Table of Contents
Overview
Most people have a love-hate relationship with honeybees. On one extreme, you may run upon hearing their 'buzz' for fear of being stung, while on the other extreme, you cannot deny that these hardworking honeybees help to produce one of the most nutritious food source we are enjoying -- honey. Actually, honeybees only attack when they are very provoked, so you are definitely safe as long as you do not disturb them when they are at work (pollinating flowers) or at home (hive). Besides, did you know that a bee loses her life with each sting? You have to agree that this is such a huge sacrifice compared to the mere swelling you get!
Some of you may think that all bees are honeybees due to their ubiquitous presence in our environment. But in fact, out of the 20,000 species of bees known in the world, there are only 10 recognized species of honeybees [1]! Honeybees belong to the genus Apis, and they possess a social organization system which is intriguingly complex yet orderly at the same time, as you would find out later!
This page features one of the most primitive species of honeybee: Apis florea. It is also known as the Red dwarf honeybee, due to the reddish-brown coloration on their abdomen, and their relatively small size compared to the other honeybees. Even though they are one of the first honeybees to ever exist in Southeast Asia, they were surprisingly only first recorded in Singapore in 2012 (NUS Bee Lab)! Now, get BEE-sy as you learn about A. florea and its behaviour, morphology and other interesting characteristics!
|1| General Information
1.1 Other honeybees in Singapore
Apart from A. florea, only three other species of honeybees can be found in Singapore. They differ in size, appearance, temperament and nesting behavior!
| Apis cerena Asian honeybee |
Apis dorsata Giant honeybee |
Apis andreniformis Black Dwarf honeybee |
| Figure 1B: Other honeybees in Singapore.Source: Flickr [Sharing allowed by users in accordance to Flikr terms] |
||
| A. cerena is one of the most common local honeybee species. With an intermediate body size, it builds multiple combs on the roof of cavities, such as tree hollows and even within concrete structures. Being highly adaptable in urban environments, it is also favored for apiculture due to the ample store of honey in its multiple combs. |
A. dorsata is known for its relatively large size and aggressive nature. Its comb tend to be single, massive, and built in the open. They are usually aggregated, where multiple colonies can be found clustered on a single tree. When provoked, a colony attack could be deadly so this is one species you definitely want to stay away from! |
Like A. florea, A. andreniformis is a small, ancestral, open-nesting honeybee species, building a single comb around a small branch. It is generally mild-tempered and harmless. Often confused with each other, it can be differentiated from A. florea by its black abdomen and the white hair (See Section 6 for more information). |
1.2 General characteristics of honeybees
A. florea shares a unique suite of characteristics with the other honeybees [2]:
(i) Eusociality - Honeybees are highly social and interdependent insects. There is a pronounced reproductive division of labour within a colony, exhibited by a caste system comprising of the female queen, male drones, and female worker bees. The queen and drones take up reproductive roles, while the sterile worker female bees carry out other important tasks like foraging and housekeeping duties. Thus, no individual queen, drone, or worker can reproduce or survive on its own - their survival are highly dependent on one another.
Some characteristics differentiating the queen, drone and worker bees of A. florea are:
 Figure 1C: Queen bee Photo: Zachary Huang [With permission] |
 Figure 1D: Male drone bee Photo: Emirates Natural History Group [Permission pending] |
 Figure 1E: Female worker beesPhoto: Dave Clark [With permission] |
| Queen - Possess massively expanded ovaries and spermatheca (sperm-storage organ) - Has the ability to control fertilization through the control of a valve which regulates sperm transfer from the spermatheca to the oviduct (where eggs are stored) - Can sting repeatedly |
Drone - Arise from unfertilized haploid eggs - Reared on periphery of brood cells - Sole function is to mate with queen - Possess endophallus: a membranous penis totally contained within the abdomen |
Worker - Possess vestigial but functional ovaries which may be activated to produce a small number of eggs - Are stepsisters to one another with different paternity due to multiple mating by the queen - Caste fate is decided by diet during larval development (See Section 3.2) |
(ii) Haplodiploidy - This is the genetic system of inheritance whereby males are haploid, possessing only half the number of chromosomes as females (diploid). As such, this allows the queen to have total control over the sex of her offspring with her ability to selective fertilize her eggs, as you would find out later (See Section 4.1).
(iii) Polyandry - Queens mate with not just one, but multiple male drones from other colonies. This ensures a genetic mix of DNA in the resultant worker bees, and thus a better colony fitness. If she were only to mate with one drone, all the daughter bees produced will essentially be clones. If she were to mate with drones from her own colony (i.e. her 'son'!), it would be a form a severe inbreeding (the DNA of drones are identical to 50% of the queen's DNA). Hence, it is necessary for a queen to have multiple sex partners for the best of her colony.
(iv) Waggle dance communication - Distance and direction - both important information necessary for foraging and migration, are communicated to the colony through a waggle dance. This communication mechanisms unique to Apis is simple yet sophisticated enough to convey the vital message. So what if they can't talk? (See Section 4.3)
1.3 Distinguishing features
In the field, A. florea can be identified by the following features, observable to the naked eye.
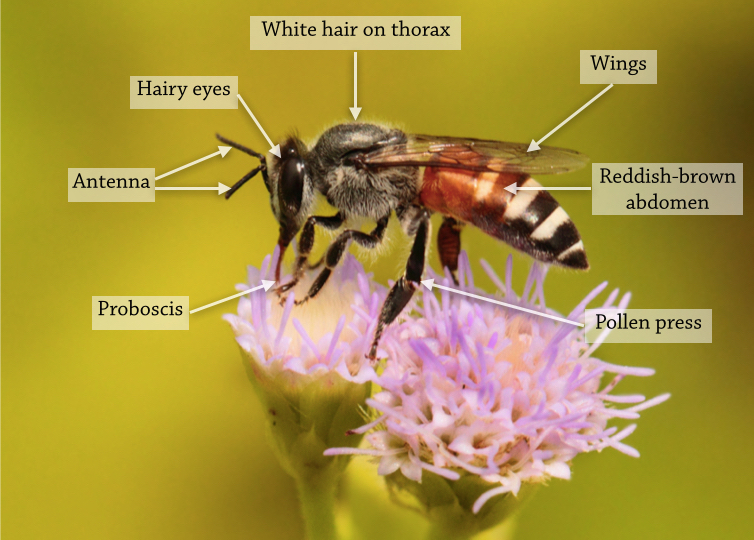 |
| Figure 1F: General features of Apis florea Photo: Albert Noorlander [With permission] Annotations by Lai Jun Li. |
| Video: Apis florea foraging on Coral vine Source: Youtube |
1.4 Distribution
Global |
|---|
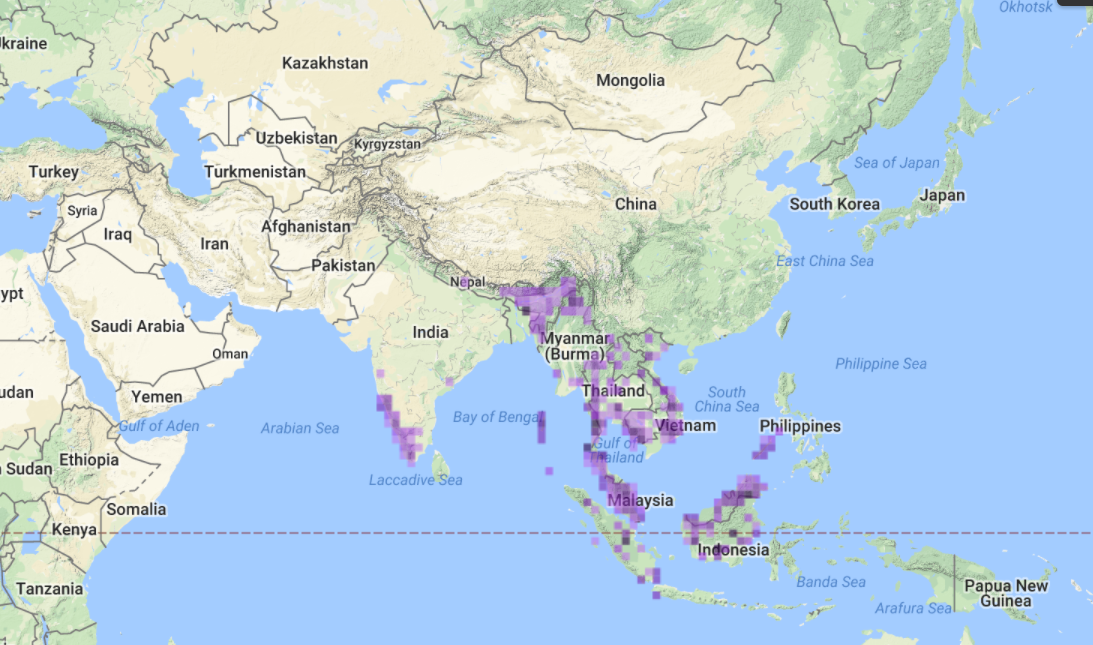
| Figure 1G. Global distribution of Apis florea. Data & Map: Discover Life [For public use] |
Native to Southern and Southeast Asia, A. florea has a fairly widespread distribution across the low latitudes due to its preference for warm climate. It can be found west in the Plateau of Iran, Oman, across mainland Asia, along the southern border of the Himalayas and further east in Vietnam, Southeast China and Peninsular Malaysia [3].
Local |
|---|
| Figure 1H. Local distribution of Apis florea.Data: NUS Bee LabMap: ScribbleMaps [For use under Creative Commons Attribution-NonCommercial-ShareAlike 2.5 license] |
Surprisingly, A. florea was only first recorded in Singapore recently (from 2012) despite its native range in Southern and Southeast Asia [3]. Thus, this makes it a new addition to our local honeybee fauna as the fourth known honeybee species in Singapore. Its current spread has been recorded, but not limited to the western and southern parts of Singapore such as: Chinese Garden, Kent Vale and Hort Park (NUS Bee Lab, personal observations).
|2| Natural History
2.1 Habitat
Nests of A. florea tend to be found in open savannah woodland, disturbed agricultural and urban areas [4].
2.2 Nesting Biology
 |
|---|
| Figure 2A: Hive of Apis florea in Thailand. Photo: Sean Hoyland [Public domain file] |
2.3 Ecosystem services
Like all honeybees, A. florea are important pollinators of tropical plants and agricultural crops, aiding in transfer of pollen from one flower to another in the process of collecting pollen for themselves. In the urban areas, these honeybees also help to pollinate many common urban plants, allowing them to thrive and bloom to create our 'Garden city'. By far, observations of A. florea pollination were recorded on the following plants (Personal observations, NUS Bee Lab):
 |
 |
 |
 |
| Figure 2B: Rose of India(Lagerstroemia indica) Photo: Kwan Han [With permission] |
Figure 2C: White weed (Ageratum conyzoides)Photo: Kwan Han [With permission] |
Figure 2D: Coral vine (Antigonon leptopus)Photo: Kwan Han [With permission] |
Figure 2E: Coat buttons (Tridax procumbens) Photo: Kwan Han [With permission] |
2.4 Predators & Parasites
 |
 |
 |
| Figure 2F: Weaver ant Photo: Muhammad Mahdi Karim [With permission] |
Figure 2G: Greater banded hornet Photo: Kwan Han [With permission] |
Figure 2H: Greater wax moth larvae Photo: Rob Synder (Bee Informed Partnership) [With permission] |
The weaver ants (Oecophylla smaragdina) are one of the most threatening predators of A. florea [6], possibly due to their common habitat type in secondary growth forest margins. Giant social wasps (e.g. Greater-banded hornet (Vespa tropica)) also have the potential to wipe out a colony of A. florea. Larvae of the greater wax moths (Galleria mellonella) are known to destroy combs of A. florea and other honeybees. They do so by burrowing into the hive, consuming the comb, pollen and brood along the way. Though worker bees continually remove them from hive, they may be overwhelmed when the larvae are present in high numbers [2]. Other attackers include flower beetles (Protaetia sp.) and stingless bees, which targets the hive for the honey resource [5].
A. florea is also known to experience parasitic attacks by mites (Euvarroa sinhai). However, there is usually minimal impact as they reproduce only on drone broods [2].
|3| Biological characteristics
3.1 Detailed morphology
As the queen bee and drones usually stay in the hive and are less commonly encountered, the detailed morphology and functions of the more common female worker bee will be discussed.
 |
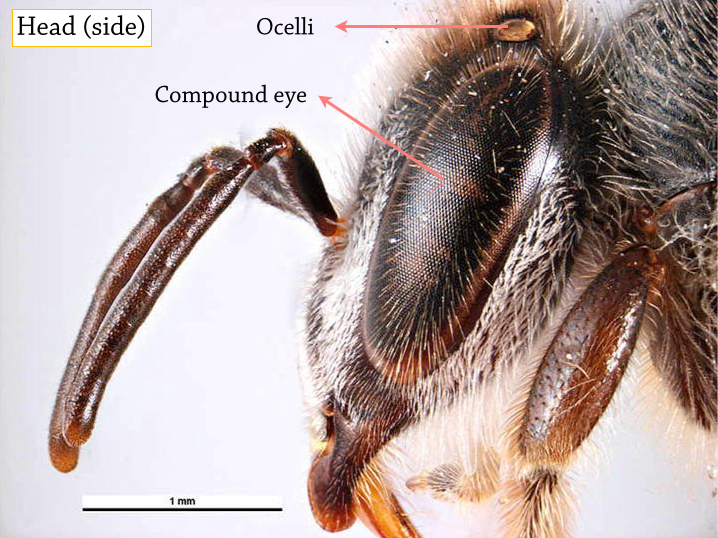 |
| Each bee has three ocelli and two compound eyes. The ocelli are used for detecting brightness, while the compound eyes provides sensitivity to ultraviolet (UV) and polarized vision. The UV vision allows them to visualize nectar guides on some flower petals, which are only visible in the UV spectrum. The polarized vision allows them to orientate and provides a sense of direction using polarized light from the sun, which is important during very cloudy days. |
|
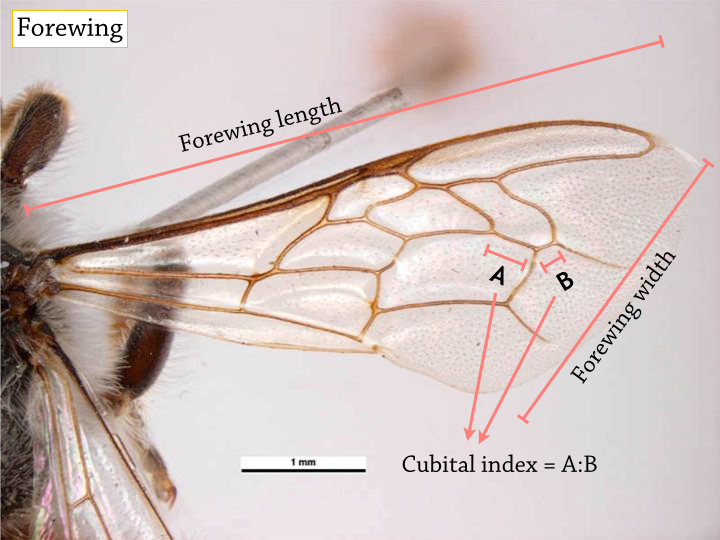 |
 |
| The following characteristics varies among honeybees and are often used in morphological identification. In the worker bees of A. florea, these characteristics measure (mm) [7][8][9]: - Forewing length: 6.17-6.74 - Forewing width: 2.12-2.32 - Hindwing length: 3.17-4.83 - Hingwing width: 1.36 ± 0.04 - Cubital Index: 2.86-3.50 - Hamuli number: 10.5-13.2 The jugal lobe is a diagnostic feature present in all honeybees. The hamuli is a series of hooks which 'clips' the hindwing together with the forewing, allowing for a more efficient and stable flight. |
|
 |
 |
| The white hair on the thorax and abdomen are characteristic of A. florea. This feature is often used to distinguish between itself and A. andreniformis (See Section 6) The sting (not annotated) is located in a chamber at the end of the abdomen. It is mild and not capable of penetrating the human skin. |
|
 |
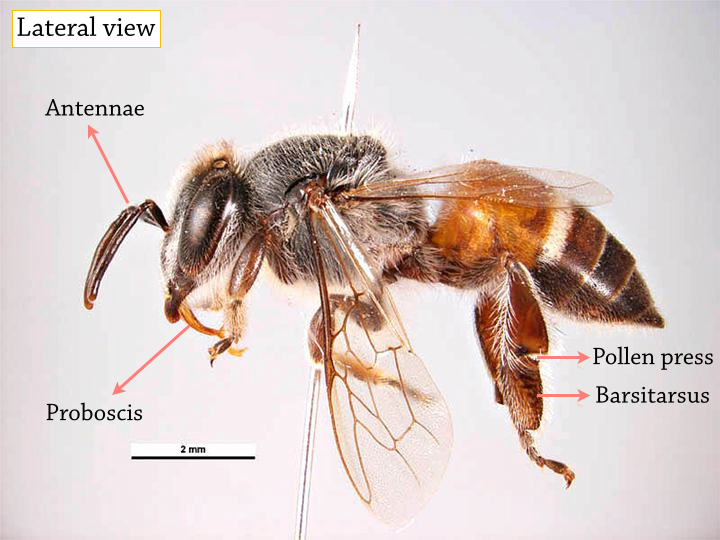 |
| The antennae contains sensitive receptors for a sense of touch and smell (to differentiate between floral and pheromone odor). The proboscis acts as the tongue of the honeybee, and is thus used to draw up liquid like water and nectar. It is withdrawn when not in use. The pollen press (or pollen 'basket') is a concave depression on the hind leg. The surrounding hairs enables the pollen to be compounded into a solid mass at the depression for transport back to the hive. While absent in the female worker bee, the barsitarsus of a male drone has a 'thumb' which is used to hold on to the hind legs of the queen during in-flight copulation [2]. |
|
| Figure 3A: Detailed morphology of female worker Apis florea Photo: Simon Hinkley & Ken Walker [For use under Creative Commons Attribution 3.0 Australia license] Annotations by Lai Jun Li |
|
3.2 Diet
Like the other honeybees, adult A. florea has a vegetarian diet consisting of mainly pollen and nectar from flowering plants.
Interestingly, the diet of developing larvae is linked to their developmental fate [2]. At the initial stage of development, all the larvae are fed with royal jelly, an extremely nutritious food source chemically-modified from nectar by the worker bees. Subsequently, only the larvae chosen to be the future queen will be continually fed with the high-quality royal jelly, boosting her reproductive fitness. Conversely, the other larvae destined to be worker bees will only be fed with lower-quality nectar and honey, and thus grows into sterile workers.
3.3 Life cycle
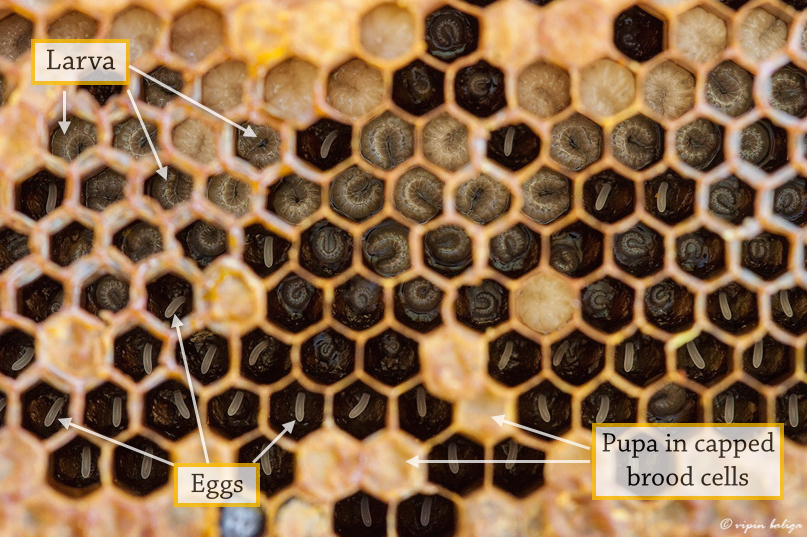
The life cycle begins when eggs are laid in individual brood cells by the queen. The development time from egg to larva to pupa to adult varies among the 3 caste [10] (Figure 3E).
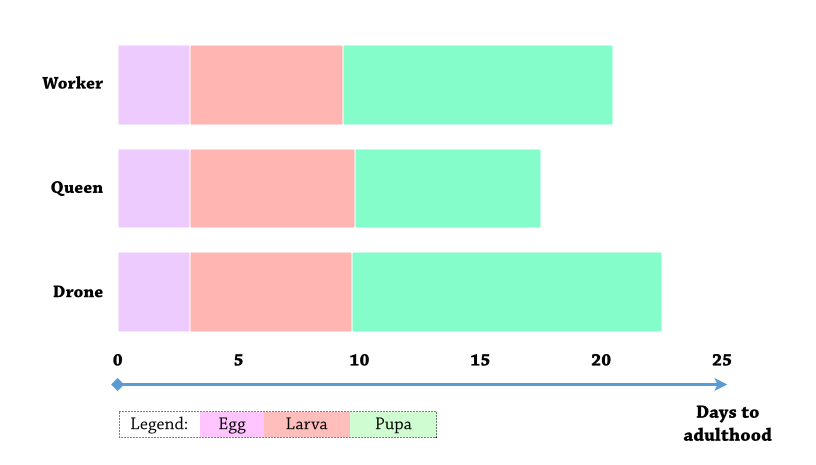 |
 |
| Figure 3B. Differential development time for castes of Apis florea. Source: (Sandhu & Singh, 1960) Illustration by Lai Jun Li. |
Figure 3C. Different stages of development in Apis florea brood cells Photo: Vipin Baliga [For use under Creative Commons Attribution 3.0 Unported license] Annotations by Lai Jun Li. |
| Video: First 21 days in the life of beesSource: Youtube |
Upon reaching adulthood, young worker bees take up the role of in-house duties such as feeding emerging larvae and cleaning of the hive. As they get older, their responsibilities include hive construction and nectar conversion to honey. Subsequently, their roles evolve to guarding of hive and foraging. Thus, the bees you see pollinating flowers are the older and more experienced ones.
|4| Behaviour
4.1 Mating & Reproduction
Mating typically occurs at a drone congregation area (DCA), where drones from different colonies gather to mate with visiting queens. During a mating flight, the male drone of Apis florea mounts itself on the queen bee midair. Its position is stabilized by using the ‘thumb’ at the basitarsus of its hind legs to firmly hold onto the queen’s legs [11][2]. After which, the drone releases its endophallus and ejaculates, depositing sperm directly into the spermatheca of the queen bee. Unfortunately, drones tend to die shortly after copulation as their abdomen is ripped apart during the release of their endophallus.
As polyandry is common practice in honeybees, the A. florea queen proceeds to mate with multiple drones before returning to her hive. She could have up to four partners, which is considered a relatively low number compared to other honeybee species [12]! Mating flights tend to be short-lasting of only about 20-30 minutes, so as to reduce chances of predation by birds [13]. The sperms deposited in the queen are then used for selective fertilization of eggs throughout her lifetime. Due to their haplodiploid genetic system, the queen has the ability to control the sex of her offspring (Figure 4A)
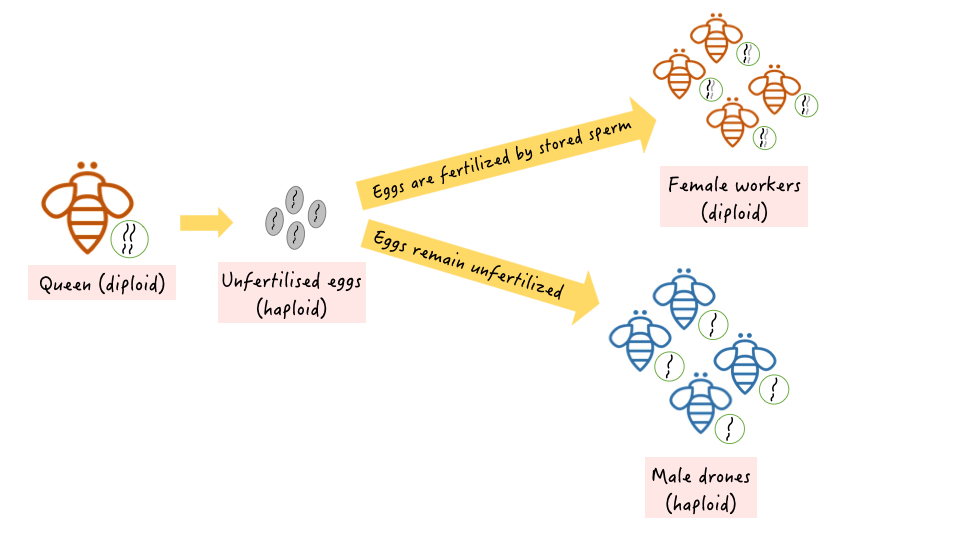 |
| Figure 4A. Illustration of sex determination & Haplodiploidy Source: Lai Jun Li |
4.2 Worker Policing
Although workers are effectively sterile, their ovaries may be activated to produce viable drones in the absence of a queen in the colony [14]. Thus, A. florea engages in worker policing, whereby worker-laid eggs are rapidly removed by oophagy (i.e. eating) to discourage worker reproduction [15]. As queen eggs are usually marked with pheromone, worker bees are able to discriminate between queen-laid and worker-laid eggs to selectively rear and destroy them respectively.
4.3 Foraging & Communication
When a foraging worker bee finds a resource-rich flower patch, she returns to the hive to inform the rest of the colony about the good news. This is done through a unique and communication mechanism called the waggle dance. Information such as the direction and distance of the resource patch is encoded in the dance. Usually, the waggle dance of A. florea is conducted on the horizontal plane at the top of the exposed hive [3]. The bees also rely on the sun’s position as an important cue in the orientation of the dance [16].
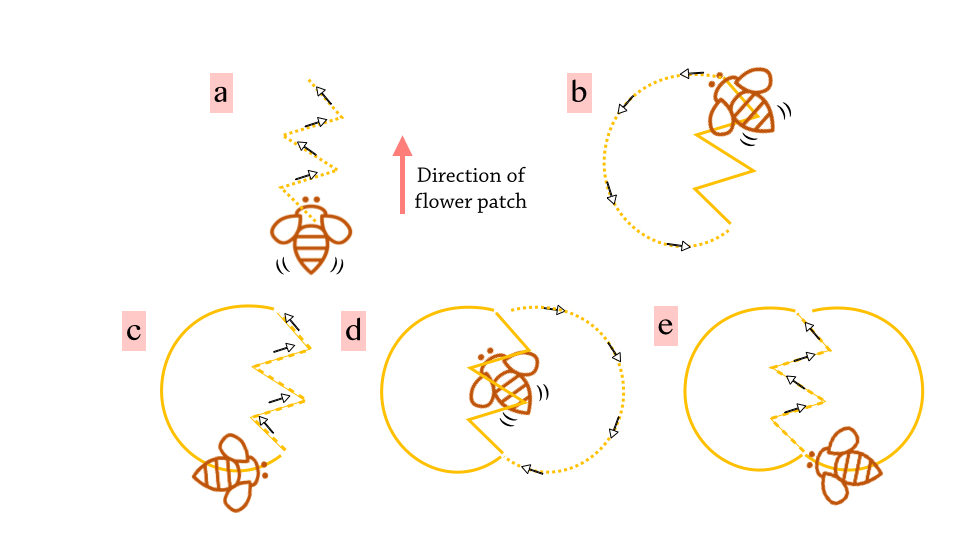 |
(a) The dance commences with the bee striding in the direction of the food source with a waggling motion. The longer the duration of the waggling, the further the distance of the food and vice versa. (b) & (c) Then, the bee would turn to either the left or right direction and encircle back to her starting point. (d) & (e) The waggling motion is repeated and this time the bee turns to the other direction and encircles back. This process is then repeated several times to ensure that the message successfully spreads to as many fellow worker bees as possible. The waggle dance occurs in the figure-of-eight pattern, as the choreography strongly resembles the number ‘8’! |
| Figure 4B. Illustration of Waggle dance Source: Lai Jun Li |
4.4 Nesting Defence
As mentioned, A. florea tends to nest in shaded locations. However, if they are unfortunately spotted by predators, the colony is easily overpowered as their stings are relatively painless and ineffective in deterring determined predators [2]. In these events, the entire colony may flee and abandon their nest.
They also use propolis as a form of defensive barrier against crawling insects, especially weaver ants. Propolis are sticky bands of resins, applied around the branch supporting the nest to block access to their hive.
Sometimes, worker bees at the hive would employ a ‘shimmering’ behavior [18], commonly to ward off flying predators. A few worker bees starts by raising and lowering their abdomens in the air. This stimulates neighboring bees to do the same, thus creating a rippling effect which spreads across the surface of the curtain of workers, very much like football fans in a stadium!
| Video: Shimmering behavior in Apis floreaSource: Youtube |
4.5 Migration and Absconding
Besides escaping from threatening predators, A. florea may also choose to abandon their nest and abscond when conditions becomes unfavorable at their current nest site, for instance loss of shade, parasitism, or lack of food [2]. Migration from the nest can be observed upon emergence of a new queen. The older queen will move to a new site with some worker bees, leaving the young queen to inherit the old hive and establish her own colony.
Prior to migration, active worker bees, also known as 'scouts', perform the waggle dance to inform the colony about the general direction of the nest-site to ensure that the swarm flies towards a coherent direction upon liftoff [19]. Once they reach a suitable area, such as a shaded tree, the swarm coalesces onto a branch randomly. The quality of the site is then assessed in situ i.e. presence of predators, availability of food. If proved unsuitable, the swarm simply moves on to seek for a better site.
If the new nesting site is less than 100-200m away from the old site, the colony would reuse the beewax of their old nest for the construction of their new nest [20]. This is a behavior known to be exhibited only by A. florea but not other honeybee species [21].
|5| Conservation status
A. florea has not yet been classified by the IUNC.
|6| Comparison with Apis andreniformis
A. florea is often confused historically with its sister species, Apis andreniformis (Black Dwarf honeybee) due to their similar morphology and overlapping distribution throughout tropical and subtropical Asia, including Southeast China, India, Burma, Laos, Vietnam, Malaysia, Indonesia, and the Philippines [22]. They were only established as separate species in the 1987 [23]. Thus, prior studies conducted in the area of sympatry are likely to have incorrect species designation, where A. andreniformis was commonly misidentified as A. florea [2]. As such, it is important to know how to distinguish these two species to avoid confusion.
Distinct morphological differences between A. florea and A. andreniformis include:
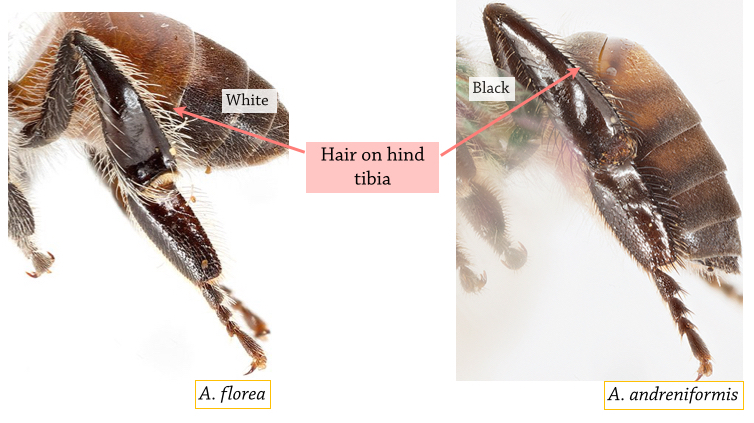 Figure 6A. Comparison of hind tibia Photos: Eunice Soh [With permission] Annotations by Lai Jun Li. |
|
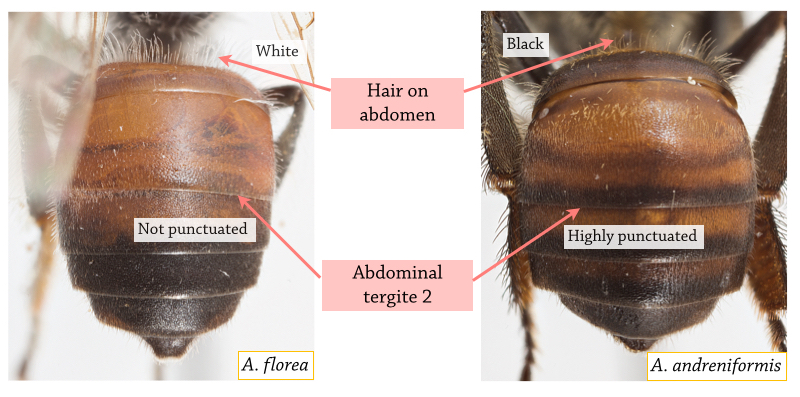 Figure 6B. Comparison of abdomen Photos: Eunice Soh [With permission] Annotations by Lai Jun Li. |
|
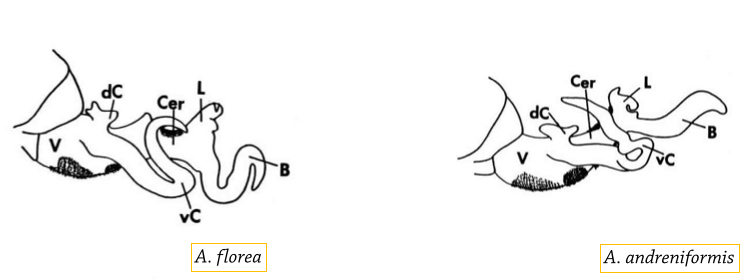 Source: Koeniger et. al. (1991) [11] |
Furthermore, A. florea and A. andreniformis also exhibit distinct behavioral differences, for instance:
(i) Separate mating time : Mating flights of A. andreniformis occur after the sun passes its zenith while for A. florea it occur later in the afternoon [24].
(ii) Fanning behaviour: To cool down the nest when it the temperature is too high (above 33 degrees Celsius), worker bees of A. andreniformis fan facing downwards while A. florea workers fan facing upwards [25].
(iii) Comb morphology: The crown of the comb of A. andreniformis contains a midrib which is absent in A. florea [26]
|7| Nomenclature
7.1 Taxonomic hierarchy
- Kingdom: Animalia
- Subkingdom: Bilateria
- Infrakingdom: Protostomia
- Superphylum: Ecdysozoa
- Phylum: Arthropoda
- Subphylum: Hexapoda
- Class: Insecta
- Subclass: Pterygota
- Infraclass: Neoptera
- Superorder: Holometabola
- Order: Hymenoptera
- Suborder: Apocrita
- Infraorder: Aculeata
- Superfamily: Apoidea
- Family: Apidae
- Subfamily: Apinae
- Tribe: Apini
- Genus: Apis (Linnaeus, 1758)
- Subgenus: Micrapis (Ashmead, 1904)
- Species: florea (Fabricius, 1787)
There are no subspecies recognized for A. florea.
7.2 Phylogenetic relationship
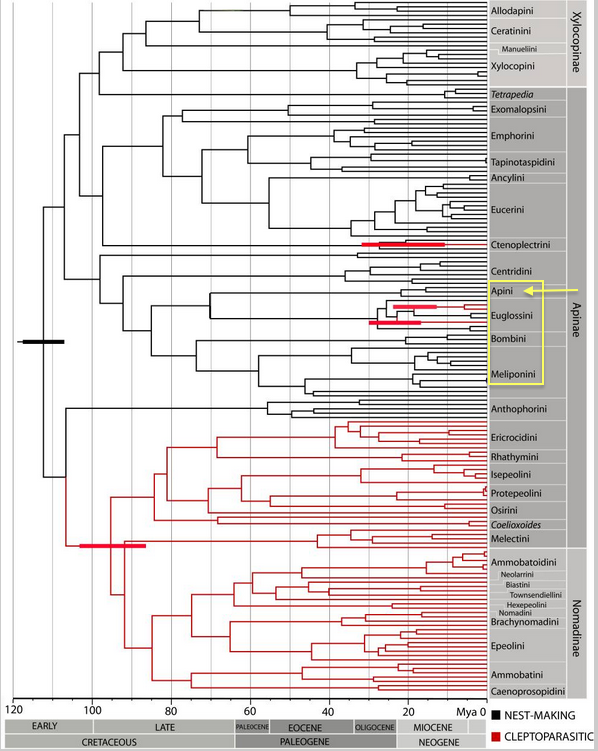 |
| Figure 7A. Maximum clade credibility tree of the Bayesian phylogenetic analysis of Apidae with a relaxed fossil-calibrated molecular-clock model. Source: Cardinal, Straka & Danforth (2010) [26] |
Phylogenetic analysis of the family Apidae revealed the emergence of a clade, which is also known as corbiculate bees, in the subfamily Apinae at approximately 90 million years ago (Figure 7A). Consisting of four main tribes (Apini, Euglossini, Bombini and Meliponini), corbiculate bees are characterized by their pollen baskets, or corbiculae, present on their hind tibia. This method of packing and carrying pollen is believed to have evolved in this clade [2]. The tribal relationships among these four tribes of (i) Apini (Honeybees), (ii) Euglossini (Orchid bees), (iii) Bombini (Bumble bees) and (iv) Meliponini (Stingless bees) remain undetermined and controversial with strikingly different conclusions. It is, however, important for understanding the evolution of eusociality [28].
Similarly, there are no consensus on the phylogeny of honeybees (genus Apis), as trees derived from molecular data sets were different from those inferred from behavioral and morphological data [2]. However, there is a general agreement on the existence of three monophyletic groups in Apis, being:
(i) Dwarf bees (A. florea and A. andreniformis),
(ii) Giant bees (A. dorsata, A. binghami, and A. laboriosa) and,
(iii) Cavity-nesting bees (A. mellifera, A. cerana, A. koschevnikovi, A. nuluensis, and A. nigrocincta).
Figure 7B shows the likely phylogenetic tree of the genus Apis, based on molecular data of nuclear (ND2) and mitochondrial DNA (EF1-α intron) data [29] and morphological data from larvae [30].
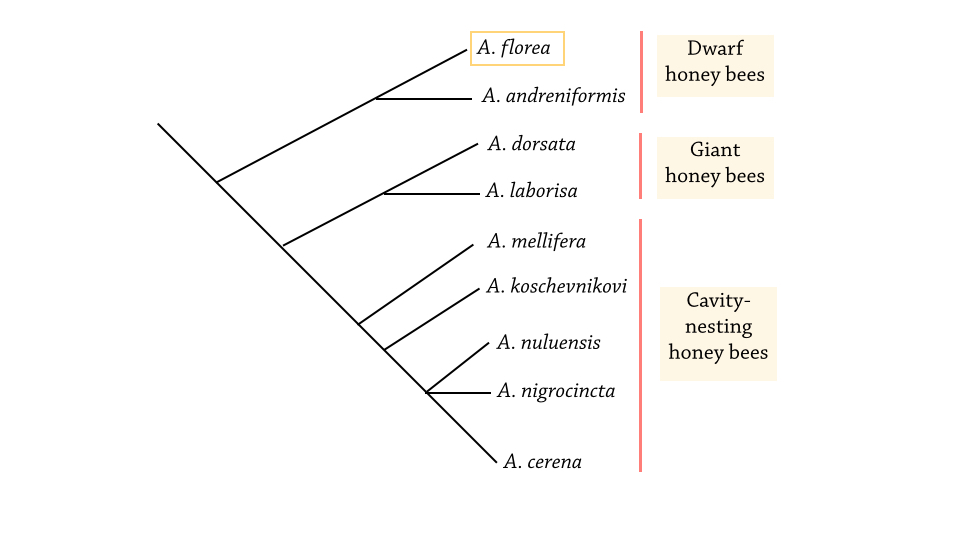 |
| Figure 7B. Phylogeny of the genus Apis. Adapted from Oldroyd & Wongsiri (2006), based on Arias & Sheppard (2005), Engel & Schultz (1997) [2][28][29] |
The basal position of A. florea is also supported by the high likelihood of open-nesting being an ancestral trait of honeybees, given that the dwarf and giant honey bees both exhibited open-nesting. Thus, it is more likely that cavity-nesting is a derived condition rather than an ancestral state, since the opposite would require the character to be lost twice in the lineages leading to the dwarf and giant honeybees and later regained in the cavity-nesting species.
7.3 Original description
Apis florea was first identified and described by Danish zoologist Johan Christian Fabricius in 1787.
 |
| Figure 7C. Original description of Apis floreaSource: Biodiversity Heritage Library: Mantissa Insectorium |
The translation of the original Latin description provides some information about A. florea then:
- A grey tufty abdomen.
- Lives in eastern India.
- Black antennae. Black head and thorax.
- Smooth red abdomen top with black base.
Though vague, these descriptions do match that of A. florea that we know today. However, it is unsure if "grey tufty abdomen" suggests:
(i) the presence of clusters of short grey hair at the abdomen, or
(ii) the presence of clusters of short hair at the grey abdomen
Scenario (i) may present a slightly deviation from the species observed today, where white hair is present on its abdomen instead. It is unknown if this deviation could be due to the subjective perception of color or a morphological variation within the species. Scenario (ii) may be valid in describing the male drone of A. florea, which possess a grey abdomen.
The location of its type specimen can be found in Kiel museum [4].
7.4 Etymology
'Apis' is the Latin word for 'bee' while 'Florea' comes from a Romanian name.
7.5 Synonyms
Apis semirufa Hoffmannsegg 1818
Apis lobata Smith 1854
Apis floralis Horne and Smith 1870
Apis testacea Bingham 1898
Apis nursei Cockerell 1911
Micrapis florea (Fabricius) Ashmead 1904
Data from [1]
|8| References
1 Engel, M. (1999). The taxonomy of recent and fossil honey bees. Journal Of Hymenoptera Research, 8(2), 165-196.
2 Oldroyd, B., & Wongsiri, S. (2006). Asian honey bees. Cambridge, Mass.: Harvard University Press.
3 Hepburn, R. H., Radloff, S., Otis, G., Fuchs, S., Verma, L., Tan, K., et al. (2005). Apis florea: morphometrics, classification and biogeography. Apidologie , 36 (3), 359-376.
4 Otis, G. W.. (1996). Distributions of Recently Recognized Species of Honey Bees (Hymenoptera: Apidae; Apis) in Asia. Journal of the Kansas Entomological Society, 69(4), 311–333. Retrieved from http://www.jstor.org.libproxy1.nus.edu.sg/stable/25085727
5 Seeley, T., Seeley, R., & Akratanakul, P. (1982). Colony Defense Strategies of the Honeybees in Thailand. Ecological Monographs, 52(1), 43. http://dx.doi.org/10.2307/2937344
6 Duangphakdee, O., Koeniger, N., Koeniger, G., Wongsiri, S., & Deowanish, S. (2005). Reinforcing a barrier – a specific social defense of the dwarf honeybee ( Apis florea ) released by the weaver ant ( Oecophylla smaragdina ). Apidologie, 36(4), 505-511. http://dx.doi.org/10.1051/apido:2005036
7 Ruttner, F. (1988). Biogeography and Taxonomy of Honeybees. Berlin, Heidelberg: Springer Berlin Heidelberg.
8 Rinderer, T., Oldroyd, B., Wongsiri, S., Sylvester, H., de Guzman, L., Stelzer, J., & Riggio, R. (1995). A morphological comparison of the dwarf honey bees of southeastern Thailand and Palawan, Philippines. Apidologie, 26(5), 387-394. http://dx.doi.org/10.1051/apido:19950504
9 Ruttner, F., Mossadegh, M., & Kauhausen-Keller, D. (1995). Distribution and variation of size of Apis florea F in Iran. Apidologie, 26(6), 477-486. http://dx.doi.org/10.1051/apido:19950604
10 Sandhu, A. S., & Singh, S. (1960). The biology and brood rearing activities of the little honeybee (Apis florea Fabricius). Indian Bee J, 22, 27-35.
11 Koeniger, G., Koeniger, N., Mardan, M., Otis, G., & Wongsiri, S. (1991). Comparative anatomy of male genital organs in the genus Apis. Apidologie, 22(5), 539-552. http://dx.doi.org/10.1051/apido:19910507
12 Koeniger, N., Koeniger, G., & Wongsiri, S. (1989). Mating and sperm transfer in Apis florea. Apidologie, 20(5), 413-418. http://dx.doi.org/10.1051/apido:19890506
13 Nagaraja N., & Brockmann, A. (2009). Drone congregation areas of red dwarf honeybee, Apis florea. Available from Nature Precedings http://hdl.handle.net/10101/npre.2009.3955.1
14 Woyke, J., & Wongsiri, S. (1992). Occurrence and size of laying worker eggs in Apis florea colonies. Journal Of Apicultural Research, 31(3), 124-127.
15 Halling, L., Oldroyd, B., Wattanachaiyingcharoen, W., Barron, A., Nanork, P., & Wongsiri, S. (2001). Worker policing in the bee Apis florea. Behavioral Ecology And Sociobiology, 49(6), 509-513. http://dx.doi.org/10.1007/s002650100325
16 Koeniger, N., Koeniger, G., Punchihewa, R., & Fabritius, M. (1982). Observations and Experiments on Dance Communication in Apis Florea in Sri Lanka. Journal Of Apicultural Research, 21(1), 45-52. http://dx.doi.org/10.1080/00218839.1982.11100515
17 Beekman, M., Gloag, R., Even, N., Wattanachaiyingchareon, W., & Oldroyd, B. (2008). Dance precision of Apis florea—clues to the evolution of the honeybee dance language?. Behavioral Ecology And Sociobiology, 62(8), 1259-1265. http://dx.doi.org/10.1007/s00265-008-0554-z
18 Butler, C. (1974). The world of the honeybee. London: Collins.
19 Makinson, J., Oldroyd, B., Schaerf, T., Wattanachaiyingcharoen, W., & Beekman, M. (2010). Moving home: nest-site selection in the Red Dwarf honeybee (Apis florea). Behavioral Ecology And Sociobiology, 65(5), 945-958. http://dx.doi.org/10.1007/s00265-010-1095-9
20 Pirk, C., Crous, K., Duangphakdee, O., Radloff, S., & Hepburn, R. (2010). Economics of comb wax salvage by the red dwarf honeybee, Apis florea. J Comp Physiol B, 181(3), 353-359. http://dx.doi.org/10.1007/s00360-010-0530-6
21 Hepburn, R., Duangphakdee, O., Phiancharoen, M., & Radloff, S. (2010). Comb Wax Salvage by the Red Dwarf Honeybee, Apis florea F. J Insect Behav, 23(2), 159-164. http://dx.doi.org/10.1007/s10905-010-9205-0
22 Gupta, R. (2014). Taxonomy and Distribution of Different Honeybee Species. In R. Gupta, W. Reybroeck, J. Veen & A. Gupta, Beekeeping for Poverty Alleviation and Livelihood Security (1st ed., pp. 63-103). Retrieved from http://link.springer.com/chapter/10.1007%2F978-94-017-9199-1_2
23 Wu, Y., & Kuang, B. (1987). Two Species of Small Honeybee—A Study of the Genus Micrapis. Bee World, 68(3), 153-155. http://dx.doi.org/10.1080/0005772x.1987.11098924
24 Wongsiri, S., Lekprayoon, C., Thapa, R., Thirakupt, K., Rinderer, T., & Sylvester, H. et al. (1997). Comparative biology of Apis andreniformis and Apis florea in Thailand. Bee World, 78(1), 23-35. http://dx.doi.org/10.1080/0005772x.1997.11099328
25 Thapa, R., & Wongsiri, S. (1994, July). Distinct fanning behaviour of two dwarf honeybees Apis andreniformis (Smith) and Apis florea (Fab.). In T. Sakai (Ed.), Proceedings of Second International Conference of the Asian Apicultural Association (pp. 344-345).
26 Rinderer, T., Wongsiri, S., Kuang, B., Liu, J., Oldroyd, B., Sylvester, H., & Guzman, L. (1996). Comparative nest architecture of the dwarf honey bees. Journal Of Apicultural Research, 35(1), 19-26. http://dx.doi.org/10.1080/00218839.1996.11100909
27 Cardinal, S., Straka, J., & Danforth, B. (2010). Comprehensive phylogeny of apid bees reveals the evolutionary origins and antiquity of cleptoparasitism. Proceedings Of The National Academy Of Sciences, 107(37), 16207-16211. http://dx.doi.org/10.1073/pnas.1006299107
28 Kawakita, A., Ascher, J., Sota, T., Kato, M., & Roubik, D. (2008). Phylogenetic analysis of the corbiculate bee tribes based on 12 nuclear protein-coding genes (Hymenoptera: Apoidea: Apidae). Apidologie, 39(1), 163-175. http://dx.doi.org/10.1051/apido:2007046
29 Arias, M., & Sheppard, W. (2005). Phylogenetic relationships of honey bees (Hymenoptera:Apinae:Apini) inferred from nuclear and mitochondrial DNA sequence data. Molecular Phylogenetics And Evolution, 37(1), 25-35. http://dx.doi.org/10.1016/j.ympev.2005.02.017
30 Engel, M., & Schultz, T. (1997). Phylogeny and Behavior in Honey Bees (Hymenoptera: Apidae). Annals Of The Entomological Society Of America, 90(1), 43-53. http://dx.doi.org/10.1093/aesa/90.1.43