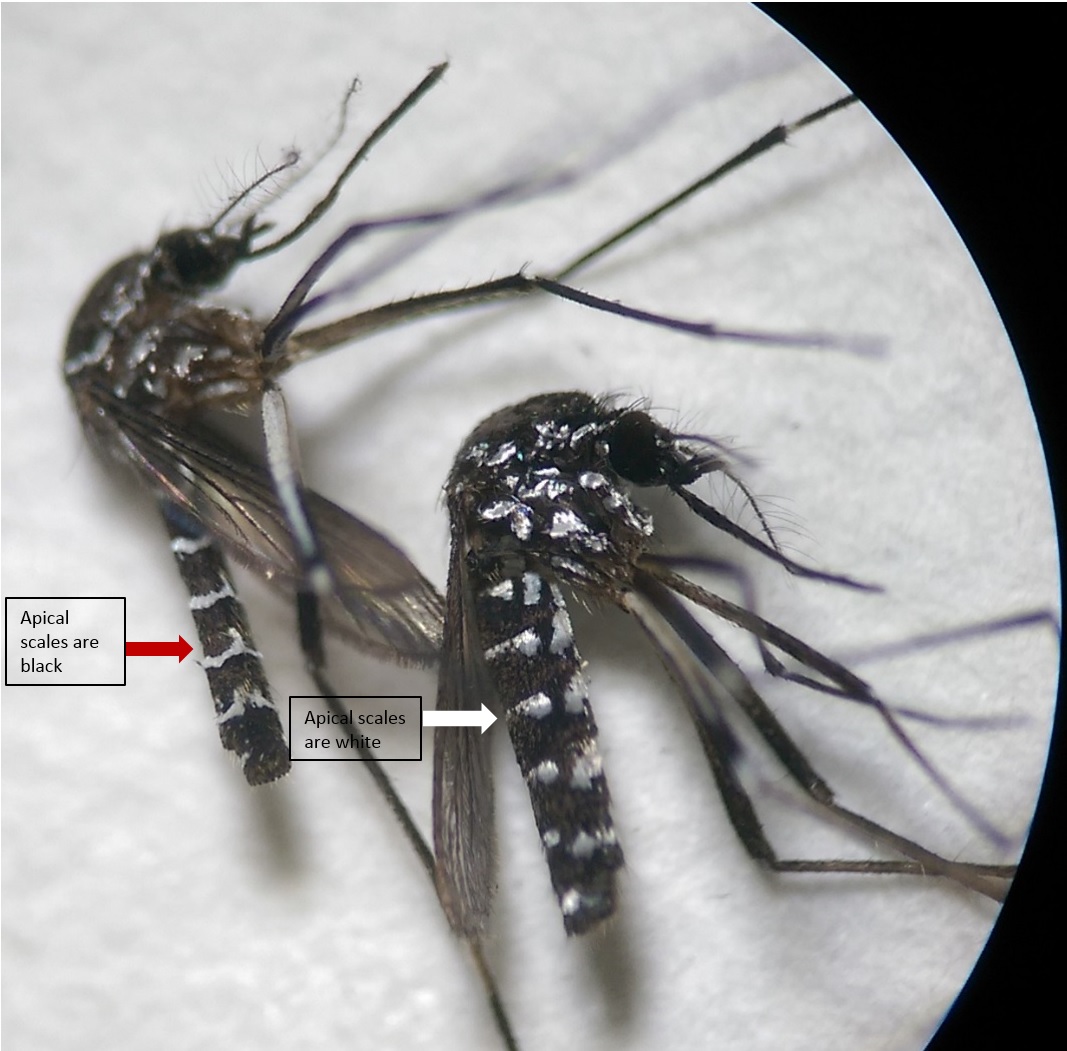 |
| Fig. 11: Female Aedes albopictus on the right, female Aedes malayensis on the left. Image by: Theodore Lee |
Note the apical scales on terga IV-VI. In Ae. malayensis (on the left of Figure 11), the apical scales are dark, with the white scales forming a U-shaped pattern through the middle of the tergum. For Ae. albopictus (on the right of Figure 11), each terga has white apical scales. This is one of the easiest methods to differentiate between Ae. malayensis and Ae. albopictus.
Sexual Dimorphism
Ae. albopictus males are generally smaller than females; when developing as pupae, they can actually be sorted as females tend to be larger and emerge slightly later. As adults, genitalia is of course different, but the most obvious difference is that males have bushier antennae compared to females (compare Figures 11 and 12).
 |
| Fig. 12: Aedes albopictus female, lateral view. Image by: Theodore Lee |
 |
| Fig. 13: Aedes albopictus male, lateral view. Image by: Theodore Lee |
Rearing & Breeding
BEWARE!
It is ILLEGAL to rear/culture/keep mosquitoes without a permit from NEA in Singapore. All information here is strictly for educational purposes only. Under the Control of Vectors and Pesticides Act Part IV Section 15: "No person shall create or cause of permit to be created any condition favourable to the propagation or harbouring of vectors." and Section 16: "No person shall breed, keep, collect, distribute, sell, import or export any vectors without the permission in writing of the Director-General". Please do not try this at home. (54)
Aedes mosquitoes are easily bred in lab conditions. Adults are stored at room temperature (25°C ± 1° C), at 70% ± 5% relative humidity (RH), with equal photoperiods of light and dark (12h:12h) (55). Adults had free access to 10% sucrose solution and water. Blood meals were given to females three times a week, and all mosquitoes within a holding cage are free to mate for maintenance cultures. Germ paper was used as a substrate to allow females to lay eggs.
Eggs were removed and incubated for 3 days to allow complete embryogenesis. When properly stored (sealed from excess moisture and heat), these eggs are viable for up to 6 months, although mortality rises sharply in the last few months.
As needed, germ papers containing eggs were hatched by placing them in a plastic tray of Milli-Q water (Figure 14). First-instar larvae usually emerge within the hour..
 |
| Fig. 14: Hatching Ae. albopictus eggs. Germ papers containing eggs placed in water in plastic trays. Image by: Theodore Lee |
Larvae are fed with yeast powder. Pupae are transferred to new holding cages to await emergence.
Trapping wild mosquitoes
While rearing mosquitoes in an insectary is useful for in-house studies, sometimes wild mosquitoes are required to add genetic diversity to insectary cultures. Instead of catching flying adults, oviposition traps (Figure 15) are used to collect eggs. The traps are small black plastic buckets about 12cm in diameter, with a hole punched through the side to allow excess water to drain out (Figure 15).
 |
| Fig. 15: Oviposition trap. Image by: Theodore Lee |
 |
| Fig. 16: Placement of oviposition traps amongst vegetation. Trap is placed at ground level. Image by: Theodore Lee |
The traps work by providing a ready source of water for mosquito females to lay eggs. Eggs laid on the germ paper can be collected and hatched in single containers, then identified and sorted by species. Traps are collected every 2-3 days to avoid eggs hatching into the water.
Taxonomy and Systematics
Synonym
Culex albopictus (Skuse, 1895) (Original description)
Stegomyia quasinigritia (Ludlow, 1911)
Stegomyia samarensis (Ludlow, 1903)
Stegomyia nigritia (Ludlow, 1910)
Aedes albopictus was first described as Culex albopictus, but has since been transferred into genus Aedes. It has 3 other synonyms.
New classification as Stegomyia albopicta was proposed by Reinert et al. in 2004, but it is not universally accepted (6) (56).
Type Information
The U.S. National Museum houses the neotype of Aedes albopictus (1 specimen). (57)
Subspecies
No subspecies rank.
Classification
The Taxo-navigation system below is provided by and referenced to UniProt Taxonomy. This is the classification of Aedes albopictus above species level.
› Metazoa
Phylogeny
Mosquito phylogeny is currently still being reassessed and several proposed reclassifications are under contention. The introduction of new molecular methods (DNA barcoding via next-gen sequencing) and the discovery of various new species also lends itself to some of the confusion seen in mosquito phylogeny. One of the studies by Reinert et al. (2004) was based on 172 character states in 119 exemplar species representing most of the genera and subgenera within tribe Aedini. The study found that genus Aedes was polyphyletic; the solution proposed was to reorganise the generic classification of Aedini, resulting in the promotion of many subgenera to genus, including Stegomyia (56).
A further study in 2009 built on this idea; this time, a cladistic analysis of 336 characters from all life stages of mosquitoes was conducted. These characters include both male and female genitalia, various scales and setae on adults and larvae, and even spots on pupae.
As defined by Reinert et al. (2009), Stegomyia is a monophyletic group, but the study showed that the two Stegomyia clades were poorly supported. While the terminal group was moderately supported, the branch supports (where "Stegomyia albopicta" is placed) leading to the terminal group are quite poorly supported (Figure 17). Much more work has to be done to resolve the proposed tree.
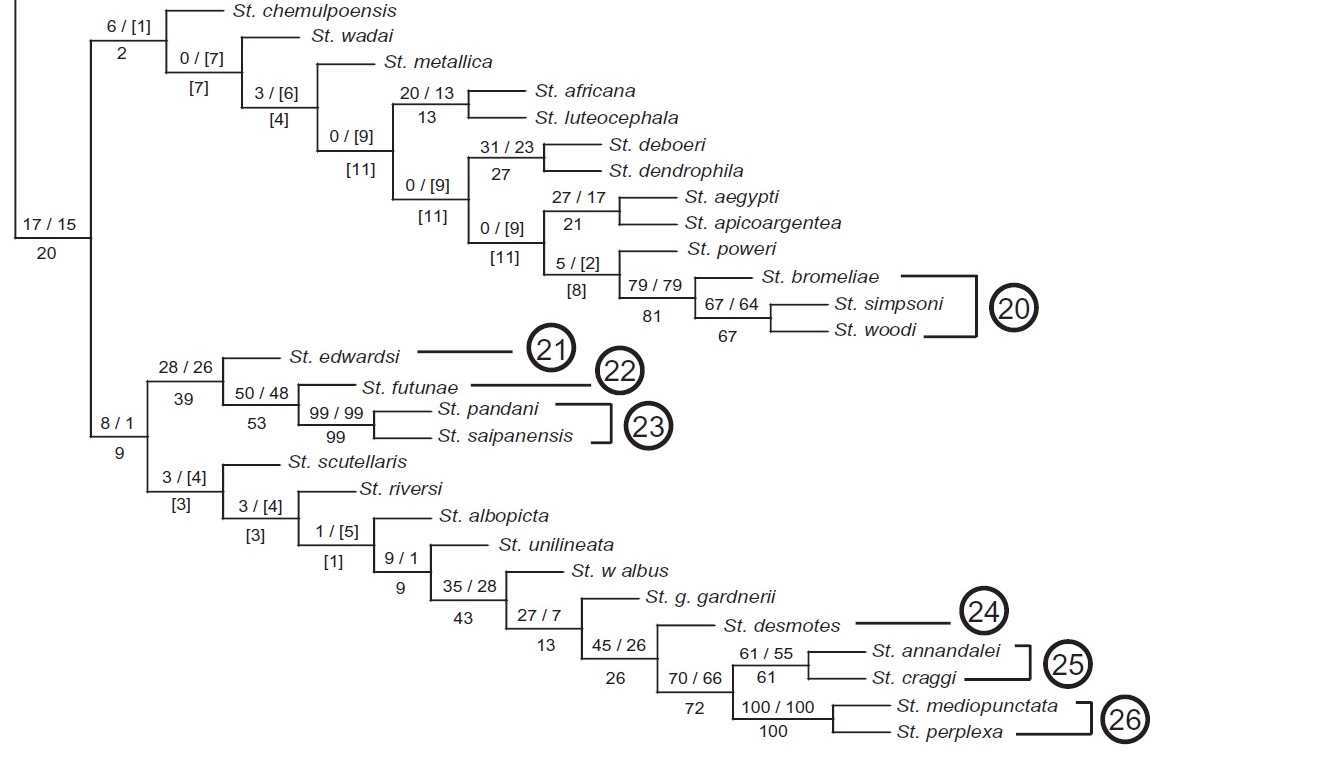 |
| Fig. 17: Proposed phylogeny of Aedes albopictus (here reclassified as Stegomyia albopicta). Notice the low/moderate support for the branch St. albopicta is on. Tree obtained from Reinert et al., 2009. Phylogeny and classification of tribe Aedini (Diptera: Culicidae). Obtained and accredited under fair use. |
References
1) Benedict, M. Q., Levine, R. S., Hawley, W. A., & Lounibos, L. P. (2007). Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne and Zoonotic Diseases, 7(1), 76–85. doi:10.1089/vbz.2006.0562.
2) Juliano, S. A. (2010). Coexistence, Exclusion, or Neutrality? a Meta-Analysis of Competition Between Aedes Albopictus and Resident Mosquitoes. Israel Journal of Ecology & Evolution, 56(3-4), 325–351. doi:10.1560/IJEE.55.3-4.325
3) Livdahl, T. P., & Willey, M. S. (1991). Prospects for an invasion: competition between Aedes albopictus and native Aedes triseriatus. Science (New York, N.Y.), 253(5016), 189–191. doi:10.1126/science.1853204
4) Lounibos, L., O’meara, G., Escher, R., Nishimura, N., Cutwa, M., Nelson, T., … SA. (2002). Testing predictions of displacement of native Aedes by the invasive Asian tiger mosquito Aedes albopictus in Florida, USA. Biological Invasions, 3, 151–166. Retrieved from http://link.springer.com/article/10.1023/A:1014519919099
5) Gratz, N. G. (2004). Critical review of the vector status of Aedes albopictus. Medical and Veterinary Entomology, 18(3), 215–227. doi:10.1111/j.0269-283X.2004.00513.x
6) Edwards, F. W. (1920). Notes on the Mosquitos of Madagascar, Mauritius and Réunion. Bulletin of Entomological Research, 11(2), 133. doi:10.1017/S0007485300044539.
7) Savage, H. M. (2005). Classification of mosquitoes in tribe Aedini (Diptera: Culicidae): Paraphylyphobia, and classification versus cladistic analysis. Journal of Medical Entomology, 42(ICZN 1999), 923–927. doi:10.1603/0022-2585(2005)042[0923:COMITA]2.0.CO;2
8) Reinert, J. F., Harbach, R. E., & Kitching, I. J. (2009). Phylogeny and classification of tribe Aedini (Diptera: Culicidae). Zoological Journal of the Linnean Society, 157(4), 700–794. doi:10.1111/j.1096-3642.2009.00570.x
9) Benedict, M. Q., Levine, R. S., Hawley, W. A., & Lounibos, L. P. (2007). Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne and Zoonotic Diseases, 7(1), 76–85. doi:10.1089/vbz.2006.0562.
10) Reiter, P., & Sprenger, D. (1987). The used tire trade: a mechanism for the worldwide dispersal of container breeding mosquitoes. Journal of the American Mosquito Control Association, 3(3), 494–501.
11) Reiter, P. (1998). Aedes albopictus and the world trade in used tires, 1988-1995: the shape of things to come? Journal of the American Mosquito Control Association, 14(1), 83–94.
12) Roiz, D., Neteler, M., Castellani, C., Arnoldi, D., & Rizzoli, A. (2011). Climatic factors driving invasion of the tiger mosquito (Aedes albopictus) into new areas of Trentino, Northern Italy. PLoS ONE, 6(4), 4–11. doi:10.1371/journal.pone.0014800
13 Alto, B. W., & Juliano, S. a. (2001). Precipitation and temperature effects on populations of Aedes albopictus (Diptera: Culicidae): implications for range expansion. Journal of Medical Entomology, 38, 646–656. doi:10.1603/0022-2585-38.5.646.
14) Paupy, C., Delatte, H., Bagny, L., Corbel, V., & Fontenille, D. (2009). Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes and Infection, 11(14-15), 1177–1185. doi:10.1016/j.micinf.2009.05.005
15) Dalla Pozza, G., & Majori, G. (1992). First record of Aedes albopictus establishment in Italy. Journal of the American Mosquito Control Association, 8(3), 318–20. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1402871
16) Li, Y., Kamara, F., Zhou, G., Puthiyakunnon, S., Li, C., Liu, Y., … Chen, X.-G. (2014). Urbanization increases Aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS Neglected Tropical Diseases, 8(11), e3301. doi:10.1371/journal.pntd.0003301
17) Chan, K., Ho, B., & Chan, Y. (1971). Aedes aegypti (L.) and Aedes albopictus (Skuse) in Singapore City: 2. Larval habitats*. Bulletin of the World Health Organisation, 44, 629–633. Retrieved from http://whqlibdoc.who.int/bulletin/1971/Vol44/Vol44-No5/bulletin_1971_44(5)_635-641.pdf.
18) Telang, A., & Wells, M. A. (2004). The effect of larval and adult nutrition on successful autogenous egg production by a mosquito. Journal of Insect Physiology, 50(7), 677–85. doi:10.1016/j.jinsphys.2004.05.001
19) Kogan, P. H., (1990). Substitute blood meal for investigating and maintaining Aedes aegypti (Diptera: Culicidae). Journal of Medical Entomology, 27, 709–712.
20) Delatte, H., Desvars, A., Bouétard, A., Bord, S., Gimonneau, G., Vourc’h, G., & Fontenille, D. (2010). Blood-feeding behavior of Aedes albopictus, a vector of chikungunya on La Réunion. Vector Borne and Zoonotic Diseases, 10(3), 249–258. doi:10.1089/vbz.2009.0026
21) Shone, S. M., Ferrao, P. N., Lesser, C. R., Glass, G. E., & Norris, D. E. (2003). Evaluation of carbon dioxide- and 1-octen-3-ol-baited Centers for Disease Control Fay-Prince traps to collect Aedes albopictus. Journal of the American Mosquito Control Association, 19(4), 445–7. doi:10.1016/j.micinf.2011.07.011.Innate
22) Hoel, D. F., Kline, D. L., Allan, S. a, & Grant, a. (2007). Evaluation of carbon dioxide, 1-octen-3-ol, and lactic acid as baits in Mosquito Magnet Pro traps for Aedes albopictus in north central Florida. Journal of the American Mosquito Control Association, 23(1), 11–17. doi:10.2987/8756-971X(2007)23[11:EOCDOA]2.0.CO;2.
23) Amiya, K. H. (2015). Pattern of Human-biting Activity of Aedes aegypti L. and Aedes albopictus Skuse in a Garden Locale from City of Kolkata, India. Journal of Mosquito Research, 5(13), 1–5. doi:10.5376/jmr.2015.05.0013.
24) Almeida, A. P. G., Baptista, S. S. S. G., Sousa, C. A. G. C. C., Novo, M. T. L. M., Ramos, H. C., Panella, N. A., Godsey, M., Simoes, M. J., Anselmo, M. L., Komar, N., Mitchell, C. J., Ribeiro, H. (2005). Bioecology and vectorial capacity of Aedes albopictus (Diptera: Culicidae) in Macao, China, in relation to dengue virus transmission. Journal of Medical Entomology, 42(3), 419–428. doi:10.1603/0022-2585(2005)042[0419:bavcoa]2.0.co;2
25) Kong, X. Q., & Wu, C. W. (2010). Mosquito proboscis: An elegant biomicroelectromechanical system. Physical Review E - Statistical, Nonlinear, and Soft Matter Physics, 82(1), 1–5. doi:10.1103/PhysRevE.82.011910.
26) Law, J. H., Ribeiro, J. M. C., & Wells, M. A. (1992). Biochemical Insights Derived From Insect Diversity.
27) Marinotti, O., Brito, M. de, & Moreiru, C. K. (1996). Apyrase and a-glucosidase in the Salivary Glands of Aedes albopictus. Comp. Biochem. Physiol., 113(4), 675–679.7
28) Ribeiro, J. M., Rossignol, P. a, & Spielman, a. (1984). Role of mosquito saliva in blood vessel location. The Journal of Experimental Biology, 108, 1–7.
29) Dieng, H., Mwandawiro, C., Boots, M., Morales, R., Satho, T., Tuno, N., … Takagi, M. (2002). Leaf litter decay process and the growth performance of Aedes albopictus larvae (Diptera: Culicidae). Journal of Vector Ecology, 27(1), 31–38.
30) Merritt, R. W., Dadd, R. H., & Walker, E. D. (1992). Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annual Review of Entomology, 37(128), 349–376. doi:10.1146/annurev.en.37.010192.002025
31) Yee, D. A., Kesavaraju, B., & Juliano, S. A. (2004). Larval feeding behavior of three co-occurring species of container mosquitoes. Journal of Vector Ecology, 29(2), 315–322.
32) Boyer, S., Gilles, J., Merancienne, D., Lemperiere, G., & Fontenille, D. (2011). Sexual performance of male mosquito Aedes albopictus. Medical and Veterinary Entomology, 25, 454–459. doi:10.1111/j.1365-2915.2011.00962.x
33) Benelli, G. (2015). The best time to have sex: mating behaviour and effect of daylight time on male sexual competitiveness in the Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae). Parasitology Research, 114(3), 887–894. doi:10.1007/s00436-014-4252-7
34) Lees, R. S., Knols, B., Bellini, R., Benedict, M. Q., Bheecarry, A., Bossin, H. C., … Gilles, J. R. L. (2014). Review: Improving our knowledge of male mosquito biology in relation to genetic control programmes. Acta Tropica, 132, S2–S11. doi:10.1016/j.actatropica.2013.11.005.
35) Estrada-Franco, J. G., & Craig, G. B. (1995). Biology, disease relationships, and control of Aedes albopictus, 49.
36) Zheng, M.-L., Zhang, D.-J., Damiens, D. D., Lees, R. S., & Gilles, J. R. L. (2015). Standard operating procedures for standardized mass rearing of the dengue and chikungunya vectors Aedes aegypti and Aedes albopictus (Diptera: Culicidae) - II - Egg storage and hatching. Parasites & Vectors, 8(1), 348. doi:10.1186/s13071-015-0951-x8
37) Fonseca, D. M., Kaplan, L. R., Heiry, R. A., & Strickman, D. (2015). Density-Dependent Oviposition by Female Aedes albopictus (Diptera: Culicidae) Spreads Eggs Among Containers During the Summer but Accumulates Them in the Fall. Journal of Medical Entomology, 52(4), 705–712. doi:10.1093/jme/tjv060
38) Eritja, R., Escosa, R., Lucientes, J., Marquès, E., Roiz, D., & Ruiz, S. (2005). Worldwide invasion of vector mosquitoes: present European distribution and challenges for Spain. Biological Invasions, 7(1), 87–97. doi:10.1007/s10530-004-9637-6
39) Gillett, J. D. (1955). Variation in the Hatching-response of Aëdes Eggs (Diptera: Culicidae). Bulletin of Entomological Research, 46(02), 241. doi:10.1017/S0007485300030881
40) Gillett, J. D., Roman, E. A., & Phillips, V. (1977). Erratic Hatching in Aedes Eggs: A New Interpretation. Proceedings of the Royal Society B: Biological Sciences, 196(1123), 223–232. doi:10.1098/rspb.1977.0038
41) Ponnusamy, L., Böröczky, K., Wesson, D. M., Schal, C., & Apperson, C. S. (2011). Bacteria stimulate hatching of yellow fever mosquito eggs. PLoS ONE, 6(9), 1–10. doi:10.1371/journal.pone.0024409
42) Elias, M., Islam, M. S., Kabir, M. H., & Rahman, M. K. (1995). Biological control of mosquito larvae by Guppy fish. Bangladesh Medical Research Council Bulletin, 21(2), 81–6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8815867.
43) Veronesi, R., Carrieri, M., Maccagnani, B., Maini, S., & Bellini, R. (2015). Macrocyclops albidus (Copepoda: cyclopidae) for the Biocontrol of Aedes albopictus and Culex pipiens in Italy. Journal of the American Mosquito Control Association, 31(1), 32–43. doi:10.2987/13-6381.1
44) Toma, T., & Miyagi, I. (1992). Laboratory evaluation of Toxorhynchites splendens (Diptera: Culicidae) for predation of Aedes albopictus mosquito larvae. Medical and Veterinary Entomology, 6(3), 281–9. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1358270.
45) Delatte, H., Desvars, A., Bouétard, A., Bord, S., Gimonneau, G., Vourc’h, G., & Fontenille, D. (2010). Blood-feeding behavior of Aedes albopictus, a vector of chikungunya on La Réunion. Vector Borne and Zoonotic Diseases, 10(3), 249–258. doi:10.1089/vbz.2009.0026
46) Ng, L.-C., Tan, L.-K., Tan, C.-H., Tan, S. S. Y., Hapuarachchi, H. C., Pok, K.-Y., … Khoo, S.-P. (2009). Entomologic and virologic investigation of Chikungunya, Singapore. Emerging Infectious Diseases, 15(8), 1243–9. doi:10.3201/eid1508.081486
47) Burattini, M. N., Chen, M., Chow, a, Coutinho, F. a B., Goh, K. T., Lopez, L. F., … Massad, E. (2008). Modelling the control strategies against dengue in Singapore. Epidemiology and Infection, 136(3), 309–19. doi:10.1017/S0950268807008667
48) Shepard, D. S., Halasa, Y. A., Tyagi, B. K., Adhish, S. V., Nandan, D., Karthiga, K. S., … INCLEN Study Group. (2014). Economic and disease burden of dengue illness in India. The American Journal of Tropical Medicine and Hygiene, 91(6), 1235–42. doi:10.4269/ajtmh.14-0002
49) Edillo, F. E., Halasa, Y. A., Largo, F. M., Erasmo, J. N. V., Amoin, N. B., Alera, M. T. P., … Shepard, D. S. (2015). Economic Cost and Burden of Dengue in the Philippines. American Journal of Tropical Medicine and Hygiene, 92(2), 360–366. doi:10.4269/ajtmh.14-0139
50) Shepard, D. S., Undurraga, E. A., & Halasa, Y. A. (2013). Economic and Disease Burden of Dengue in Southeast Asia. PLoS Neglected Tropical Diseases, 7(2), e2055. doi:10.1371/journal.pntd.0002055
51) Chung, Y. K., & Pang, F. Y. (2002). Dengue virus infection rate in field populations of female Aedes aegypti and Aedes albopictus in Singapore. Tropical Medicine & International Health : TM & IH, 7(4), 322–330. doi:10.1046/j.1365-3156.2002.00873.x
52) Wong, P. S. J., Li, M. Z. I., Chong, C. S., Ng, L. C., & Tan, C. H. (2013). Aedes (Stegomyia) albopictus (Skuse): A Potential Vector of Zika Virus in Singapore. PLoS Neglected Tropical Diseases, 7(8), 1–5. doi:10.1371/journal.pntd.0002348
53) Rattanarithikul, R., Harrison, B. A., Panthusiri, P., & Coleman, R. E. (2005). Illustrated Keys to the Mosquitoes of Thailand I. Background; Geographic Distribution; Lists of Genera, Subgenera, and Species; and a Key to the Genera. The Southeast Asian Journal of Tropical Medicine and Public Health, 36 Suppl 1, 1–80. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15916030. Images reproduced from page 54-55.
54) http://statutes.agc.gov.sg/aol/search/display/view.w3p;ident=1ba4f228-c5f8-4786-b557-50f62e9f1be5;query=DocId%3A1e7cfa51-b6ab-4e63-b6e7-7c846b4f9175%20Depth%3A0%20Status%3Ainforce;rec=0#pr15-he-.
55) Zheng, M.-L., Zhang, D.-J., Damiens, D. D., Lees, R. S., & Gilles, J. R. L. (2015). Standard operating procedures for standardized mass rearing of the dengue and chikungunya vectors Aedes aegypti and Aedes albopictus (Diptera: Culicidae) - II - Egg storage and hatching. Parasites & Vectors, 8(1), 348. doi:10.1186/s13071-015-0951-x
56)Reinert, J. F., Harbach, R. E., & Kitching, I. J. (2004). Phylogeny and classification of Aedini (Diptera: Culicidae), based on morphological characters of all life stages. Zoological Journal of the Linnean Society, 142(3), 289–368. doi:10.1111/j.1096-3642.2004.00144.x
57) Huang, Y. (1968). Neotype designation for Aedes (Stegomyia) albopictus (Skuse) (Diptera: Culicidae). Ent Soc Wash, 297–302.
Contact Me
Theodore Lee is contactable here