Red Silver Spider
Argyrodes flavescens (Pickard-Cambridge) 1880
 |
| Figure 1. Gif image from imgflip.com |
How many spiders are there on the web?
Look closely at the sides of the web! Are these red spiders the spiderlings of the big spider in the middle of the web? Are these spiders the male spiders instead?
Read on to find out more!
 |
| Figure 2. A close up look of Argyrodes flavescens hanging on the periphery of a web (Copyright © 2009 Starmer, F.) |
1. Introduction of Argyrodes flavescens (Red silver spider)
These small red-brown spiders are not the spiderlings nor males of the host spider. They are instead another species of spiders that behave as kleptoparasites and lives on the web of a larger web building spider host (Commonly Nephila spp.). The term kleptoparasitism or cleptoparasitism originated from Greek root words kleptēs thief + parasite, which means parasitism by theft [10]. In terms of etymology, kleptoparasitism should refer to the generalized theft of any resource, not just food [11]. Therefore from the term itself, it describes Argyrodes flavescens' dependent relationship on the host spider for resources. An additional information is that within the spiders of the genus Argyrodes, all except //Argyrodes flagellum// live on the webs of the larger web building spiders [2]!Table of Contents
In the Gif above (Figure 2), it showcases how small these kleptoparasite are as compared to the web building host spider. The host spider Nephila pilipes abdomen is approximately 5cm long while these small kletoparasites' abdomen are only approximately 3mm!
2. Distribution
2.1 Local distribution
Argyrodes flavescens are found in locations where the large web building host could be found. The common host would be the golden orb weavers which are found in secondary and primary forest in Singapore such as Pulau Ubin, Sungei Buloh Nature Reserve, Labrador Park etc. Refer to //Nephila pilipes// species page for the details of their local distribution.
2.2 Asia distribution
 |
| Figure 3. Distribution point map of Argyrodes flavescens indicated by the yellow dots (Picture from Encyclopedia of Life © Discover Life and original sources). Last updated on 22/7/2012. |
The distribution of Argyrodes flavescens reflected in Figure 3 above were based on occurrence reports by the Global Biodiversity Information Facility (GBIF) network as of 22nd July 2012. The distribution includes countries in Asia: Malaysia [3], Thailand, Lao People's Democratic Republic [4], South Korea [5]. However, the list is not exhaustive as throughout the years Argyrodes flavescens has been recorded in other parts of Asia:Japan [6], China [7], Taiwan [8] and Singapore [9] (Figure 4 below) .
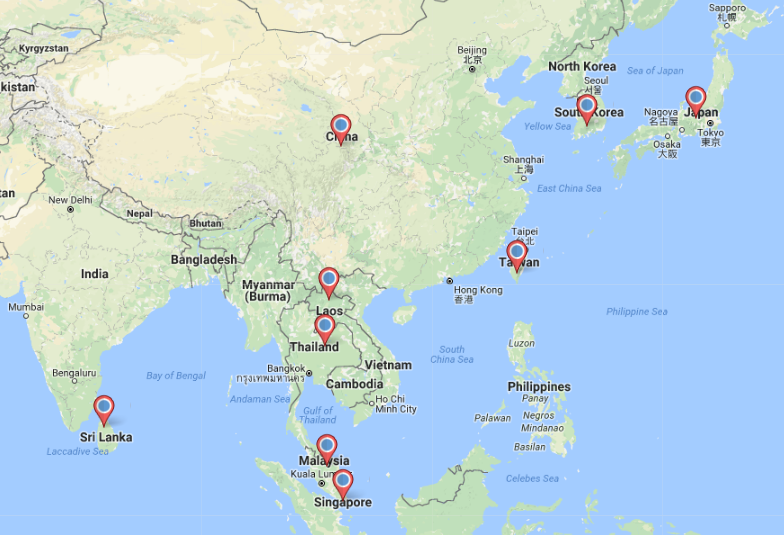 |
| Figure 4. Distribution map of Argyrodes flavescens in Asia. (Created by Lim Zhi Yun using Maptive). |
3. Biology
3.1 Kleptoparasitism
 |
| Figure 5. Numerous Argyrodes flavescens hanging on the web of a Nephila host (Copyright © 2007 Starmer, F.) |
As mentioned in the introduction, Kleptoparasitism or cleptoparasitism orginated from Greek root words kleptēs thief + parasite, means parasitism by theft [10]. In terms of etymology, kleptoparasitism should refer to the generalized theft of any resource, not just food [11].
Kleptoparasitism is one of the most common form of exploitation between animals which involves stealing of already captured food items from another animal [12,13]. In the past, most kleptoparsitic studies focused mainly on birds but the prevalence of this behavior is widespread in many animal taxa [14].
For the interaction to be termed kleptoparasitism, the kleptoparasite must incur a benefit and the host must be negatively affected by the loss of food [14]. In the case that the host does not incur any energetic cost via the loss of the resource, the interaction is not considered to be kleptoparasites [14]. In general, kleptoparasites do not injure the hosts in any direct way other than through loss of nourishment [14].
3.1.1 Kleptoparasitism in web building spiders
A spider web serves as a microhabitat to obligatory kleptoparasites and provides them with a variety of resources, such as captured prey, prey remains, and silk [9,15]. The Theridiidae spider, Argyrodes flavescens, is known to conduct nearly all their activities on webs of other spiders and is commonly associated to the large web-weaving spider from the genus Nephila [9,16]. Figure 6 showcases a few scenarios of the different behaviors of Argyrodes inhabitants on a Nephila web.
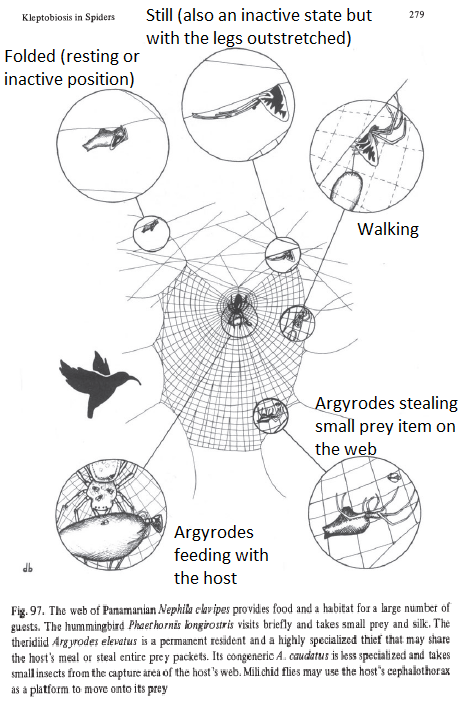 |
| Figure 6. A pictorial diagram to show how a Nephila clavipes hostweb serves as a habitat for other web associates. Note that the Argyrodes in this diagram are Argyrodes elevatus and Argyrodes caudatus. (Copyright © 1987 Vollrath.F[17]) (Image edited with wordings by Lim Zhi Yun). |
3.1.2 Argyrodes-Nephila Kleptoparasitism relationship
Previous studies have shown that the presence of A. flavescens is detrimental to N. pilipes in terms of reduced weight gain, increased web damage, rate of web relocation and ultimately mortality [9].
3.1.2.1 Susceptibility of Nephila to kleptoparasitism
Although Argyrodes kleptoparasites are found to occupy other web building spiders, spiders from the genus Nephila are especially susceptible to kleptoparasites [18–20]. There exist a variety of reasons behind why Nephila webs are suitable or favourable for Argyrodes [17]. Factors such as the web being a relatively permanent fixture and having an extensive three-dimensional barrier web helps to provides ample shelter with a large carrying capacity for Argyrodes to live on [17] (Refer to Figure 7).The significance of barrier webbing for Argyrodes were also proposed by several researchers [21,22]. Moreover, the mesh of the Nephila web is very fine and able to trap even the smallest insects [17]. The capture area of the orb web is also large, extremely stiff, and has diverging radii rendering it difficult for Nephila to monitor [17].In Singapore, there are two Nephila species that are susceptible to kleptoparsities: Nephila pilipes and //Nephila antipodiana//. Readers can refer to the Nephila pilipes species page for other reasons on why the genus Nephila are susceptible to kleptoparasitism.
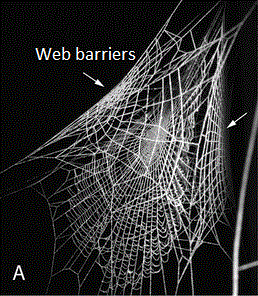 |
| Figure 7. A picture of a Nephila pilipes web with web barriers by the side (Copyright © 2010 Kuntner et al. [57]). (Image edited with wordings by Lim Zhi Yun). |
3.1.2.2 Factors influencing Argyrodes-Nephila relationships
The Argyrodes-Nephila relationships are known to be influenced by many factors, such as life stage, web characteristics, prey availability and environmental conditions [9]. In general, larger webs (and hence sub adult- adult Nephila) are found to carry a higher load of kleptoparasites [9].3.1.2.2.1 Abundance of Argyrodes
It is well known that the abundance of Argyrodes considerably varies within host species and web characteristics, web structure and occupancy of host webs [8,18,23,24].In the case of the golden orb weavers Nephila clavipes (from Neotropics), the abundance of kleptoparasites on the clustered webs is entirely predictable based on the size of the host’s web. However, it has been shown that on the isolated webs of N. clavipes, there exists a considerable variation in the number of kleptoparasites and host web size is a poor indicator of the kleptoparasite load [36]. In Singapore context, previous studies have shown that the number of A. flavescens is positively correlated with body size, web size and ambient light intensity surrounding the webs of host spider Nephila pilipes [9].
3.1.2.2.2 Host availability
A study in Japan have demonstrated the seasonal dynamics of Nephila-Argyrodes systems by showing that host availability influences the abundance and dynamics of kleptoparasites [25].Argyrodes flavescens in sub-tropical Japan were observed to prefer different hosts at different period of the year due to the availability of the host (Differing life stages of the two different Nehpila species) [25]. In August and November, Nephila clavate was preferred while Nephila maculata was preferred in July. This was attributed to the larger size of Nephila maculata than Nephila clavate in July [25]. A study by Grostal & Walter revealed a similar result that Nephila spp. were the preferred hosts for Argyrodes antipodianus [24].
3.1.2.2.3 Inter-specific competition
In the same study, the authors found out that the peak density of two Argyrodes (A. flavescens and A. bonadea) were different due to inter-specific competitions. The authors demonstrated experimentally, that in a small spatial scale, the number of individuals of A. flavescens removed from the group was positively correlated with the rate of increase in A. bonadea [25]. As of 2016, A. bonadea sighting has yet to be recorded in Singapore. |
| Figure 8. Argyrodes bonadea (Left image by Akio Tanikawa, available under a Creative Commons Attribution-Noncommercial-Share Alike license) (Right image by Patrick Randall, available under a Creative Commons Attribution-Noncommercial-Share Alike license. Copyright © 2013 Patrick Randall. ) |
3.2 Feeding Behaviour (Foraging strategies)
Kleptoparasitic spiders in the genus Argyrodes are known to form social groups around their host spider’s web [26]. It is potentially hazardous to forage as a kleptoparasite on the host web as the host spider itself is a potential predator [27]. Host spiders are known to readily eat Argyrodes [22] and often chase them away [21,28,29].Argyrodes spider not only utilise a range of kleptoparasitic foraging techniques to exploit host spiders, they are also known to utilise araneophagic techniques such as attacking the vulnerable hosts that are moulting or lunging at spiderlings [26]. Kleptoparsitic foraging techniques such as “feeding with the host” are riskier than the other tactics such as “silk stealing” and they are known to give the most benefit to the kleptoparasites [21].
Argyrodes are known to display the following foraging strategies:
| 1. Collect small prey which are apparently ignored by hosts or eat prey remains which were abandoned by hosts [8,9] (Refer to Figure 9) |
|
||
| 2. Steal freshly captured or freshly stored prey by hosts [9,30] (Refer to Figure 10) |
|
||
| 3. Feed with the host to exploit digested food [9,21,23,24,26] (Refer to Figure 11) |
Video 1. Argyrodes flavescens feeding with hosts to exploit digested food (Copyright © 2009 Starmer, F.) |
||
| 4. Silk stealing [9,31] (Tso & Severinghaus 1998; Koh & Li 2002) (Refer to Figure 12) |
|
||
| 5. Hunt for spiderlings of host or attack the moulting host [21,26,32] (Rarely observed) |
No image was found for this at the moment. |
3.4. Reproduction
Argyrodes flavescens conduct nearly all their activities on webs of their host spider, which includes reproduction. During mating, the adult female grabs the head of the adult male A. flavescens with her chelicerae while , the male repeatedly prod his pedipalp one at a time into the female sexual organ (epigynum) (Refer to video 2 and 3). During their mating process, the male and female relaxes and back off from time to time (Refer to video 2 and 3). There was no publication found on the consequences of mating on the host web or if it makes them more vulnerable to the host detection. There are also very limited publications on the exact time it takes for A. flavescens to reach sexual maturity but one publication mentioned that with the consistent high prey availability, spiderlings of A. flavescens are able to reach maturity and produce egg case in a month [25]. |
Video 2. A male and female A. flavescens mating (Copyright © 2008 Starmer, F.) Video 3. A close up look of a male and female A. flavescens mating (Copyright © 2016 Su Yong-Chao) |
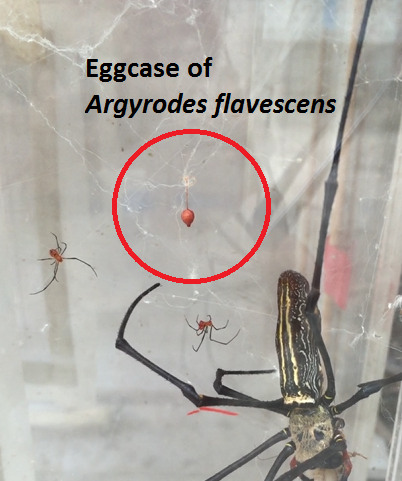
3. 4.1 Egg case
|
As mentioned previously, Argyrodes flavescens is known to conduct nearly all their activities on webs of their host spider, which includes laying of egg case. In the wild, A. flavescens builds a small tent web away from the host web or use the abandoned portion of the host web to place their egg sac [33]. The egg case of A. flavescens are pale white to light brown in appearance and spherical, suspended by a long stalk [33]. The upper part of the eggcase is cone shaped, rounded and tapers well into a point. Inside the egg sac, the eggs are loosely deposited into the middle of a little puff of flossy silk [33]. After hatching, the offsprings crawl through the hole at the bottom of the egg sac [33]. |
3.4.2 Life stages
There are 4 instars stage in A. flavescens before the final moult to become the adult. The sex of the A. flavescens is only distinguishable at the third instar onwards. Notice the pedipalps on the third and fourth instar image (Figure 15).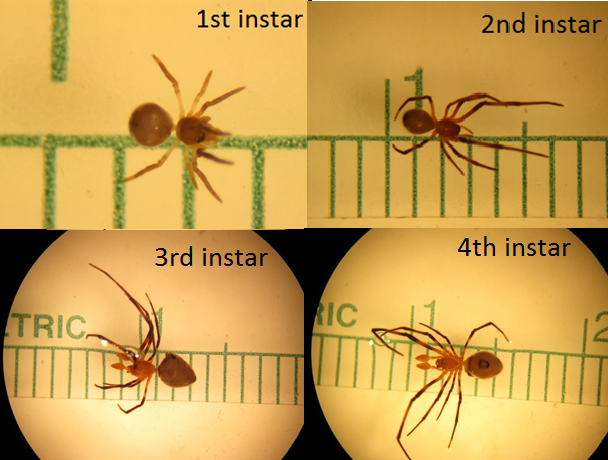 |
| Figure 15. Different stages of instars (1st to 4th) of A. flavescens (Copyright © Su Yong-Chao) |
3.5 Dragline and Locomotion
Draglines are important for Argyrodes as they have been observed to escape and run away from the attack of the host via its dragline [34]. Dragline are stiff strand of silk produced by spiders especially to form the framework of its web and as a means of lowering itself and returning to a height [54]. In addition, Argyrodes has been observed to swing from their draglines to get closer to the prey item [34]. Rotary probe movement are also observed in Argyrodes, including A. flavescens. They perform investigative behaviors as “rotary probe” by rotating the first pair of legs, because the host web is an unknown environment to the invader [34]. (Refer to Video 4 to observe the rotary probe action and the dragline from their spinnerets.) |
Video 4. A.flavescens pulling out dragline from its spinnerets and performing rotary probing (Copyright © 2009 Starmer, F.) |
Figure 6 from section 3.1.1 has showcased some but not all of the scenarios of Argyrodes behavioural activities on the host's web. To facilitate reader's experience, Figure 6 is inserted below this section for easy reference.
In general, eight behavioral activities of Argyrodes (Specifically Argyrodes Ululans) were recognized and recorded [30]:
“(1) Rotary probing (rotating the first pair of legs at the coxatrochanter joint);(Refer to video 4 above)
(2) Feeding (extracting food from prey); (Refer to Feeding behaviour section 3.2)
(3) Folded (resting or inactive position in which the spider remains motionless in the web with the legs folded up near the body; (Refer to Figure 6)
(4) Still (also an inactive state in which the spider sits in the web motionless with the legs outstretched, (Refer to Figure 6)
(5) Grooming (cleaning legs by passing them through the chelicerae); (Refer to Figure 6)
(6) Mating (courtship and copulation); (Refer to Reproduction section 3.4 )
(7) Stealing behaviors (including leg waving, web shaking, and clearing silk); and (Refer to Feeding behaviour section 3.2)
(8) Walking (locomotion)” [30]
 |
| Figure 6. A pictorial diagram to show how a Nephila clavipes hostweb serves as a habitat for other web associates. Note that the Argyrodes in this diagram are Argyrodes elevatus and Argyrodes caudatus. (Copyright © 1987 Vollrath.F[17]) Image edited with wordings by Lim Zhi Yun. |
4. Morphology
This section describes the morphology of A. flavescens. There are limited detailed description or drawings on the morphology of A. flavescens, hence this section does not consist of full comparison between the male and female A. flavescens.4.1 Morphology of A. flavescens (Male and Female)
 |
| Figure 17. Male (left) and female (right) Argyrodes flavescens (Copyright © 2014 Taiwan Spider Picture Data) |
|
|
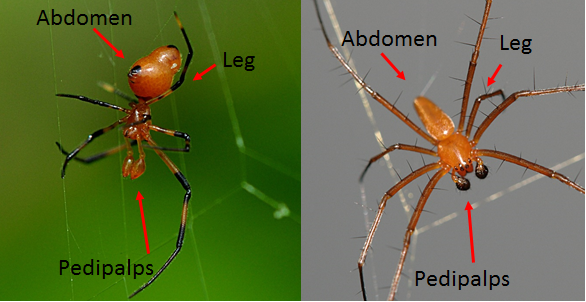
4.1.1 Legs (Tarsus)
Although most of the Theridiid ae family members have a "comb like structure" of serrated bristles on the last segment (Tarsus) of their fourth leg (Figure 20), a look under the dissection microscope shows that Argyrodes flavescens lack such structures or that it is indistinguishable (Figure 21). Only tarsal claws were distinctively found on their leg instead.5. Diagnosis
5.1 Male Nephila VS Argyrodes flavescens
As A. flavescens (Figure 22) and males N. pilipes (Figure 23) are commonly found on the same web of the host (female) N. pilipes,there is a need to know how to differentiate the two species. At first glance, they may be mistaken to be same species since both are much smaller than female N. pilipes host, of similar reddish brown colour and are commonly observed at the periphery of the web. However, they can be clearly differentiated in a few ways (Figure 24).
|
|
| The following features can be used to distinguish between the two species: |
|
|||
| Size |
Approximately 2mm smaller than male N.pilipes |
Approximately 2mm larger than male A.flavescens |
||
| Legs morphology: size and colour |
Slender, deep blackish brown hue and the extremity of the femora yellow |
Thicker and of reddish brown hue throughout. |
||
| Legs morphology: spinations |
No spinations |
Visible spinations |
||
| Pedipalps |
Smaller and less visible |
Larger and more visible |
||
| Abdomen shape |
Extremity produced into a somewhat cylindrical prominence, rounded at its extremity with a black spot at the end of the prominence region and one at the spinneret |
Conical shape and is uniformly colored |
||
| Abdomen colour |
The distinctive silvery markings |
No silvery markings |
||
5.2 Argyrodes miniaceus VS Argyrodes flavescens
 |
| Figure 25. Argyrodes miniaceus (Left) photo by Nick Monaghan under Creative Commons 3.0 Australia License and Argyrodes flavescens (Right) (Copyright © 2014 Taiwan Spider Picture Data) |
Argyrodes flavescens and Argyrodes miniaceus are of similar coloration and both have silvery markings on their abdomen. Therefore A.flavescens closely resembles A. miniaceus but it can be distinguished by examining the structure of female genitalia and the shape of embolus of male palps [6] (Figure 26).
.
![Figure 23. Morphology diagrams of Argyrodes flavescens vs Argyrodes miniaceus ( Tanikawa etal, 1996) [6].png Figure 23. Morphology diagrams of Argyrodes flavescens vs Argyrodes miniaceus ( Tanikawa etal, 1996) [6].png](files/Figure%2023.%20Morphology%20diagrams%20of%20Argyrodes%20flavescens%20vs%20Argyrodes%20miniaceus%20%28%20Tanikawa%20etal%2C%201996%29%20%5B6%5D.png) |
| Figure 26. Morphology diagrams of Argyrodes flavescens vs Argyrodes miniaceus ( Tanikawa etal, 1996) [6] |
6. Taxonomy
The spider family Theridiidae, popularly known as comb-footed spiders, ranks as one of the most species-rich families of spiders [35]. One of the reason for the high species number in the Theridiidae is due the diversity of foraging and lifestyle strategies [35].The taxonomy of the subfamily Argyrodinae has always been challenging [36] [37]. The current spider subfamily Argyrodinae (family Theridiidae) comprises of 239 named species [38] in six genera, Argyrodes, Faiditus, Neospintharus, Ariamnes, Rhomphaea, and Spheropistha [35]
Two montoypic genera, Argyrodella and Deelemanella were proposed by their authors to be members of the Argyrodinae [39][40]. There are limited information known about the biology of either species and the authors mentioned that their genitalia and other anatomical structures that are somewhat different from typical Argyrodines [39][40][41]. (Note that these two genera were not included in the phylogeny tree below.)
In 1894, Simon regarded the genera Argyrodes, Ariamnes and Rhomphaea as a group he named Argyrodeae [43]. Subsequently in 1962, the New World species was revised by Exline and Levi. Argyrodes, Ariamnes and Rhomphaea were lumped into a single genus, Argyrodes [36]. Within this genus, Exline and Levi recognised six species groups: Argyrodes, Ariamnes, Cancellatus, Cordillera, Trigonum and Rhomphaea [36]. In 1998, Tanikawa added Spheropistha to the genus Argyrodes [42].
In 2001, Exline and Levi’s one genus system was elevated to the subfamily level (Argyrodinae) by Yoshida [44]. In this subfamily Argyrodinae, Yoshida retained the genus Argyrodes and resurrected the following genera: Ariamnes, Rhomphaea and Spheropistha [44]. In 2004, Agnarsson’s morphological phylogeny of Theridiidae strongly supported the monophyly of the Argyrodinae which also aligned to Yoshida’s genera [35][44]. A molecular phylogeny of the Theridiidae family showed similar result [45]. In 2004, Agnarsson also elevated the Cancellatus and Cordillera species groups to a single genus, Faiditus, and elevated the Trigonum species group to genus Neospintharus [35]. This then formed the modern six genus system of Argyrodinae (which excluded the consideration of the two monotypic genera Argyrodella and Deelemanella mentioned previously).
The genera Ariamnes and Neospintharus utilise araneophagy as their main foraging strategy. Rhomphaea mainly utilises araneophagy and occasionally kleptoparasitism. The foraging strategies or habits of Spheropistha remain understudied. The genera Argyrodes and Faiditus use kleptoparasitism as their main foraging strategy [46][47].
6.1 Phylogenetics
In 2014, Su and Smith conducted the first study of group-living behaviour among kleptoparasitic spiders in a molecular phylogenetic context [16]. This is also the only paper (as of 2016) that included Argyrodes flavescens in the phylogeny tree of Argyrodinae as other authors worked mainly with Neotropical species. Their study included 41 species of Argyrodinae in their analyses: 23 species in Argyrodes, six species in Faiditus, five species in Rhomphaea, two species in Neospintharus, two in Ariamnes and three Spheropistha [16]. They also used published DNA sequence data from five species [45].
The DNA was extracted from the legs and prosoma of the specimens. In their analyses, they utilised 2511 bp of DNA sequence data from two mitochondrial genes, cytochrome c oxidase subunit I (COI, 776 bp) and 16S rRNA (16S, 633 bp), two nuclear genes, 28S rRNA (28S, 777 bp) and histone 3 (H3, 325 bp) [16]. They used published data and personal observations to character code three behavioural characters – foraging strategy, sociality and size of host web used – into discrete character states [16]. They categorised foraging strategies into "kleptoparasitism", "araneophagy", or ‘typical’ predation using self-constructed webs.
Su and Smith estimated the divergence time using the concatenated data matrix under a gene-tree framework using BEAST v. 1.8.0 [50]. In the concatenated analyses , the clock models and substitution rates of each gene was unlinked and the substitution models were set up in MrBayes. The oldest fossil occurrence for the Argyrodines, Argyrodes parvipatellaris [51][52] , was used as the reference calibration time for the divergence time estimations. The phylogenetic trees constructed by Su and Smith were then inferred using a Bayesian method in MrBayes v. 3.2.1 [48] and maximum likelihood in GARLI v. 2.0 [49].
In Figure 27, both Bayesian and maximum likelihood (ML) analyses of concatenated data strongly support monophyly of the Argyrodinae (Bayesian posterior probability = 1.00 and ML bootstrap support = 100; Figure. 27) [16]. Both analyses also show that three genera – Ariamnes, Neospintharus and Rhomphaea – are each supported as clades, while the other three – Faiditus, Argyrodes and Spheropistha – are not [16]. There are multiple well-supported species groups within the Argyrodes + F. xiphias+ Spheropistha clade (Fig. 27, consensus tree), but the relationships among these groups are not resolved in the ML analysis (refer to Figure. 28 for summary tree) [16].
Argyrodes flavscens falls under one of this unresolved clade, Miniaceus clade, which is indicated by the consensus and summary trees (Figures 27 and 28); this clade contains seven species, including Argyrodes flavescens and three species currently in the genus Spheropistha [16]. However, due to the uncertainty in the placement of the Spheropistha species, this clade is not recovered in the maximum clade credibility tree illustrated in Figure. 29 [16].
The Bayesian stochastic search variable selection (BSSVS) reconstruction of the foraging strategy (Figure. 29) indicates that the ancestral foraging strategy in Argyrodinae is araneophagy [16]. BSSVS allowed the authors to evaluate that a single origin of kleptoparsitism in the the Argyrodes + Spheropistha + Faiditus + Ariamnes clade with a secondary loss of kleptoparasitism in the genus Ariamnes is more likely as compared to two origins of kleptoparasitism, one in the genus Faiditus and one at the base of the Argyrodes + F. xiphias + Spheropistha clade [16].
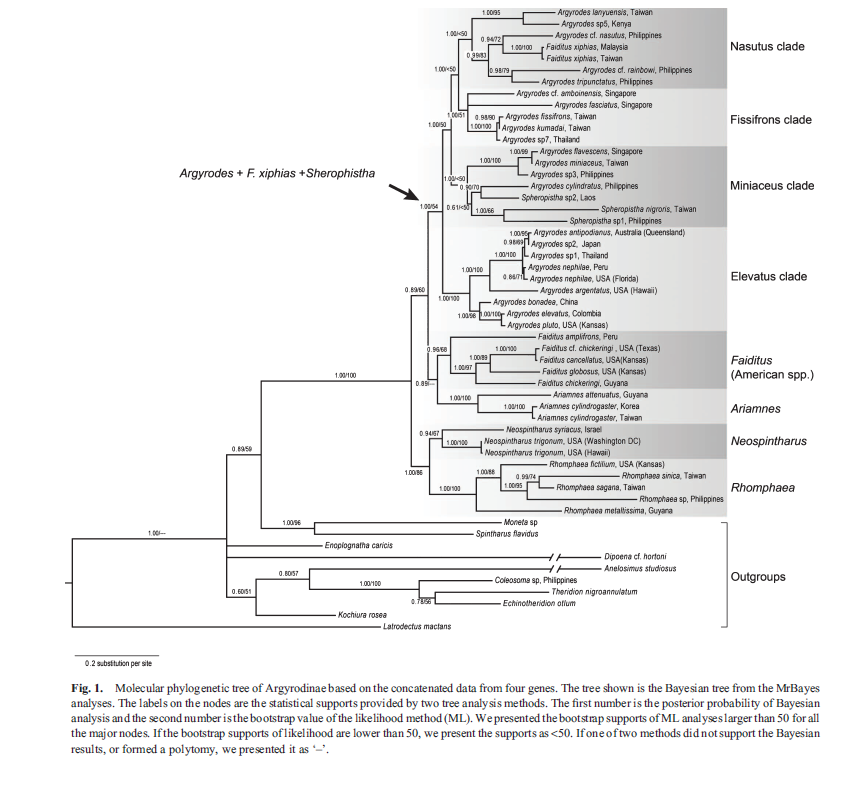 |
| Fig. 27 . Consensus tree. Molecular phylogenetic tree of Argyrodinae based on the concatenated data from four genes. The tree shown is the Bayesian tree from the MrBayes analyses. The labels on the nodes are the statistical supports provided by two tree analysis methods. The first number is the posterior probability of Bayesian analysis and the second number is the bootstrap value of the likelihood method (ML). (Su and Smith 2014) |
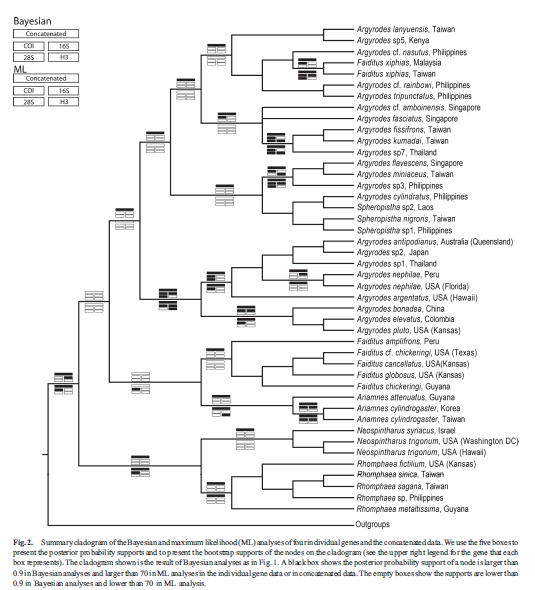 |
| Fig. 28 . Summary cladogram of the Bayesian and maximum likelihood (ML) analyses of four individual genes and the concatenated data. The authors used the five boxes to present the posterior probability supports and to present the bootstrap supports of the nodes on the cladogram (see the upper right legend for the gene that each box represents). The cladogram shown is the result of Bayesian analyses as in Fig. 27. A black box shows the posterior probability support of a node is larger than 0.9 in Bayesian analyses and larger than 70 in ML analyses in the individual gene data or in concatenated data. The empty boxes show the supports are lower than 0.9 in Bayesian analyses and lower than 70 in ML analysis (Su and Smith 2014). |
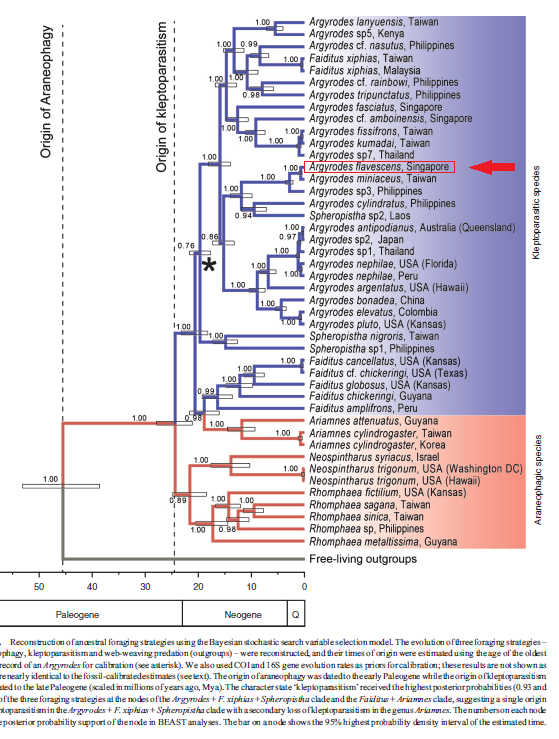 |
| Fig. 29 . Reconstruction of ancestral foraging strategies using the Bayesian stochastic search variable selection model. The evolution of three foraging strategies – araneophagy, kleptoparasitism and web-weaving predation (outgroups) – were reconstructed, and their times of origin were estimated using the age of the oldest fossil record of an Argyrodes for calibration (see asterisk). (Su and Smith 2014). |
6.2 Vernacular (Common) Names
Argyrodes flavescens is commonly called the "red and silver spider" or "red and silver dewdrop spider" due to the their colouration and the shape of its' abdomen [2].6.3 Etymology
There are limited resources online that explains the etymology behind the family “Theridiidae”. Many sources simply gave it the definition of “Theridiidae-a family of comb-footed spiders”[2].The genus name Argyrodes is derived from Greek where argyros represent "silver" and -odes represent "like" [55]. Although this "silver like" coloration does not match Argyrodes flavescens's reddish-brown colouration, it is likely named due to the other silvery Argyrodes species. In Latin, flavescens means “become yellow” [56], which could be due to the yellow colouration on their femora or their reddish-brown colouration.
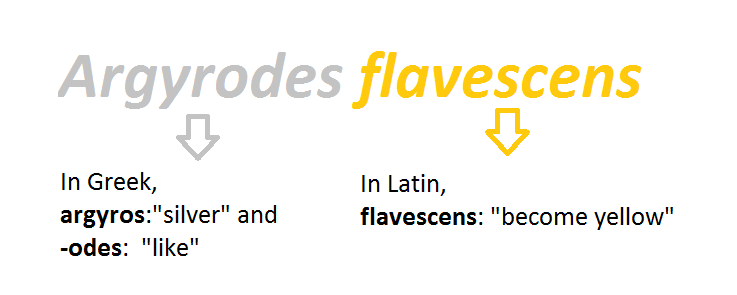 |
| Figure 30. Explanation of how the name Argyrodes flavescens was derived. (Created by Lim Zhi Yun) |
6.4 Type Specimen
Argyrodes flavescens was first described by Pickard-Cambridge, O. in 1880 (Refer to Figure 28) [53]. The syntypes used in the description was from Sri Lanka, and preserved in British Museum of Natural History (BMNH) [6] There was no information found on the lectotypes. |
| Figure 31. Original description of Argyrodes flavescens by Pickard-Cambridge, O. in 1880. |
7. Conservation Status
This taxon has not yet been assessed for the IUCN Red List.8. Literature and References
1. E. Sue Andersen 2014 Captive Breeding and Husbandry of The Golden Orb Weaver Nephila inaurata madagascariensis at Woodland Park Zoo.2. Joseph K. H. Koh 2000 A Guide to Common Singapore Spiders. BP Guid. to Nat. Ser. Publ. by Singapore Sci. Cent. Spons. by Br. Pet.
3. Nasir, D. M., Su, S., Mohamed, Z. & Yusoff, N. C. 2014 New distributional records of spiders (Arachnida: Araneae) from the west coast of Peninsular Malaysia. Pakistan J. Zool 46, 1573–1584.
4. Peter Jäger & Bounthob Praxaysombath 2011 Spiders of Laos part 3.pdf.
5. Namkung, J., Yoo, J. S., Lee, S. Y., Lee, J. H., Paek, W. K. & Kim, S. T. 2009 Bibliographic Check list of Korean Spiders (Arachnida: Araneae) ver. 2010. J. Korean Nat. 2, 191–285. (doi:http://dx.doi.org/10.1016/S1976-8648(14)60055-4)
6. Tanikawa, A., Chida, T. & Kumada, K. 1996 New Records of A rgyrodes flavescens ( Araneae : Theridiidae ) from Japan. 45, 47–52.
7. Song, D. X., Zhu, M. S. & Chen, J. 1999 The Spiders of China. Hebei Univ. Sci. Techology Publ. House , 640 pp.
8. Tso, I. M. & Severinghaus, L. L. 2000 Argyrodes fissifrons inhabiting webs of Cyrtophora hosts: Prey size distribution and population characteristics. Zool. Stud. 39, 236–242.
9. Koh, T. H. & Li, D. 2002 POPULATION CHARACTERISTICS OF A KLEPTOPARASITIC SPIDER. RAFFLES Bull. …
10. In press. Definition of kleptoparasite in English. Oxford Dict.
11. Burger, L. W. 2000 Long-term effects of red-cockaded woodpecker cavity-entrance restrictors.
12. Rothschild, M. & Clay, T. 1952 Fleas, flukes and cuckoos. A study of bird parasites. Fleas, flukes cuckoos. A study bird parasites. (doi:10.1001/jama.1952.03680150102046)
13. Brockmann, H. J. & Barnard, C. J. 1979 Kleptoparasitism in birds. Anim. Behav. 27, 487–514. (doi:10.1016/0003-3472(79)90185-4)
14. Iyengar, E. V. 2008 Kleptoparasitic interactions throughout the animal kingdom and a re-evaluation, based on participant mobility, of the conditions promoting the evolution of kleptoparasitism. Biol. J. Linn. Soc. 93, 745–762. (doi:10.1111/j.1095-8312.2008.00954.x)
15. Pasquet, A., Leborgne, R. & Cantarella, T. 1997 Opportunistic Egg Feeding i n the Kleptoparasitic Spider Argymdes gibbosus. Ethology 170, 160–170. (doi:10.1111/j.1439-0310.1997.tb00015.x)
16. Su, Y. C. & Smith, D. 2014 Evolution of host use, group-living and foraging behaviours in kleptoparasitic spiders: Molecular phylogeny of the Argyrodinae (Araneae:Theridiidae). Invertebr. Syst. 28, 415–431. (doi:10.1071/IS14010)
17. Vollrath, F. 1987 Kleptobiosis in Spiders. In Ecophysiology of Spiders (ed W. Nentwig), pp. 274–286. Berlin, Heidelberg: Springer Berlin Heidelberg. (doi:10.1007/978-3-642-71552-5_20)
18. Henaut, Y. 2000 Host selection by a kleptoparasitic spider. J. Nat. Hist. 34, 747–753. (doi:10.1080/002229300299390)
19. Whitehouse, M. 2011 Kleptoparasitic spiders of the subfamily Argyrodinae: a special case of behavioural plasticity. Spider Behav. Flex. Versatility.(ME Herberstein, ed.). Cambridge Univ. Press. Cambridge, UK , 348–386.
20. Robinson, M. H. & Robinson, B. 1973 Ecology and behavior of the giant wood spider. Nephila maculata , 1–76.
21. Whitehouse, M. 1986 The foraging behaviours of Argyrodes antipodiana (Theridiidae), a kleptoparasitic spider from New Zealand. New Zeal. J. Zool. 4223, 37–41. (doi:10.1080/03014223.1986.10422658)
22. Whitehouse, M. E. A. 1997 The benefits of stealing from a predator: foraging rates, predation risk, and intraspecific aggression in the kleptoparasitic spider Argyrodes antipodiana. Behav. Ecol. 8, 665–667. (doi:10.1093/beheco/8.6.665)
23. Whitehouse, M. E. a 1988 Factors influencing specificity and choice of host in Argyrodes antipodiana (Theridiidae, Araneae). J. Arachnol. 16, 349–355. (doi:10.2307/3705921)
24. Grostal, P. & Walter, D. E. 1999 Host Specificity and Distribution of the Kleptobiotic Spider Argyrodes antipodianus (Araneae, Theridiidae) on Orb Webs in Queensland, Australia. J. Arachnol. , 522–530. (doi:10.2307/3706051)
25. Miyashita, T. 2002 Population dynamics of two species of kleptoparasitic spiders under different host availabilities. J. Arachnol. 30, 31–38. (doi:10.1636/0161-8202(2002)030[0031:PDOTSO]2.0.CO;2)
26. Whitehouse, M. E. A. 2016 Sex-linked differences in learning to improve foraging techniques in the group-living kleptoparasitic spider Argyrodes antipodianus (Theridiidae). New Zeal. J. Zool. 422343, 96–11. (doi:10.1080/03014223.2015.1127264)
27. Elgar, M. a 1993 Inter-specific associations involving spiders: kleptoparasitism, mimicry, and mutualism. Mem. Queensl. Museum 33, 411–430.
28. Cangialosi, K. R. 1991 Attack strategies of a spider kleptoparasite: effects of prey availability and host colony size. Anim. Behav. 41, 639–647. (doi:10.1016/S0003-3472(05)80902-9)
29. Vollrath, F. 1981 Energetic considerations of a spider parasite-spider host system. Rev. Arachnol 3, 37–44.
30. Cangialosi, K. R. 1990 Life Cycle and Behavior of the Kleptoparasitic Spider, Argyrodes Ululans (Araneae, Theridiidae). J. Arachnol. 18, 347–358.
31. Tso, I.-M. & Severinghaus, L. L. 1998 Silk stealing by Argyrodes lanyuensis (Araneae: Theiriidae): A unique form of Kleptoparasitism. Anim. Behav. 56, 219–225.
32. Tanaka, K. 1984 Rate of predation by a kleptoparasitic spider Argyrodes fissifrons upon a large host spider Agelena limbata. J. Arachnol. 12, 363–367. (doi:10.2307/3705367)
33. Javed, S. M. M., Srinivasulu, C. & Tampal, F. 2010 Addition to araneofauna of Andhra Pradesh , India : occurrence of three species of Argyrodes Simon , 1864 ( Araneae : Theridiidae ). J. Threat. Taxa 2, 980–985.
34. Silveira, M. C. & Japyassú, H. F. 2012 Notes on the behavior of the kleptoparasitic spider Argyrodes elevatus. Rev. Etol. 11, 56–67.
35. Agnarsson, I. 2004 Morphological phylogeny of cobweb spiders and their relatives (Araneae, Araneoidea, Theridiidae). Zool. J. Linn. Soc. 141, 447–626.
36. Exline, H. & Levi, H. W. 1962 American spiders of the genus Argyrodes (Araneae, Theridiidae). Arañas americanas del género Argyrodes (Araneae, Theridiidae). Bull. Museum Comp. Zool. 127, 75–202.
37. Yoshida, H. 2001 A revision of the Japanese genera and species of the subfamily Theridiinae (Araneae: Theridiidae). Acta Arachnol. 50, 157–181.
38. Platnick, N. (2014). The world spider catalog, version 15 American Museum
of Natural History. Retrieved November 23, 2016, from Available at http://research.amnh.org/iz/spiders/
catalog/
39. Yoshida, H. (2003). A new genus and three new species of the family Theridiidae (Arachnida: Araneae) from North Borneo. Acta Arachnologica 52, 85–89. doi:10.2476/asjaa.52.85
40. Saaristo, M. I. (2006). Theridiid or cobweb spiders of the granitic Seychelles
islands (Araneae, Theridiidae). Phelsuma 14, 49–89.
41. Saaristo, M. I. (1978). Spiders (Arachnida, Araneae) from the Seychelle
islands, with notes on taxonomy. Annales Zoologici Fennici 15(2), 99–126.
42. Tanikawa, A. (1998). The new synonymy of the spider genus Argyrodes (Araneae: Theridiidae) and a description of a new species from Japan. Acta Arachnologica 47, 21–26. doi:10.2476/asjaa.47.21
43. Simon, E. (1892–1895). ‘Histoire Naturelle des Araignées. Second Edn.’ Volume 1 (section 3, published 10 October 1894), Librairie Encyclopédique de Roret, rue Hautefeuille, 12, Paris, pp. 496–503.
44. Yoshida, H. (2001). The genus Rhomphaea (Araneae: Theridiidae) from Japan, with notes on the subfamily Argyrodinae. Acta Arachnologica 50(2), 183–192. doi:10.2476/asjaa.50.183
45. Arnedo, M. A., Coddington, J., Agnarsson, I., and Gillespie, R. G. (2004). From a comb to a tree: phylogenetic relationships of the comb-footed spiders (Araneae, Theridiidae) inferred from nuclear and mitochondrial
46. Elgar, M. (1993). Inter-specific associations involving spiders: kleptoparasitism, mimicry and mutualism. Memoirs of the Queensland Museum 33(2), 411–430.
47. Whitehouse, M., Agnarsson, I., Miyashita, T., Smith, D., Cangialosi, K., Masumoto, T., Li, D., and Henaut, Y. (2002). Argyrodes: phylogeny, sociality and interspecific interactions: a report on the Argyrodes symposium, Badplaas 2001. The Journal of Arachnology 30(2), 238–245. doi:10.1636/01618202(2002)030[0238:APSAII]2.0.CO;2
48. Ronquist, F., and Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19(12), 1572–1574. doi:10.1093/bioinformatics/btg180
49. Zwickl, D. J. (2006).GARLI: genetic algorithm for rapid likelihood inference. Available at http://www.bio.utexas.edu/faculty/antisense/garli/Garli.html [Verified July 2014]
50.Drummond, A. J., Suchard, M. A., Xie, D., and Rambaut, A. (2012). Bayesian
phylogenetics with BEAUti and the BEAST 1.7. Molecular
51. Wunderlich, J. (1988). Die fossilen Spinnen im dominikanischen Bernstein. Beiträge zur Araneologie 2, 1–378.
52. Penney, D., and Green, D. I. (2011). Fossils in Amber: Remarkable Snapshots of Prehistoric Forest Life. (Siri Scientific Press.) 226 pp.
53. CAMBRIDGE, R. O. P. 1880 3. On some new and little-known Spiders of the Genus Argyrodes, Sim. Proc. Zool. Soc. London 48, 320–344. (doi:10.1111/j.1469-7998.1880.tb06563.x)
54. Species details : Argyrodes flavescens O. Pickard-Cambridge, 1880. (2016, October 31). Retrieved November 23, 2016, from http://www.catalogueoflife.org/col/details/species/id/c5b3aaa311332520ad13085fe8e5dd90
55. Argyrodes. (n.d.). Retrieved November 23, 2016, from https://en.wikipedia.org/wiki/Argyrodes
56. Flavescens. (n.d.). Retrieved November 23, 2016, from https://en.wiktionary.org/wiki/flavescens
57. Kuntner, M., Gregorič, M. & Li, D. Naturwissenschaften (2010) 97: 1097. doi:10.1007/s00114-010-0736-1
9. Copyright of images and illustrations
1. Starmer, F. (2007) (2008) (2009). Permission is granted to copy, distribute and/or modify this document under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License2. Su Yong-Chao (2016) Permission to publish image sought and granted by author on Nov 2016. Copyright remains with the author.
3. Tanikawa etal (1996) Permission to use the images from published paper sought and granted by author on Nov 2016. Copyright remains with the author.
4. Taiwan Spider Picture Data (2014) Permission to publish image sought and granted by author on 6 Nov 2016. Copyright remains with Taiwan Spider Picture Data.
5. Brandeis University Field Biology Electronic Field Guides(N.d) Permission to publish image sought but not granted because author is uncontactable. Image will be taken down if the author requests to.
Author
Name: Lim Zhi Yun (Miss)
Email: a0115015@u.nus.edu