|
| Pencil-roots (top-left, source: Cheong J.M.D), Teardrop shaped fruits (bottom-left, source:© www.NatureLoveYou.sg), Yellowish-orange flower (top-right, source:© www.NatureLoveYou.sg), Elongated leaves with pointed tip (bottom-right, source: © Jessica Tay). |
General Introduction
Table of Contents
Etymology
'Avicennia' is said to honour Iranian philosopher and physician, Avicenna (980-1037)². ‘Api’ means ‘fire’ in Malay and it originates from the observation that this particular mangrove attracts fireflies. ‘Putih’ and ‘alba’ means ‘white’ in Malay and Latin respectively and it is from the fact that the leaves are pale-coloured on the underside.
Taxonavigation
Class: Magnoliopsida
Order: Lamiales
Family: Avicenniaceae
Genus: Avicennia
Species: alba
Distribution
Global distributionApi-api putih has a wide distribution from Western India to Indochina, the Malay Archipelago, the Philippines, New Guinea and Northern Australia (Figure 1)³.
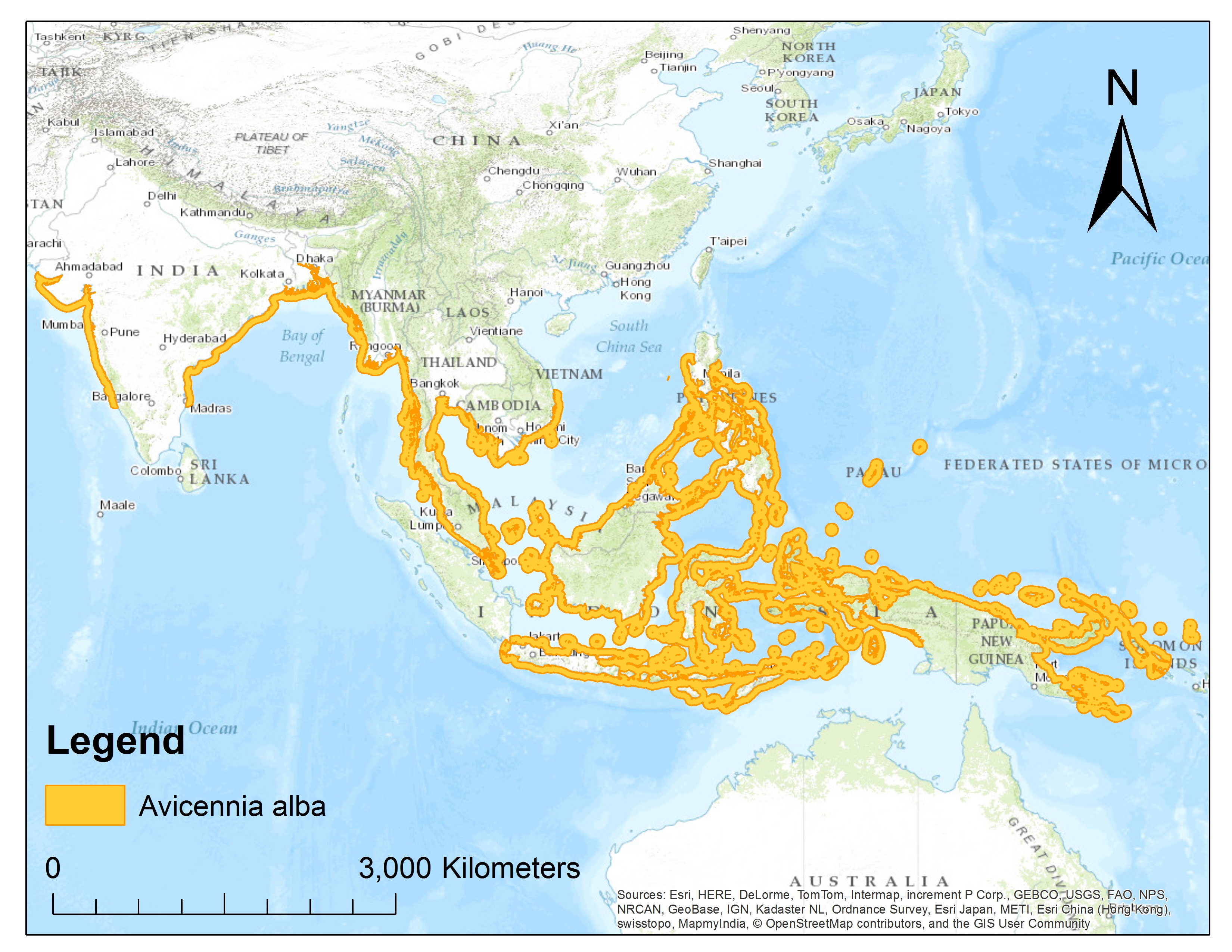 |
| Figure 1: Global distribution of Avicennia alba. (Source: Adapted from IUCN red list) |
Distribution within Singapore
This particular species is very common in Singapore and can be found in almost all the mangrove patches in Singapore, as seen in Figure 2. The map below shows the distribution of mangrove forest in Singapore and the places bold in red are mangroves where Api-api putih can be found, with information gathered from various online sources⁴.
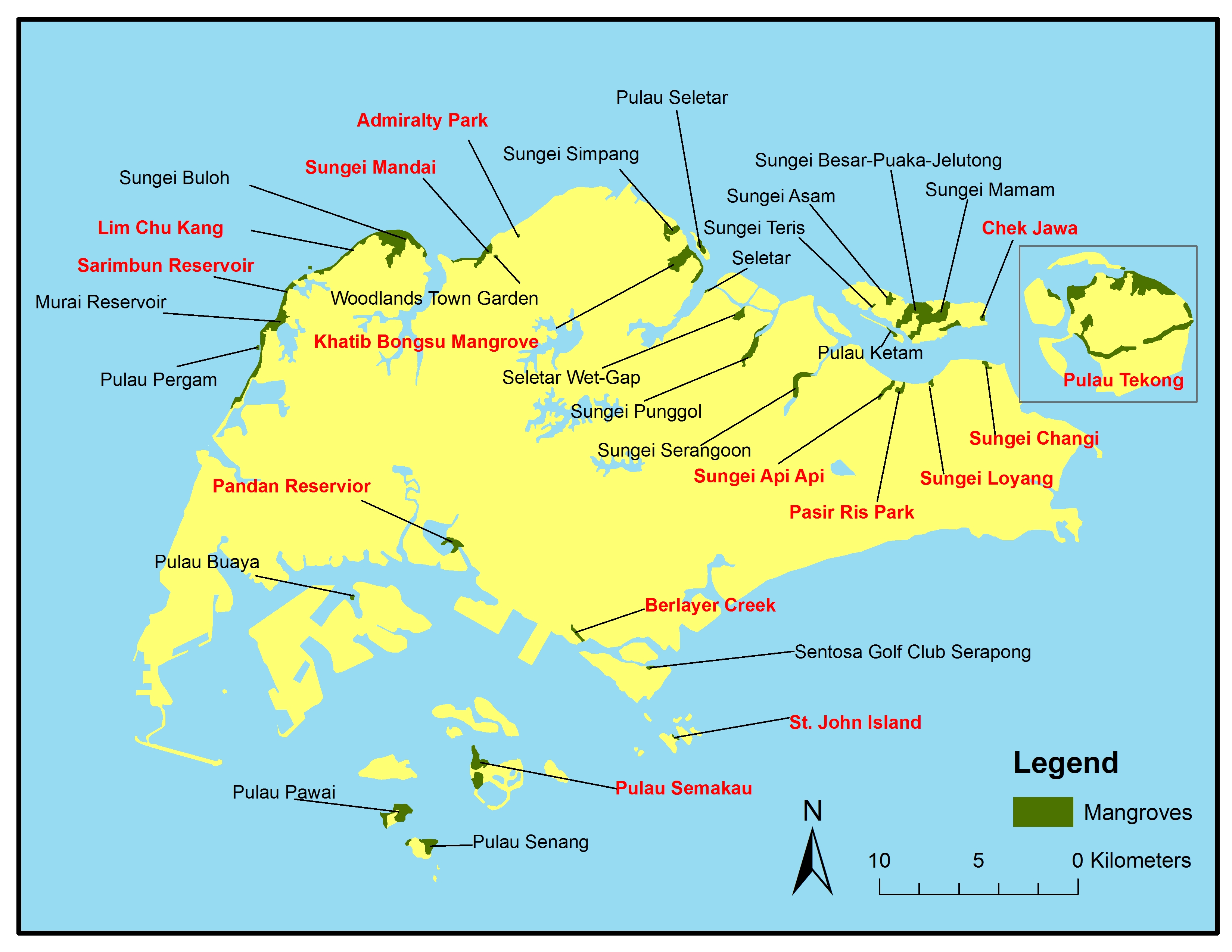 |
| Figure 2: Local distribution of mangrove forest. Places bolded in red are mangroves where A. alba can be found, with information gathered from various online sources. (Source: Base map from Tan W.T. Data adapted from Yee et al. (2010)) |
1) Sungei Buloh Wetland Reserve;
2) Chek Jawa;
3) Pasir Ris Mangrove and;
4) Berlayer Creek
Visit National Parks Board website for directions!
Back to Top
How to identify Api-api putih!
Api-api putih is a tree that can grow up to around 10 to 25 metres in height. With the description of the plant below, you can learn how to identify Api-api putih as well³!| Leaves -Characteristically lanceolated (long and pointed at the apex) -Around 8-12cm long -Dark green on upperside, pale-coloured, whitish on the underside -Leaves are simple (undivided leaf blade) and opposite (a pair of leaves attached at a node)
|
||
| Flowers - Orange-yellow colour with around 10-30 flowers on each unit. - Each flower is only about 0.5cm in diameter with four equal lobed petals that are smooth on the inside. - Flowers of Api-api putih is distinguished from other Avicennia species by its long spicate distal flower units that are about 1.5 to 3cm long. - Stamens are short, only about 2mm long and does not protrude or thrust outwards - Ovary is about 2mm long and without a style.
|
||
Fruits
- Distinctive conical shape that extends to a pronounced pointed tip. - It is especially obvious during the early stages of fruit development. - About 1-4cm long - Smooth velvety outer skin. - Each fruit contains a single seed. |
||
- About 20–30 cm tall with a tapered bluntly pointed end. - Lenticels, for gaseous exchange, are found on the roots. |
||
Bark
- Smooth or slightly roughened but it is not fissured. - A distinct character of Api-api putih tree is the growth of sooty mould on older stem parts. |
Biology
Habitat type
 |
| Berlayer Creek mangrove at low tide (top-left), Mandai mangrove at low tide (bottom-left), Pandan mangrove a high tide (right). (Source: © Jessica Tay) |
Reproduction
Api-api putih, like all other Avicennia species are insect-pollinated. They are commonly pollinated by flies and bees. These pollinators serve an important purpose for successful sexual reproduction for greater genetic variability because Api-api putih is capable of self-pollination⁸. Although flowers of Api-api putih are very similar to flowers of Avicennia marina and Avicennia officinalis, studies showed that their flowering time is non-synchronised to minimise competition for pollinators and spread availability of nectar over a longer period of time⁹.After pollination, Api-api putih reproduces via cryptovivipary. This means that the single seed does not remain dormant in the fruit but actively produces chlorophyllous seedling within the fruit⁹, without breaking through the fruit wall¹⁰. When the fruit detaches from the parent plant, seedling will burst through the fruit wall and are dispersed by water. Each tree produces numerous seedlings which are small and light to remain buoyant and are dispersed widely via tidal currents though establishment of seedlings remained mainly along the coastline⁹. As seedlings are chlorophyllous, they can photosynthesise as soon as they take root.
For successful establishment of seedlings, a ‘window of opportunity’ is required where disturbance (such as tide level, erosion rate, weather conditions) is at minimum¹¹. Figure 3 below shows the three challenges that an Api-api putih seedling needs to overcome for successful establishment. 1) Seedlings need to grow a minimum root length during an inundation-free period to prevent floating during a tidal inundation; 2) after which, roots need to be long enough to resist against hydrodynamics forces of waves and currents; and lastly 3) when roots penetrate into the sediments, it has to resist sediment erosion to prevent being dislodged¹¹.
| Figure 3: 1) Seedling burst through the fruit wall to take root as soon as there is an inundation-free period; 2)roots grow long enough to resist against waves and currents; 3) seedlings have to resist against sediment erosion to prevent dislodgement. (Source: Balke et al., 2011) |
Back to Top
Ecology
| Damage/Pest |
Mechanism/Organism |
||||
|---|---|---|---|---|---|
| Avicennia seed moth Autoba alabastrata¹² |
|
||||
| Foliage damage by herbivores¹² |
|
||||
| Structural damage¹² |
|
Adaptations
Api-api putih are usually found near the coast and its habitat is frequently inundated. Therefore, it has special adaptations to survive in areas that have high salinity, waterlogged muddy soil and high wave actions¹³.Roots
 |
| Figure 4: Pencil roots with lenticels (circled in red) which are important for direct gaseous exchange a the surface to provide plant with additional oxygen. (Source: © Tan Heok Hui). |
Vertical pencil roots (pneumatophores) help to transport oxygen to underground root system¹⁴.
During a tidal inundation, waterlogged soils are often poorly oxygenated. Therefore, Api-api putih has specialised roots, also known as breathing roots, to help in gaseous exchange. These roots are vertical pencil roots, known as pneumatophores, which grows vertically upwards from the submerged lateral underground root system (Figure 5). Pencil roots are covered with lenticels that enables gaseous exchange for oxygen directly from the surface (Figure 4). Hence, pencil roots provide the plant with additional oxygen which cannot be taken from the soil. However, gaseous exchange can only occur when pencil roots are not submerged because at high tides, surface tension of water blocks lenticels and prevent gaseous exchange¹⁴. Therefore, permanent inundation in the mangrove habitat will eventually kill the root system and the plant.
Increases stability of tree
Pencil roots and underground lateral root system (Figure 5) spread over large areas which help to stabilise tree on the unstable waterlogged ground as well as to withstand high wave actions. Additionally, extensive pencil roots network help to stabilize the sediments and prevent erosion.
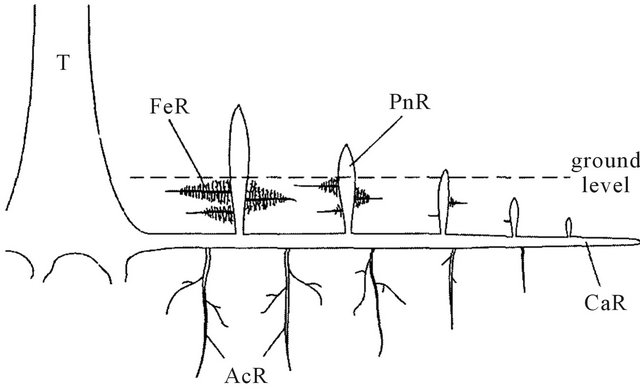 |
| Figure 5: Simplified diagram of root system of Avicennia species. T: Trunk. CaR (cable roots), primary roots with lateral underground root system, horizontal growth. PnR (pneumatophores), pencil roots with upward vertical growth from CaR. FeR (feeding roots), lateral horizontal roots grown from PnR. AcR (anchor roots), vertical roots grown downward from CaR. (Source: Purnobasuki, 2013³⁸) |
Important for salt exclusion
Mangroves evolved specialised survival strategies to thrive under high salinity environments. For Avicennia species, they are salt secretor, which means that they exclude excess salt from their roots and leaves. The roots exclude 85-95% of the salt that are carried towards the roots via transpiration. This is because roots develop barriers against apoplastic movement that increase resistance to water flow and exclude salt from entering the roots¹⁵. This helps to maintain salt concentration balance within the whole plant.
| Figure 6: Excess salt excreted as salt crystals on the underside of a Avicennia marina leaf. (Source: Wikimedia commons, Public Domain) |
Api-api putih, like other Avicennia species, secretes excess salt that is not excluded at the roots from its leaves¹⁶ (Figure 6). Salts are expelled through specialized salt-secreting glands which are small slit-like openings found on the upper surface of the leaves¹⁷ ¹⁸. This further regulates salt concentration in the plant.
Fast root and seedling development
From information mentioned in the section on Reproduction, Api-api putih is known to have fast root and seedlings development. These are essential to anchor seedlings into sediment and survive against wave action¹¹ and to start photosynthesising as soon as seedling take root, to grow quickly. These adaptations make Api-api putih a good coloniser of bare tidal flats.
Back to Top
Conservation status
Despite the threats listed above, Api-api putih has a conservation status of being the least concern, according to the IUCN Red list¹⁹. This species is widespread (as seen in Figure 1), although it is still threatened by the loss of mangrove habitats.Taxonomy
Original descriptionThe original description of Api-api putih is in Latin by Blume C.L.(1826) Bijdragen tot de flora van Nederlansch Indië Vol. 14: 821.
| Screenshot of original description by Blume (1826). Source: Biodiversity Heritage Library. Public domain. |
From the description above, it roughly translates to:
'Leaves are oblong-lanceolate and acute, smooth snowy underside. It increases towards the shore.
Flowers blossoms all year-round and the name is Api Api'
Type Information
The type specimen of Api-api putih can be found in Botanic Garden Meise Herbarium in Belgium (Barcode number: BR0000009088016).
One can refer to this type specimen (Figure 7) to confirm the identification of Api-api putih plant.
 |
| Figure 7: Type specimen of Avicennia alba. (Source: © Botanic Garden Meise Herbarium, Belgium) |
Phylogenetic analysis
In the genus Avicennia L., there are eight species. From a study by Schwarzbach and McDade (2002)²⁰ to find the phylogenetic relationship of mangrove family Avicenniaceae with analysis using chloroplast and nuclear ribosomal DNA sequence, four Avicennia species was sampled, and Avicennia was found to be strongly monophyletic as shown in Figure 8 below.
| Figure 8: Phylogenetic Relationships of the Mangrove Family Avicenniaceae. Phylogenetic position of Avicennia alba is circled in red. Genus Avicennia is shown to be a monophyletic group. (Source: Schwarzbach and McDade, 2002). |
Genetic studies to help improve efficiency of breeding programmes
In another study by Ghose et al. (2014)²¹, a random amplified polymorphic DNA (RAPD) analysis was conducted on three species of Avicennia, Avicennia alba (Api-api putih), Avicennia marina (Forsk.) Vierh. and Avicennia officinalis L. As molecular markers are not affected by environmental factors, unlike morphological markers, DNA markers can be used to quantify interspecific genetic diversity and establish phylogenetic relationships among species²¹.
From the study, results showed that:
- A. alba and A. officinalis are more closely related to each other and share a common mode on the phylogenetic tree at 73.4% of similarity (Figure 9).
- A. marina showed most genetic variability.
| Figure 9: Dendrogram showing phylogenetic relationship between three species of Avicennia base on RAPD analysis. (Source: Ghosh et al. (2014)). |
Hybridization in Avicennia species
There are no evidence of hybridization among different species of Avicennia, even if more than one species are found in close proximity to one another, thus demonstrating their reproductive isolation²².
Back to Top
Importance of Mangroves
Other than Api-api putih, there are many other species of mangrove plant. In fact, that are roughly 70 species of mangroves around the world²². Mangroves are valuable ecology and economic resource, providing humans and other organisms with many goods (e.g. wood for fuel, food items) and ecosystem services (e.g. coastal protection, flood abatement)²³ . Read on to find out more about the importance of mangroves!General importance
 |
| Figure 10: Network of roots in the mangrove forest that are important to trap sediments for land-building as well as trap litters to prevent them from being washed into the sea. (Source: © Tan Heok Hui) |
1) They are ‘land builders’²⁴
- Mangroves roots help to trap sediments and reclaim land naturally. Trapping of sediments also contribute to soil formation which also facilitates in stabilizing the coastline²⁵.
- Mangroves act as a filter for upland run-off via trapping of sediments and litters between their network of roots²⁶ (Figure 10). This helps to prevent sediments and litters from being flushed into the sea and cause marine pollution.
- Filtration of water improves water quality and maintain water clarity which are important for organisms like clams and oysters to thrive in the mangrove and intertidal area²⁶.
3) Nursery for juvenile organisms
- Mangroves serve as important nurseries for juvenile of many marine organisms, such as reef fishes, crabs and oysters²⁷ ²⁸, by providing food and shelter against predators before juveniles grow big enough to swim out to patch reefs or the ocean²⁹.
- They are also important feeding grounds for transient birds, reptiles and fishes²⁵.
- Mangroves produce large amounts of detritus that serves as the basis of the food web and hence possibly contributes to productivity in offshore waters²⁶.
- Mangrove forests are is one of the largest storage of carbon³⁰. Thus they play an important role in the global carbon cycle. Therefore, increasing the extent of mangrove forests can be used as effective means against climate change.
- Mangroves are found along coastlines and they serves as a barrier of protection against shoreline erosion by strong waves and currents and help to protect coastal communities against storms²⁵.
- From Figure 11, it shows that wave height can be reduced as it travels through the mangrove forest. For example, wave height is reduced by 35% as it travels through 80 metres of mangrove forest³¹.
| Figure 11: Wave height reduces as it travels through the mangrove forest. (Source: McIvor et al. 2012) |
7) Contribute nutrient to environment
- Avicennia species have leaves that are known to be thinner and contain fewer types of tannin (less chemical defense against herbivory). Therefore they decomposed at a faster rate than other mangrove species³², contributing more nutrient to the environment.
Important feeding ground for migratory birds³³
- An important mangrove habitat in Singapore is Sungei Buloh Nature Reserve which serves as a major bird roosting site. Every year, Sungei Buloh function as an important stop-over site for around 12 – 15 thousand million migratory birds flying from the northern Eurasian continent to Africa and the Southern parts of Asia to escape the winter months. The flyway (migratory route) along Singapore is known as the East Asian Australasian Flyway.Back to Top
Threats to Mangroves
Despite the importance of mangroves, this ecosystem still faces threats which have the potential of pushing some species to extinction.1) Conversion of mangrove forest for shrimp farming
- Due to the high biomass productivity in the mangrove forest, trees are often cleared away for conversion into shrimp ponds (Figure 12). In the past, Sungei Buloh also had shrimp ponds where prawns spawn in the mangroves that were rich in organic nutrients³⁴. However, shrimp aquaculture has many harmful impacts on the mangrove forest, including direct loss of mangrove forest to construct ponds, increase in sedimentation and turbidity from ponds, reduction in water quality and influx of excess nutrients which could lead to harmful algal bloom²³.
 |
| Figure 12: Farmers constructing a shrimp farm. As seen in the photo, part of the mangrove forest have been cleared away to build the shrimp farm, leaving only strips of mangroves at the sides and middle. (Source: Creative Commons, CC BY 2.0) |
2) Overexploitation of ecological goods and services
- Mangrove forest has long been exploited by coastal communities for its ecological goods such as timber, tannin and food, and services such as water filtration and flood abatement. The increase in human population has led to encroachment of community into coastal area. This may results in increased volume of waste being dumped into mangroves and adjacent coastal habitats. While mangrove forest can adsorb some of the excess nutrients, toxic waste are harmful to their growth³⁵.
- Mangrove forests are also deforested for timber as well as to make space for growing agriculture crops, building of housing or for aquaculture (Figure 13). Sediments usually get accumulated at flooded estuaries and mangrove forests.When mangroves are removed, the loss of a sediment sink may lead to increase sediments in the sea³⁶.
 |
| Figure 13: Mangrove deforestation in Cambodia, Koh Kong. (Source: © Peter Bennet, permission pending) |
4) Coastal squeeze
- Mangrove forests are vulnerable to sea level rise and they progress landwards at a rate that is determined by the rate of 1) sea level rise, 2) sediment accretion and 3) rate of erosion²³. However, as countries build seawalls against sea level rise, they prevent migration of mangroves toward adjacent upland³⁷ (Figure 14). Coastal squeeze will slowly decrease the area of mangrove forest and eventually causing them to drown if sediment erosion by sea level rise is greater than sediment accretion³⁷.
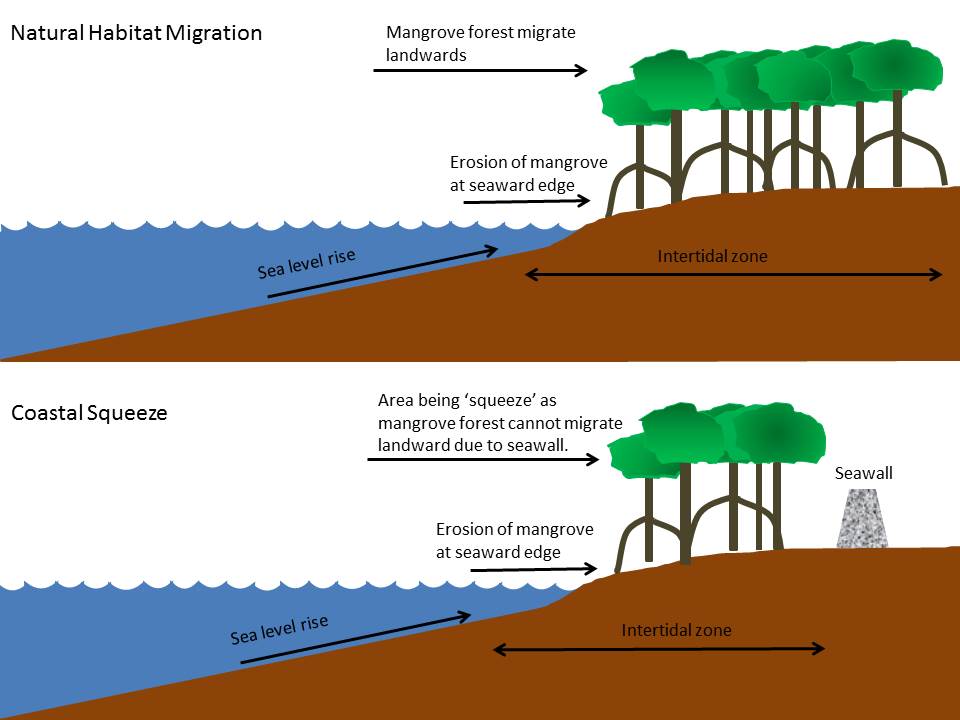 |
| Figure 14: Illustration of effect of coastal squeeze on mangrove forest due to the presence of seawall which prevents mangrove from migrating landwards when sea level rises. (Source: © Jessica Tay, adapted from Coastal Wiki, Coastal squeeze) |
Back to Top
Glossary
Here are definitions of some of the terms used in the page to help you better understand the content³⁹!Apex: The tip or point.
Spicate: Arranged in spikes
Fissured: Narrow opening produced by cleavage, to split.
Lenticels: A body of cells formed on the outer layer of woody stems and roots, appearing as a lens-shaped spot and serving as a pore.
Chlorophyllous: of or containing chlorophyll for photosynthesis.
Inundation: To flood; cover or overspread with water
Apoplastic movement: Movement of water and solutes through the cell walls and intercellular space, without crossing the plasma membrane.
Hybridization: To produce hybrids which is the offspring of two different species, breeds or varieties.
Detritus: organic matter produced by the decomposition of organisms.
Encroachment: To advance beyond proper or usual limits.
Back to Top
Contact me
source: © Jessica Tay) or you have any questions regarding the page. Also, please check out the other species pages that were done by other students who have taken this module!References
[1] Ng P.K.L and Sivasothi N. (1999). A Guide to the Mangroves of Singapore I: The Ecosystem and Plant Diversity. Singapore Science Centre.[2] Umberto Q. (2000). CRC World dictionary of plant names: Common names, scientific names, eponyms, Synonyms and etymology. Volume 1 A-C. CRC Press, 1999. pp. 242.
[3] Tomlinson P. B. (eds). (1994). The botany of mangroves. Cambridge tropical ecology series. Cambridge university press. pp. 419. Tomlinson, B. P., 1986. The Botany of Mangroves. Cambridge University Press, Cambridge
[4] Yee A.T.K., Ang W.F., Teo S., Liew S.C., Tan H.T.W. (2010). The present extent of mangrove forest in Singapore. Nature in Singapore 3:139–145.
[5] Smith T.J.III. (1992). Forest structure. In: Robertson AI, Alongi DM (eds) Tropical mangrove ecosystems. American Geophysical Union. American Geophysical Union, Washington, pp 101–136.
[6] El-Khouly A.A. and Khedr A.A. (2007). Zonation pattern of Avicennia marina and Rhizophora mucronata along the red sea coast, Egypt. World applied sciences journal 2:283–288.
[7] Lugo A.E. and Snedaker S.C. (1974). The ecology of mangroves. Annual review of ecology and systematics 5:39–64.
[8] Matthijs S., Tack J., van Speybroeck D. and Koedam N. (1999). Mangrove species zonation and soil redox state, sulphide concentration and salinity in Gazi Bay (Kenya), a preliminary study. Mangroves and salt marshes 3: 243–249.
[9] Raju A.J.S., Rao P.V.S., Kumar R. and Mohan S.R. (2012). Pollination biology of the crypto-ciciparous Avicennia species (Avicenniaceae). Journal of threatened taxa 4:3377–3389.
[10] Ong J.E. and Gong W.K. (2013). Structure, function and management of mangrove ecosystems. ISME mangrove educational book series No. 2. International society for mangrove ecosystems (ISME), Okinawa, Japan, and International Tropical Timber Organisation (ITTO), Yokohama, Japan.
[11] Balke T., Bouma T.J., Horstman E.M., Webb E.L., Erftemeijer P.L.A. and Herman P.M.J. (2011). Windows of opportunity: thresholds to mangrove seedling establishment on tidal flats. Marine ecology progress series 440:1–9.
[12] Murphy D.H. (1990). The natural history of herbivory on mangrove trees in and near Singapore. Raffles Bulletin of Zoology 38:119–203.
[13] Joshi H. and Ghose M. (2003). Forest structure and species distribution along soil salinity and pH gradient in mangrove swamps of the Sundarbans. Tropical ecology 44:197–206.
[14] Mangrove roots (2014). Retrieved from: http://www.mangrove.at/mangrove_roots.html. (Accessed on 18th October 2014)
[15] Krishnamurthy P., Jyothi-Prakash P.A., Qin L., He J., Lin Q., Loh C., and Kumar P.P. (2014). Role of root hydrophobic barriers in salt exclusion of a mangrove plant Avicennia officinalis. Plant, Cell and Environment.
[16] Parida A.K. and Jha B. (2010). Salt tolerance mechanisms in mangroves: a review. Trees 24:199–217.
[17] Shimony C., Fahn A. and Rheinhold L. (1973). Ultrastructure and ion gradients in the salt glands of Avicennia marina (Forsk) Vierh. The New Phytologist 72:27–36.
[18] Tan W.K., Lin Q., Lim T.M., Kumar P. and Loh C.S. (2013). Dynamic secretion changes in the salt glands of the mangrove tree species Avicennia officinalis in response to a changing saline environment. Plant, Cell & Environment 36:1410–1422.
[19] International Union for Conservation of Nature (2014). Avicennia alba. Retrieved from: http://www.iucnredlist.org/details/178830/0 (Accessed on 26th October 2014).
[20] Schwarzbach A.E. and McDade L.A. (2002). Phylogenetic Relationships of the Mangrove Family Avicenniaceae based on chloroplast and nuclear ribosomal DNA sequences. Systematic Botany 27:84–98.
[21] Ghosh P., Das S. S. and Das S. (2014). Phylogenetic relationship among three species of the mangrove genus //Avincennia// found in Indian Sundarban, as revealed by RAPD analysis. Asian Journal of Plant Science and Research 4:25-30.
[22] Duke N.C., Benzie J.A.H., Goodall J. A. and Ballment E.R. (1998). Genetic structure and evolution of species in the mangrove genus Avicennia (Avicenniaceae) in the Indo-West pacific. Evolution 52: 1612–1626.
[23] Alongi D.M. (2002). Present state and future of the world’s mangrove forests. Environmental Conservation 29:331–349.
[24] McKee K. L. (1996). Mangrove ecosystem: Definitions, distribution, zonation, forest structure, trophic structure, and ecological significance. In: Feller I. C. and Sitnik M. (eds). Mangrove ecology: a manual for a field course. A field manual focused on the. biocomplexity on mangrove ecosystems. 1–6.
[25] Ewel K.C., Twilley R.R. and Ong J.E. (1998). Different kinds of mangrove forest provide different goods and services. Global ecology and biogeography letters 7:83–94.
[26] Sulochanan B. (2013). Mangrove ecosystem and its impact on fisheries. Central Marine Fisheries Research Institute. 57–62.
[27] Robertson A. I. and Blaber S. J. M. (1992) Planktons, epibenthos and fish communities. In: Robertson A. I., Alongi D. M. (Eds.), Tropical Mangrove Ecosystems. Coastal and Estuarine Studies No. 41, American geophysical union, Washington, DC (1992), pp. 173 – 224.
[28] Macnae W. (1974). Mangrove forest and fisheries. FAO. Rome. IOFC/DEV/74/34, 35 pp.
[29] Roach J. (2004). Mangroves are nurseries for reef fish, study finds. National Geographic news.
[30] Donato D. C., Kauffman B.J., Murdiyarso D., Kurnianto S., Stidham M., and Kanninen M. (2011). Mangroves among the most carbon-rich forests in the tropics. Nature Geoscience, 4: 293–297.
[31] McIvor A., Möller I., Spencer T., and Spalding M., (2012). Reduction of wind and swell waves by mangroves. Natural Coastal Protection Series: Report 1: The Nature Conservancy and Wetlands International.
[32] Sivakumar, A. and Kathiresan, K. (1990). Phylloplane fungi from mangroves. Indian Journal of Microbiology, 30(2) : 229 – 231.
[33] Sivasothi M. (2003). Mandai mangrove and mudflat. Retrieved from: http://mangrove.nus.edu.sg/mandai/significance.html. (Accessed on 20th October 2014).
[34] Lee Kong Chian Natural History Museum (2014) Prawn farming. Retrieved from: http://lkcnhm.nus.edu.sg/dna/activities/details/32 (Accessed on 3rd November 2014).
[35] Yim M.W. and Tam N.F. Y. (1999). Effects of wastewater-borne heavy metals on mangrove plants and soil microbial activities. Marine Pollution Bulletin 39:179–186.
[36] van Maren D.S., Liew S.C. and Hasan G.M.J. (2014). The role of terrestrial sediments on turbidity near Singapore’s coral reefs. Continental Shelf Research 76:75–88.
[37] Torio D.D. and Chmura G.L. (2013). Assessing coastal squeeze of tidal wetland. Journal of Coastal Research 29:1049–1061.
[38] Purnobasuki H. (2013). Characteristics of root caps in four root types of //Avicennia marina// (Forsk.) Vierh. American Journal of Plant Science 4: 853–858.
[39] http://dictionary.reference.com/. Accessed on 23rd November 2014.