|
| Photo by April Nobile. Used under CC-BY-SA-3.0. |
Introduction
Table of Contents
This page will elaborate on the yellow crazy ants' behaviour, ecological interactions, known life history, ecological impact (invasive or otherwise), and distribution. This page also records existing research of A. gracilipes in Singapore and explains its taxonomic history. Also provided are diagnostic tools for identifying the yellow crazy ant worker (and queen if needed), and to differentiate it from some other common ant species. A phylogenetic analysis of the ant's position on the tree of life can be found at the end of the article.
Basic Facts about Ants (Formicidae)
- Ants are from the same order as bees and wasps (Hymenoptera). Most ant species (like bees and wasps) are eusocial, i.e. they form colonies, raise each others' young, and most individuals forgo reproduction in order to take care of the offspring of the queen(s).
- There are castes in ant society with different morphologies: workers, drones, and queens. Some species also have a soldier caste (A. gracilipes does not have one).
- Ant workers are all female! So is the queen. Males do not work, and only exist to fertilise the queen.
- Unlike bees and wasps, the common morph of ants have evolved to become wingless. Only the alates (sexually reproducing morphs, male or female) are born with wings, although they are deciduous (will drop after their mating flights and/or when they settle down in new colonies).
- As a rule, ants are haplodiploid; fertilised eggs are born as diploid females (with 2 sets of chromosomes) while unfertilised eggs are born as haploid males (with one set of chromosomes). However, for A. gracilipes and some other ant species, there are some diploid males.
The rest of the article will elaborate specifically on Anoplolepis gracilipes.
Behaviour
Eusociality
Like most hymenopterans, A. gracilipes are highly cooperative eusocial insects. They form supercolonies that are unicolonial (merged colonies that interact as one colony without intraspecific aggression) [1; 2] and polygynous (multiple queens in one colony) [3]. Each nest usually has about 40 queens, although a few may have almost 300 queens; there are usually a few thousand workers, and up to ten thousand in some cases [4]. Workers usually constitute more than 80% of the individuals in a nest [4]. Only one instance of intraspecific aggression has been found recently on Tokelau between two A. gracilipes lineages which arrived on Tokelau at different times [5]. The more related two colonies are, the less intraspecific aggression they will have for each other and the more likely they are able to form supercolonies [2]. Evidence suggests that the caste an ant is born into is determined by its genes [2; 6]..
A. gracilipes are able to survive flooding by forming rafts with their bodies (see the video below from 1 min 18 sec):
Feeding
A. gracilipes have feeding strategies that are generalist in both method and source. They engage in predation, scavenging, and foraging [7]. They prey on small vertebrates such as newborn pigs, dogs, cats, rabbits, rats, and chickens, as well as hatchling birds and reptiles [8]. They scavenge dead invertebrates [9] and also prey on them while alive [10]. They harvest honeydew from hemipterans, and also bring back sugary fluids from fruits and plants to the nest [4]. They usually scavenge or attack in swarms (personal observation; see also the photo below and this documentary of A. gracilipes on Christmas Island attacking red land crabs).| Yellow crazy ants pulling away a dead lizard. Use under CC0 1.0 Universal. |
It attacks prey by spraying formic acid on their sensitive areas, such as eyes or mouthparts [3]. This action can be used for defense against competitors for food sources or attackers of the nest as well [10]. Here is a video showing A. graciilipes attacking birds with formic acid in Hawaii:
Mutualism
Like other invasive tramp ants, A. gracilipes forms mutually beneficial relationships with various sap-sucking hemipterans, which are responsible for stress on plants and trees. These hemipterans provide honeydew in return for protection from A. gracilipes [3]. A. gracilipes also has mutualistic relationships with Allotinus major in Sulawesi and Lophocephala guerini in India [11]. Some pictures of their mutualisms are available at Alexander Wild's website.Life History
Workers are produced perennially, while alate production usually occurs with the beginning of the wet season, but may be produced perennially [4]. The colony usually disperses by budding: queens leave the nest by foot with a group of workers to start new colonies. Not all queens leave, some shed their wings and remaining in the nest where they are born. Mating flights and/or winged dispersal have not been formally observed, and it is not known whether alates are pre-fertilised or have to meet alates from other colonies in order to start a new colony [10].Workers are usually heterozygous (sharing alleles from a father and mother) while queens are homozygous (same maternal alleles) [2; 6].
There are a large proportion of diploid males found in A. gracilipes colonies, which is unusual since Hymenopteran males are usually haploid [2; 6]. However, this is not unique to A. gracilipes and has been observed in the red fire ant Solenopsis invicta [2]. A large proportion of diploid males suggest inbreeding, which would result in greater relatedness, and hence explain lowered intraspecific aggression [2]. They do not reproduce by obligate hybridisation i.e. two lineages do not have to breed to produce workers [6].
The worker eggs 'reach maturity' in 76-84 days according to Fluker and Beardsley [12]. Other studies show a shorter total time estimate for full development from egg to adult (refer to the table below). The known details of the life cycle of A. gracilipes in a colony mentioned in the literature [4; 10] are illustrated in the table below:
| Egg Stage |
Larval Stage |
Pupal Stage |
Adult Lifespan |
Time of Production |
Reproduction |
|
| Workers |
18-20 days |
16-20 days |
20 days |
6 months |
Perennial |
May reproduce asexually; not enough evidence [6] |
| Queens |
18-20 days |
? |
30-34 days |
Several years |
1-2 months before wet season (perennial in some places e.g. Seychelles) |
700 eggs annually |
| Males |
? |
? |
? |
? |
2 months before wet season (perennial in some places e.g. Seychelles) |
Sexual reproduction (with foreign or familiar queens is not known) |
Ecological Impact
The yellow crazy ant is considered an invasive species, as it has infiltrated ecosystems all over the world (see Distribution).It is considered one of the hundred worst invasive species in the world by IUCN and the Invasive Species Specialist Group [13].
They encourage forest destruction by their mutualistic relationship with phloem-feeding, honeydew-producing hemipterans such as aphids. Such destruction also extends to agricultural crops [8]. The excessive production of honeydew also encourage the growth of Capnodiaceae moulds that hamper photosynthesis [14]. The overall impact observed includes reduced canopy cover, higher temperature and humidity, and a decrease in diversity of endemic animals [15].
A. gracilipes also earns its status as an invasive species by destroying keystone species populations in multiple ecosystems, such as the red land crabs (Gecarcoidea natalis) on Christmas Island, at a rate of 2 million a year [3; 13] The eradication of red land crabs led to the deregulation of ecosystem mechanisms that G. natalis was responsible for, such as litter breakdown. Other key invasion case studies occurred at Seychelles [14].
Documentary of the invasive impact of A. gracilipes on G. natalis on Christmas Island:
Ironically, it has also been used for biological control. In Papua New Guinea, it was used to control pod weevil (Pantorhytes szentivanyi) in cacao plantations [16]. It also has a controlling effect on other pests such as cockroaches, centipedes, and rats in Seychelles, and was intentionally dispersed by some locals for that purpose [14]. There is also some evidence that A. gracilipes may suppress folivores, but the net effect on trees is negative due to their positive interactions with Hemiptera [17].
This video summarises the problem of invasive species and focuses on Anoplolepis gracilipes:
Positive ecological impacts of A. gracilipes have not been formally documented.
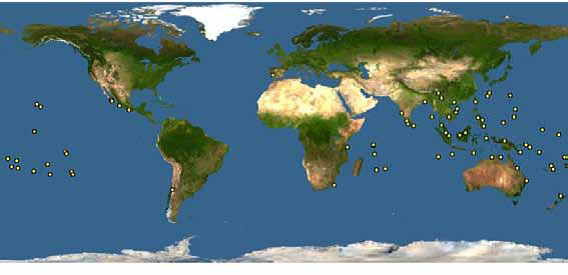
Distribution
A. gracilipes thrives in moist lowland tropical areas below 1200 m, especially tropical rainforest habitats. However, it has been known to survive in arid climates such as in California, United States [8]. It is unclear in which areas of its range it is native or exotic as it was already discovered to cover most of its current known distribution by 1895 [8]. The foraging productivity of A. gracilipes increases with ambient temperature and relative humidity, reaching a maximum between 26 to 30°C, which explains why it dominates tropical ecosystems [7]. A. gracilipes nest both on the ground and in the trees [4].
Above elevations of 1200 m, it is only found in Philippines, China, Tibet, and Mexico, and Hawaii. It is found mostly under the latitudes of 26-27oN. It is absent from most of the Neotropics and West Central Africa even though it may potentially be able to succeed there Anoplolepis gracilipes is believed to have originated from Africa or tropical Asia. Most species of Anoplolepis are found only in Africa, although A. gracilipes itself is not as abundantly found in Africa as it is in Asia [8].
Anoplolepis gracilipes in Singapore
It was first documented in Singapore by Smith in 1857 from Wallace's specimen collections in the region as Formica gracilipes [18].
It has an entry in the book 'Guide to Urban Pest Ants in Singapore', and is also considered a pest ant by pest control agencies in Singapore (Microland, Maximum Pest Management).
Tan and Corlett conducted a study in 2012 to identify the major animal taxa that scavenge dead invertebrates in different habitats with different levels of urbanisation in Singapore. A. gracilipes often had one of the most scavenging events ahead of other scavenging species at primary forest, old secondary forest, new secondary forest, and artificial impervious surfaces, while entirely absent at grassland and recreational park habitats [9]. The ant was also documented as one of the pest ants that infest healthcare premises [19]. There have been no other studies to determine its full range and concentrations in various habitats in Singapore.
Can A. gracilipes still be considered a pest or invasive in Singapore if it has been present in Singapore for more than a century? Its current ecological impacts in Singapore are not well-research, and more studies into its ecological interaction in Singapore's forests should be conducted.
Diagnosis
The main features of ant anatomy are illustrated in the picture below. Technically, the abdomen includes the petiole and one segment of the alitrunk, which is why it is more scientifically correct to call the hind section of the ant that is separated by the petiole the gaster, and call the midsection the alitrunk instead of the thorax. Learn basic ant identification and anatomy here.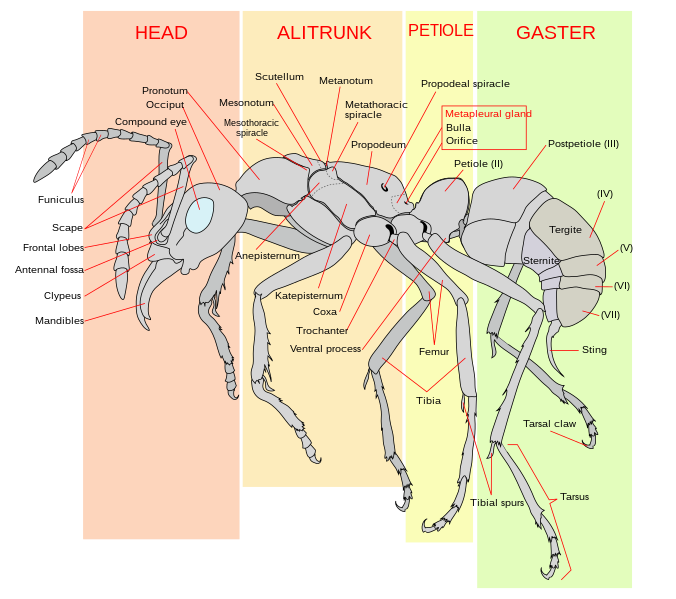 |
| Image of generic ant under public domain. |
Descriptions
Worker
They are approximately 1-5 mm long (usually about 4mm) and have slender bodies. They are coloured yellow-brown with a gaster that is a darker shade. Their antennae have 11 segments, and are almost as long as their bodies, with a scape that is twice the length of their small heads [8]. Their eyes are also large and black relative to their head. They do not have a sting, but have an acidopore in its place from which they can spray formic acid. Their mandibles have 8 teeth each. Only one petiole present; it is thick and the top of it is sharply curved [10]. Workers are monomorphic, i.e. they do not have specialised morphologies even if they perform different worker roles [11]. It has hairs on its head and gaster, but mostly absent on the alitrunk [20].A dichotomous key for identifying ants on Pacific islands (which includes A. gracilipes) can be found here. More photos of the yellow crazy ant can be found on Alex Wild's website.
|
|
Alates
The queen is much bigger than the worker. The antennae are still long, but do not extend beyond the length of the body. The scape is still longer than the length of the head. The alitrunk has a significant hunch and is not slender like the worker morph. The gaster, like most ant queens, is large. It is a much darker shade of brown compared to the rest of the body; the difference in shade is more pronounced in the queen than the worker. However, its general colour is about the same yellow-brown as the worker. |
| Side view of A. gracilipes queen (without wings) by Amy Carmichael, Queensland University of Technology. Used under CC BY 3.0 AU. |
No pictures of the male are readily available.
Morphologically similar ants
A. gracilipes (workers) may be confused with the following genera/species that can be found in Singapore [21]:| Similar ant species |
Property of similarity |
Differentiated by |
|||
| Acropyga sp. |
11 antennae segments |
A. gracilipes are
|
|
||
| Oecophylla sp. |
Similar in size and leg length |
A. gracilipes
|
|
||
| Paratrechina longicornis |
Erratic movement |
P. longicornis are
|
|
||
| Camponotus maculatus |
Similar size and shape |
C. maculatus
|
|
Nomenclature
Vernacular Names
A. gracilipes is known as the yellow crazy ant, because of its yellow-brown colouration and its frenetic movements. Other English names include 'long-legged ant' or 'crazy ant'. 'Crazy ant' may also apply to Paratrechina longicornis because it is also known for its frenetic movements [8]. It is also called the Maldive ant (in Seychelles), Gramang ant (Indonesia), and ashinaga ki-ari (Japan) [10].History of Scientific Names
The renaming of the species followed two different original type specimens discovered separately by Thomas C. Jerdon and Fredrick Smith, before being consolidated under one specific epithet by Barry Bolton. This is expressed below.
Note: 'Combination' in a genus refers to the association of a genus-rank and species-rank name to form the binomial name of a species [22]. Synonyms are alternate names for the ant; senior synonyms are older than the reference name, junior synonyms are younger than the reference name.
| Formica longipes Jerdon 1851 Thomas C. Jerdon, a zoologist working for the East India Company in Madras, India, described the worker: "Worker, length 1-5th of an inch; in form exceedingly similar to the last; head more oblong than triangular; eyes more posterior; antennae very long; abdominal pedicle shorter, proportionally; abdomen a longer oval; legs very long of a pale rufous colour throughout, tinged with dusky on the abdomen. This Ant is found in all the forests of India living in holes in the ground, in tolerable numerous societies, and feeding on vegetable secretions. i have not seen it at any distance from the jungles. At Tellicherry for example, I have never seen it, but as soon as you go a little inland and get into the jungle you meet with it. It is often found in bungalows and out-houses." [23] 'Longipes' comes from the Latin word 'longus' (legs) and 'pes' (foot), and means 'long-legged'. It is a junior homonym of Formica longipes Latreille, 1802a, which has since been reassigned to Pheidole longipes (another species). Anoplolepis longipes Emery 1925 Combination in Plagiolepis by Carlo Emery in 1887. Anoplolepis was created as a subgenus of Plagiolepis in 1914 by Felix Santschi [22], i.e. combination in Plagiolepis (Anoplolepis). In 1925, Carlo Emery promoted Anoplolepis from a subgenus under Plagiolepis to a full genus, i.e. combination in Anoplolepis [22]. As a result, literature on Anoplolepis gracilipes before 1995 will be found under this name. See AntCat entryfor Anoplolepis longipes. |
Formica gracilipes Smith 1857 Fredrick Smith, cataloguing the hymenopterans collected by Alfred Russel Wallace in Southeast Asia, described this worker ant from Singapore: "Pale ferruginous, abdomen dark rufo-piceous; antennae longer than the body; head ovate, and wider than the thorax, narrowed behind; the eyes black and prominent. Thorax elongate and compressed; the prothorax narrowed into a slender neck; legs very much elongated, the posterior pair one-third longer than the insect, the tibiae and tarsi pale testaceous; the abdominal scale incrassate, rounded in front and truncate behind; the abdomen dark nifopiceous, short and ovate; the base more or less pale ferruginous." [18] 'Gracilipes' means 'slender-legged'. Prenolepis gracilipes Mayr 1862 Combination in Prenolepis. Plagiolepis gracilipes Mayr 1867a Combination in Plagiolepis. Formica trifasciata Smith 1858b is also recognised as a senior synonym [24]. |
| Anoplolepis gracilipes Smith 1857 (current name) Plagiolepis gracilipes and Anoplolepis longipes are recognised to be senior synonyms (through comparison of syntypic workers) and combined in Anoplolepis. Designated gracilipes as a replacement name for longipes by Barry Bolton [22]. See AntCat entry for Anoplolepis gracilipes. |
|
Systematic Information
Phylum: ArthropodaClass: Insecta
Order: Hymenoptera
Family: Formicidae
Tribe: Plagiolepidini
Subfamily: Formicinae
Genus: Anoplolepis
Species: gracilipes
Type Information
Holotype
Specimen ID: ANIC32-017722Collected from: Fiji (-16.8oN, 179.433oE)
Location: Australian National Insect Collection (ANIC)
Syntypes
According to AntWiki, two syntypes of Formica gracilipes collected by F. Smith are held in the Oxford University Museum of Natural History (OUMNH) labelled 'Sing. 30'. Specimens are also labelled 'Aru' if from Aru Islands (Indonesia) and 'N' if from New Guinea. It is likely that CASENT0102951 and CASENT0103001 are the two referred here.Specimen ID: CASENT0102951
Collected from: Singapore
Location: Oxford University Museum of Natural History (OUMNH)
Specimen ID: CASENT0103001
Collected from: Singapore
Location: Oxford University Museum of Natural History (OUMNH)
Specimen ID: CASENT0903237
Collected from: Singapore
Location: British Museum of Natural History (BMNH)
Phylogeny
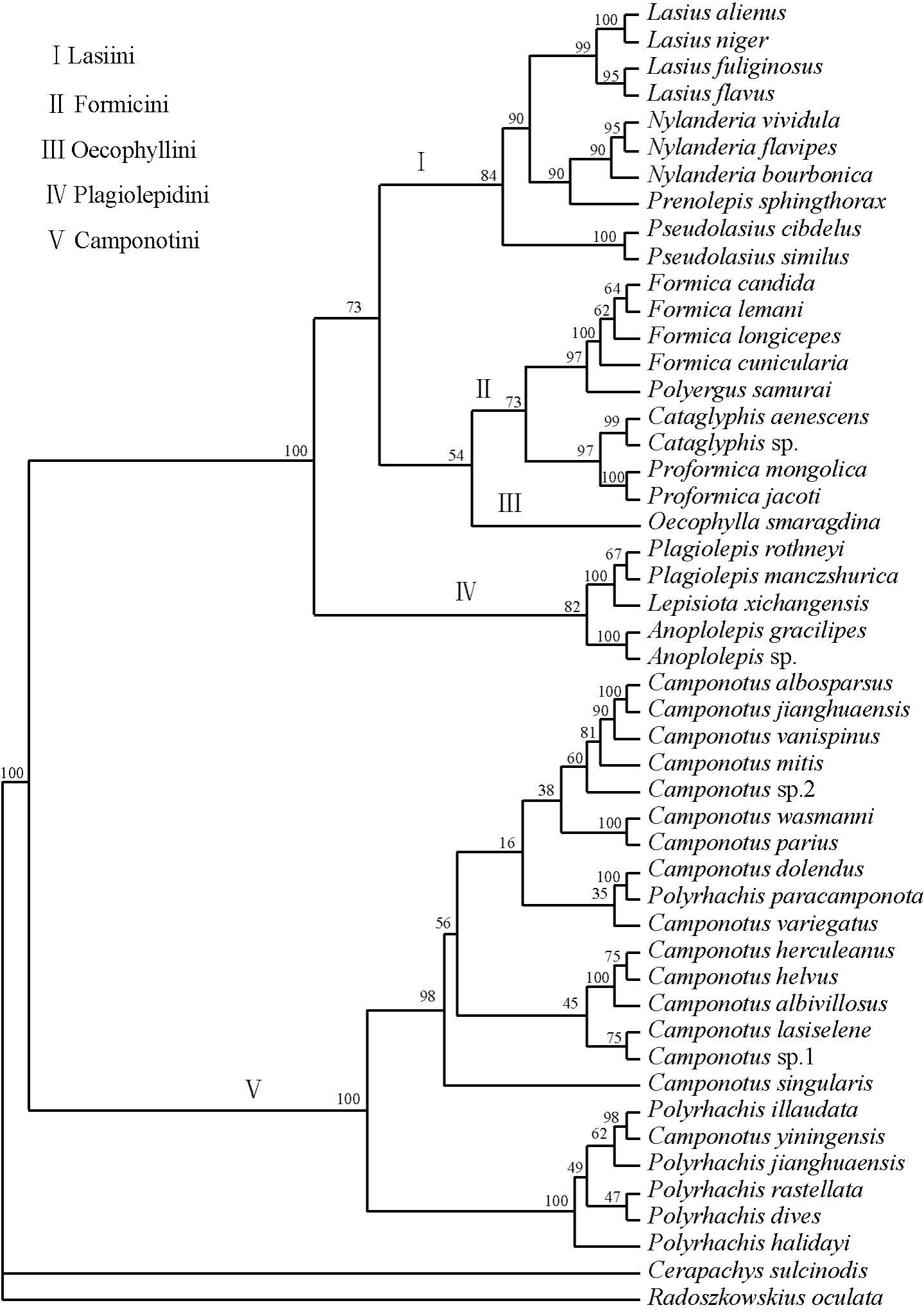
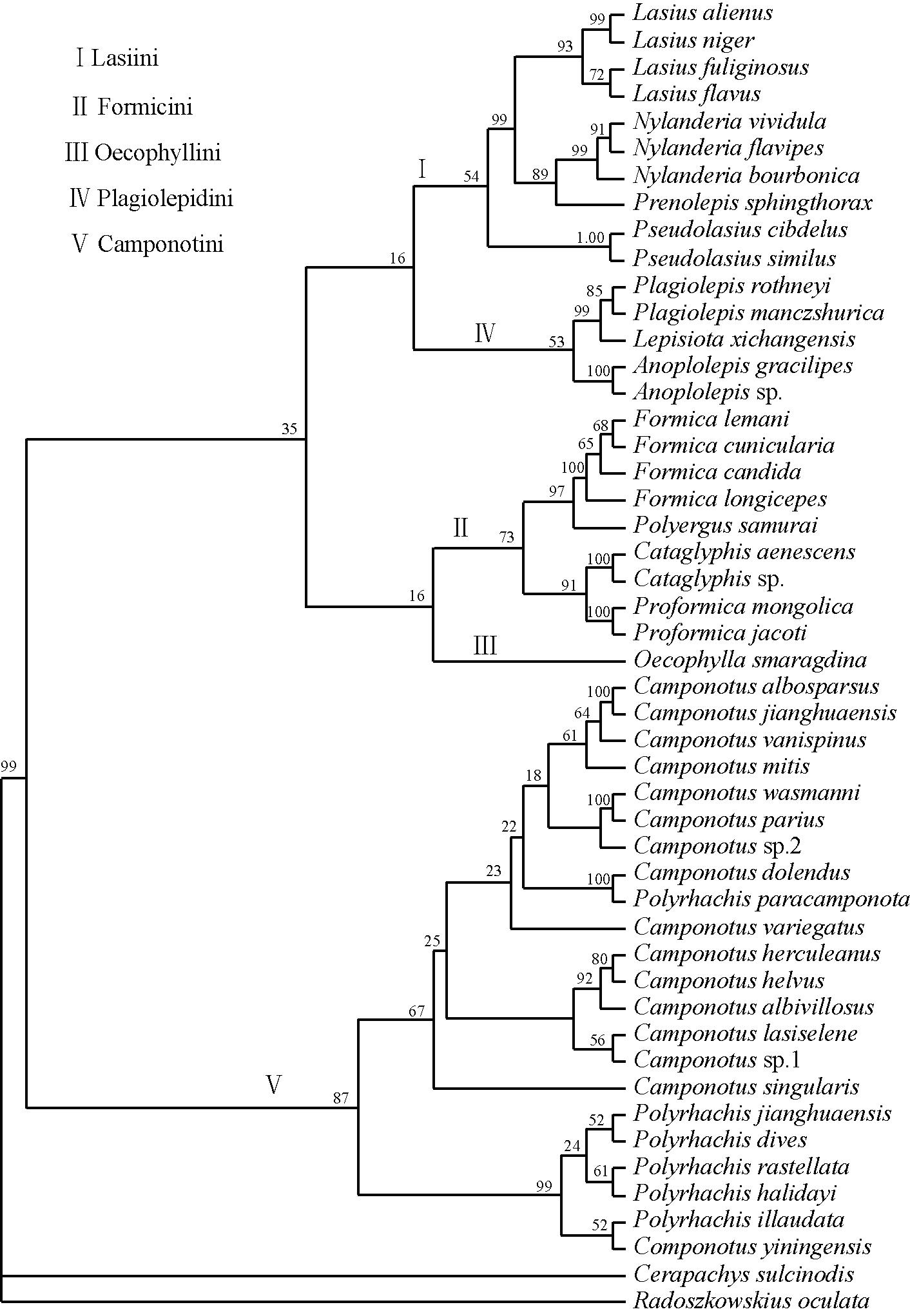
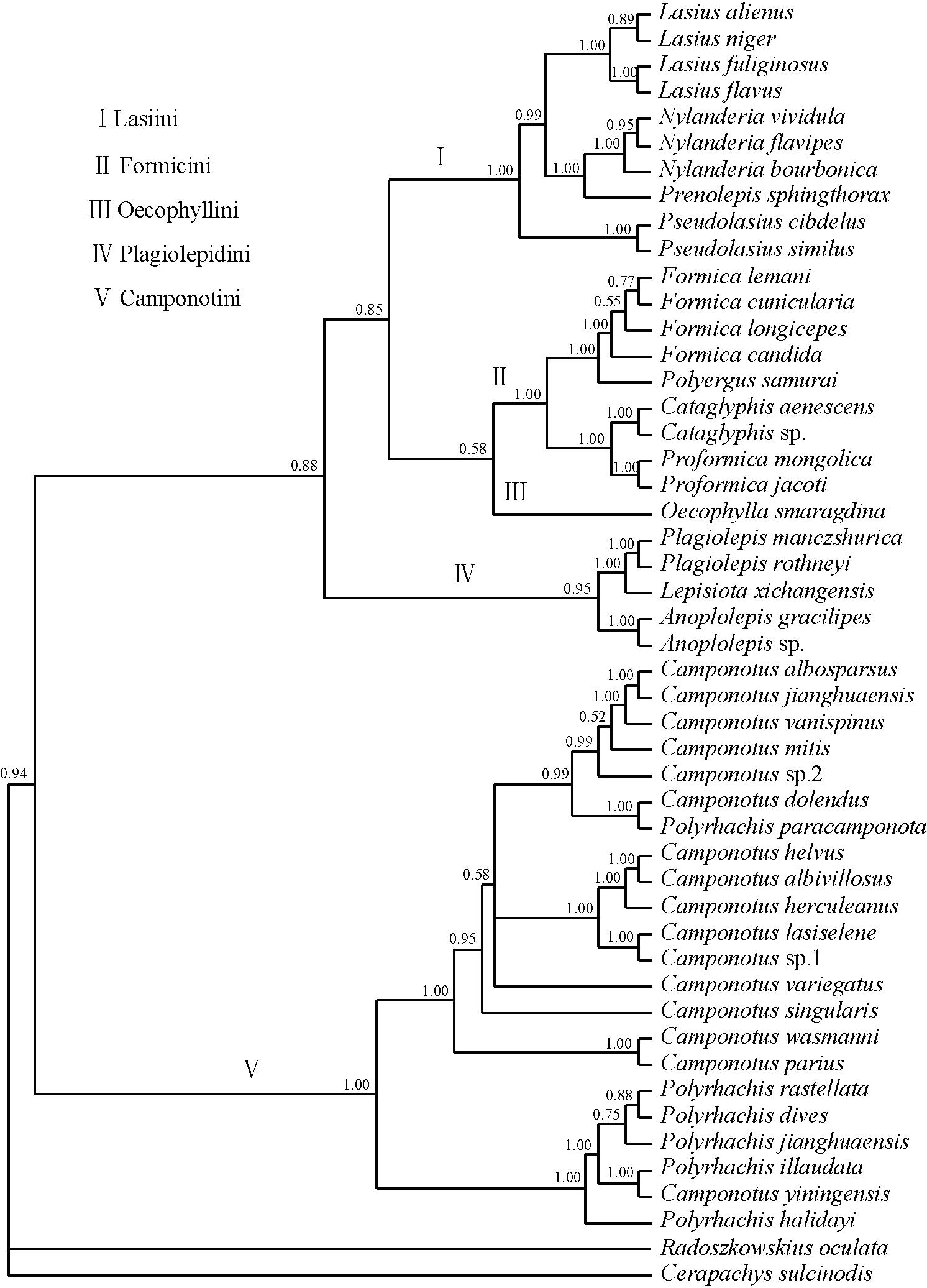
The trees below (maximum parsimony (MP), Bayesian majority-rule (BI), neighbour-joining (NJ)) were generated by running a 1000 bootstrap replicates using concatenated sequences of Cytb (44y bp), COI (825 bp), and COII (552 bp) genes [25]. There is very strong support for the position of Anoplolepis sp. in the tribe Plagiolepidini on the Tree of Life (Bootstrap values for MP = 100; BI = 100; NJ = 1.00). These phylogenetic analyses vindicate Bolton & Ficken's [26] earlier taxonomic placement based on morphological analysis.
|
|
||||
|
References
1. Lach, L., Parr, C. L., & Abbott, K. (2010). Ant ecology. Oxford University Press.2. Drescher, J., Blüthgen, N., & Feldhaar, H. (2007). Population structure and intraspecific aggression in the invasive ant species Anoplolepis gracilipes in Malaysian Borneo. Molecular Ecology, 16(7), 1453-1465.
3. O'Dowd, D. J., Green, P. T., & Lake, P. S. (2003). Invasional ‘meltdown’on an oceanic island. Ecology Letters, 6(9), 812-817.
4. Haines, I. H., & Haines, J. B. (1978a). Colony structure, seasonality and food requirements of the crazy ant, Anoplolepis longipes (Jerd.), in the Seychelles.Ecological Entomology, 3(2), 109-118.
5. Abbott, K. L., Greaves, S. N., Ritchie, P. A., & Lester, P. J. (2007). Behaviourally and genetically distinct populations of an invasive ant provide insight into invasion history and impacts on a tropical ant community.Biological Invasions, 9(4), 453-463.
6. Gruber, M. A. M., Hoffmann, B. D., Ritchie, P. A., & Lester, P. J. (2013). The conundrum of the yellow crazy ant (Anoplolepis gracilipes) reproductive mode: no evidence for dependent lineage genetic caste determination. Insectes sociaux, 60(2), 135-145.
7. Chong, K. F., & Lee, C. Y. (2009). Influences of temperature, relative humidity and light intensity on the foraging activity of field populations of the longlegged ant, Anoplolepis gracilipes (Hymenoptera: Formicidae). Sociobiology, 54(2), 531.
8. Wetterer, J. K. (2005). Worldwide distribution and potential spread of the long-legged ant, Anoplolepis gracilipes (Hymenoptera: Formicidae). Sociobiology,45(1), 77-97.
9. Tan, C. K., & Corlett, R. T. (2012). Scavenging of dead invertebrates along an urbanisation gradient in Singapore. Insect Conservation and Diversity, 5(2), 138-145.
10. Abbott, K., Harris, R., & Lester, P (2005). Invasive ant risk assessment: Anoplolepis gracilipes. Landcare Research contract report for Biosecurity New Zealand, Ministry of Agriculture and Forestry, Wellington, New Zealand.
11. Holway, D. A., Lach, L., Suarez, A. V., Tsutsui, N. D., & Case, T. J. (2002). The causes and consequences of ant invasions. Annual review of ecology and systematics, 181-233.
12. Fluker, S. S., & Beardsley, J. W. (1970). Sympatric associations of three ants: Iridomyrmex humilis, Pheidole megacephala, and Anoplolepis longipes in Hawaii. Annals of the Entomological Society of America, 63(5), 1290-1296.
13. Lowe S., Browne M., Boudjelas S., De Poorter M. (2000) 100 of the World’s Worst Invasive Alien Species A selection from the Global Invasive Species Database. Published by The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN), 12pp.
14. Haines, I. H., & Haines, J. B. (1978b). Pest status of the crazy ant, Anoplolepis longipes (Jerdon)(Hymenoptera: Formicidae), in the Seychelles. Bulletin of entomological Research, 68(04), 627-638.
15. Kaiser-Bunbury, C. N., Cuthbert, H., Fox, R., Birch, D., & Bunbury, N. (2014). Invasion of yellow crazy ant Anoplolepis gracilipes in a Seychelles UNESCO palm forest. NeoBiota, 22, 43-57.
16. McGregor, A. J., & Moxon, J. E. (1985). Potential for biological control of tent building species of ants associated with Phytophthora palmivora pod rot of cocoa in Papua New Guinea. Annals of applied biology, 107(2), 271-277.
17. Hill, M., Holm, K., Vel, T., Shah, N. J., & Matyot, P. (2003). Impact of the introduced yellow crazy ant Anoplolepis gracilipes on Bird Island, Seychelles.Biodiversity & Conservation, 12(9), 1969-1984.
18. Smith, F. (1857). Catalogue of the hymenopterous insects collected at Sarawak, Borneo; Mount Ophir, Malacca; and at Singapore, by AR Wallace.Journal of the Proceedings of the Linnean Society of London. Zoology, 2(6), 42-88.
19. Man, L. S., & Lee, C. Y. (2012). Structure-Invading Pest Ants in Healthcare Facilities in Singapore. Sociobiology, 59(1), 241.
20. Slip, D., & Comport, S. (2001). The status of the Yellow Crazy ant (Anoplolepis gracilipes) on the Cocos (Keeling) Islands. Report to Parks Australia North, Cocos (Keeling) Islands.
21. Csurhes, S., Hankamer, C. (2012). Pest Animal Risk Assessment: Yellow Crazy Ant Anoplolepis gracilipes. Biosecurity Queensland.
22. Bolton, B. (1995). New general catalogue of the ants of the world. Harvard University Press.
23. Jerdon, T. C. (1854). X.—A catalogue of the species of ants found in Southern India. Journal of Natural History, 13(74), 100-110.
24. Mayr, G. (1867a). Adnotationes in monographiam formicidarum Indo-Neerlandicarum. Tijdschrift voor Entomologie, 10, 33-117.
25. Chen, Z., Zhou, S. Y., Ye, D., Chen, Y., & Lu, C. (2013). Molecular Phylogeny of the Ant Subfamily Formicinae (Hymenoptera, Formicidae) from China Based on Mitochondrial Genes. Sociobiology, 60(2), 135-144.
26. Bolton, B., & Ficken, L. (1994). Identification guide to the ant genera of the world (Vol. 222). Cambridge: Harvard University Press.
See Also
AntkeyAntWeb
AntWikiAustralian National Insect Collection Database
CABI Invasive Species CompendiumISSG Database
Pacific Island Ant Key
| Page | Date Edited |
|---|---|
| Anoplolepis gracilipes | Dec 21, 2015 |
| Home | Dec 3, 2017 |