1. Introduction
Table of Contents
 |
| Figure 1: Oriental fruit flies on a fruit. Photo credits: Florida Division of Plant Industry [1] |
The Tephritidae family comprises non-fruit feeding tephritids, which are rarely pestiferous [2], and frugivorous tephritids, which contain several genera which are of major economic concern globally [3]. Some notorious fruigivorous tephritid species include: the Mediterranean fruit fly or Medfly (Ceratitis capitata (Wiedemann)), the Cherry fruit fly (Rhagoletis cingulata (Loew)), the Mexican fruit fly, (Anastrepha ludens (Loew)) and the Oriental fruit fly (Bactrocera dorsalis (Hendel)).
The Oriental fruit fly belongs to the Bactrocera dorsalis species complex, a group of almost 100 morphologically similar tephritid taxa [4, 5]. Amongst the generally inconspicuous species in this species complex, the Oriental fruit fly and the Carambola fruit fly (B. carambolae) stand out in their economic impact. These species have been recognised as possibly the world’s most important pests of horticulture [6, 7]. This page focuses solely on the Oriental fruit fly.
Due to fairly recent updates to the scientific nomenclature of the Oriental fruit fly, information with regards to the Oriental fruit fly can also be found online under the following names: the Asian Papaya fruit fly (Bactrocera papayae), the Philippine fruit fly (Bactrocera philippinensis) and the Invasive fruit fly (Bactrocera invadens). For more information with regards to the many names of this species, head over to the Scientific Name & Synonyms section.
1.1. Disclaimer: Fruit fly ≠ Drosophila melanogaster
Whenever the phrase "fruit fly" is mentioned, the image of the model organism, Drosophila melanogaster, comes to mind. However, albeit their similar common names, Drosophila melanogaster, the Common fruit fly, does not belong to the same family as Bactrocera dorsalis, the Oriental fruit fly; Drosophila melanogaster belongs to the family Drosophilidae whilst Bactrocera dorsalis belongs to the family Tephritidae (See Figure 3).
| Common fruit fly |
Oriental fruit fly |
Size comparison |
 |
 |
|
| Scientific name: Drosophila melanogasterFamily: Drosophilidae Significance:Important model organism in genetics and developmental biology research Adult's appearance:Red eyes; Yellow or brown (tan) colour; Black stripes on dorsal surface of abdomen Larvae's appearance:Minute white maggots lacking legs and a defined head |
Scientific name: Bactrocera dorsalisFamily: Tephritidae Significance:Notorious pest species known for causing immense economic losses Adult's appearance:Variable colour & pattern; prominant yellow and dark brown to black markings on dorsal surface of thorax; Generally with two horizontal black stripes and a longitudinal mark on dorsal surface of abdomen Larvae's appearance:10 mm long; creamy white colour |
Drosophila melanogaster size:1-3 mm body length Bactrocera dorsalis size:6-8 mm body length Credits: Drosophila melanogasterInformation: Animal Diversity Web [8]Image: Malcolm Storey © Copyright Bactrocera dorsalisInformation: Plant Health Australia [9]Image: Arina Adom |
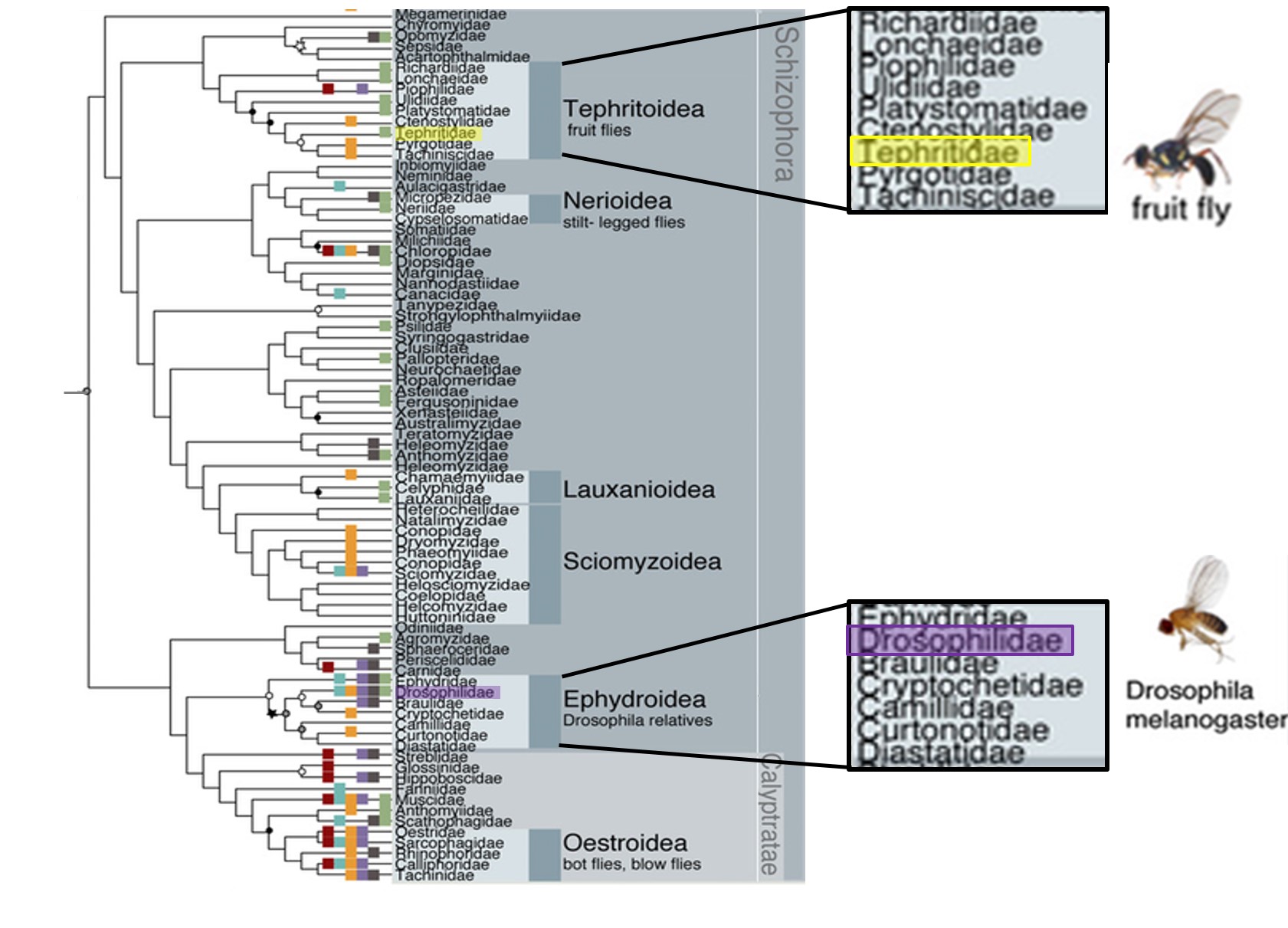 |
| Figure 3: Phylogeny tree indicating positions of the Tephritidae and Drosophilidae families on the Schizophora phylogeny tree. Image adapted from Wiegmann et al. [10] |
2. Biology
2.1. Life History
In tropical environments, Oriental fruit flies breed throughout the year. Throughout her lifetime (typically 1-3 months), a female fly is able to typically lay between 1,200 to 1,500 eggs (under field conditions) and over 3,000 eggs (under optimum conditions) [11].
Figure 4: Gif showing adult Oriental fruit flies on an orchid. Video credits: YouTube [25]
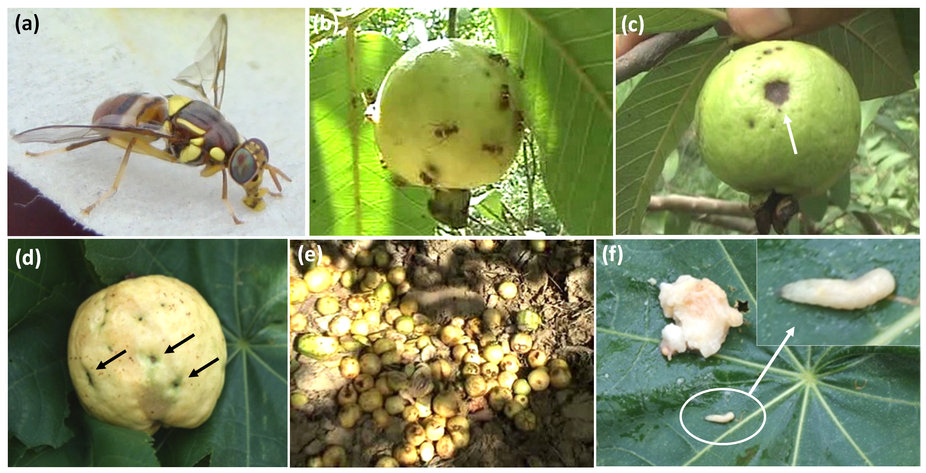
Eggs are laid in batches of 1-20 eggs per fruit, just under the fruit's skin, where fruit-decaying bacteria is subsequently deposited. Although ripe fruit are preferred for oviposition, oviposition on immature fruits has also been observed. Development from egg to adult is usually completed within about 16 days, however cooler weather conditions are known to delay development. Larvae (commonly known as maggots) develop within the fruit [11, 12].
 |
| Figure 5: Image of oriental fruit fly larvae within a citrus fruit. Photo credit: Scott Bauer (permission pending) [15] |
When mature, larvae emerge from the fruit and jump or drop onto the ground (watch the video below to see how the larvae jumps!).
Video 1: Video showing jumping tephritid larvae found inside a squash. Video credits: YouTube [26]
In the soil, they form tan to dark brown puparium. Once mature, the flies emerge from their puparia as adult flies. Young males often disperse over several kilometres before reaching sexual maturity and finding its mate! For this reason, Oriental fruit flies are known to spread far and fast, causing much damage to crop fruits [11, 12]. The Oriental fruit fly life cycle is summarised in the following figure.
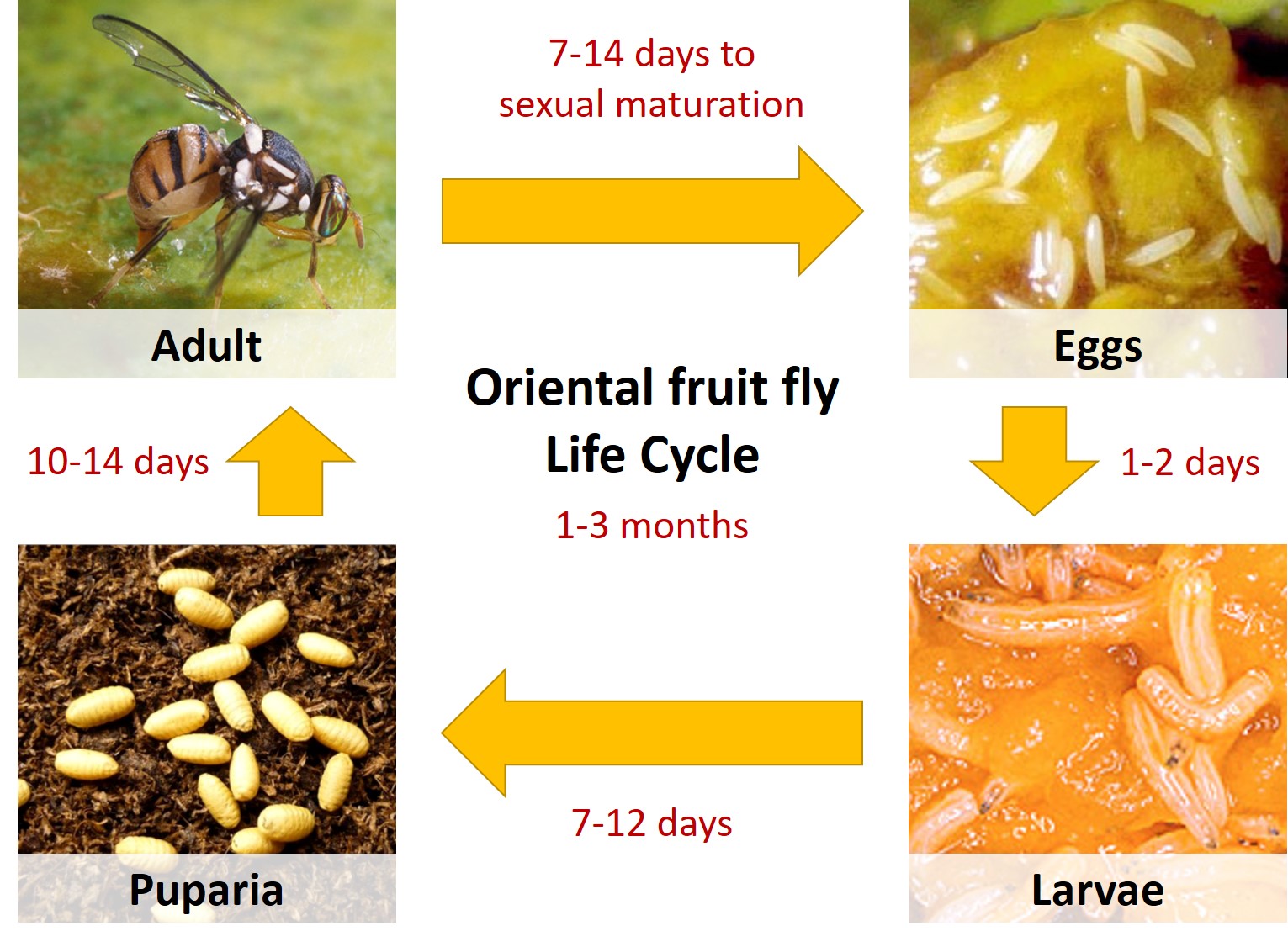 |
| Figure 6: Life Cycle of Tephritid fruit fly. Image credits: Adapted from various sources; Permission pending [13-16]. |
2.2. Feeding Habits
The Oriental fruit fly is highly polyphagous and is able to feed on and infest a large variety of produce from fruits, vegetables and other plants. The USDA has recorded its' occurence on 478 kinds of fruit and vegetables as of 2016 [17].
|
|
|
Figure 8: Gif showing a damaged fruit infected with a B. dorsalis larva [28].
3. Economic Importance
The Oriental fruit fly has been known to cause up to billions of dollars in financial losses due to (1) the direct damage caused to fruit crops, (2) exportation and quarantine restrictions from areas with a history of fruit fly infestations and (3) management costs to eradicate existing infestations [15].3.1. Damage to Crops
Regarded as the "most damaging pest of tropical horticulture in the world ", Oriental fruit flies are feared for their indiscriminate infestations on a wide range of produce, whether ripe or unripe. Damage caused by the fruit fly result from 1) oviposition in fruit and soft tissues of vegetative parts of certain plants, 2) feeding by the larvae, and 3) decomposition of plant tissue by invading secondary microorganisms [19]. Often, much damage can occur before any obvious signs of infestation within a fruit are even observed [12].The first signs of an infestation are small discoloured patches on the skin fruit skin, which develop from the "sting" of the female fly during ovipositon. Once hatched, fruit fly larvae able to tunnel deeply into the fruit flesh for feeding, spreading widely within infested fruit and making the whole fruit unpalatable. In certain fruit types, maggot infestation results in tissue breakdown and internal rotting associated with maggot infestation, but this varies with the type of fruit attacked. Affected young fruit tend to become distorted, callused and usually drop prematurely whereas affected mature fruits develop a water soaked appearance. The larval tunnels subsequently provide entry points for bacteria and fungi that cause the fruit to rot further. When only a few larvae develop, damage consists of an unsightly appearance and reduced marketability because of the egg laying punctures or tissue break down due to the decay [20]. Damage to crops has been estimated at between 50-80% in West Pakistan and even up to 100% in Malaysia [21].
Watch the video below to see the impacts of Oriental fruit fly infestations on the community in Mpumalanga, South Africa.
Video 2: News report of the impacts of Oriental fruit fly infestations in Mpumalanga, South Africa. Video credits [27]
3.2. Quarantine
Due to the potential damage caused by these flies, coupled with their ability to be active dispersers and breed agressively as adults, the Oriental fruit fly is regarded as an invasive species in many countries around the world. These countries guard heavily against the introduction of the Oriental fruit fly through immigration in hopes of avoiding massive economic loss to agricultural farmers. Fresh agricultural produce being transported into each country is meticulously monitored and quarantined in order to isolate and eliminate the accidental introduction of these flies. As a result, much economic loss is caused by trade restrictions. Malaysian fruits, for example, cannot be freely exported to lucrative markets like USA, Japan and Australia, because of strict quarantine regulations in these countries, which prohibit entry of fresh fruits from the fruit flies infested countries unless proper disinfestation treatments are carried out [23]. This situation is also faced in the Philippines, Thailand, Indonesia, India, Taiwan and China.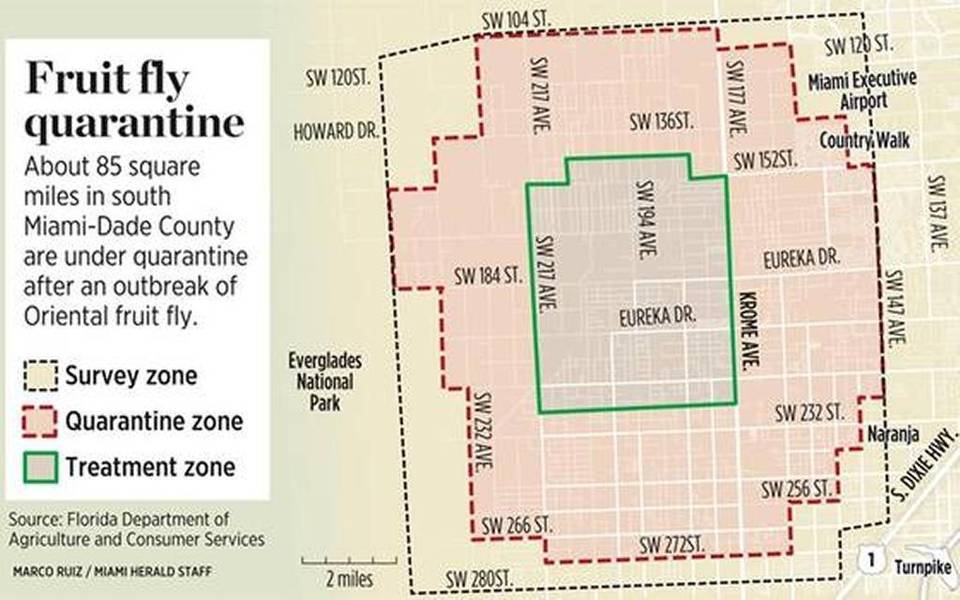 |
| Figure 10: Extract from a newspaper article highlighting quarantine measures taken after an Oriental fruit fly outbreak. Image credit: Florida Department of Agriculture and Customer Services [24]. |
3.3. Management
Fortunately, populations of the Oriental fruit fly are manageable and can be eradicated, albeit a huge financial cost incurred. In 1985, all Japanese territories were declared free of the Oriental fruit fly after an 18-year program of eradication combining insecticide-impregnated fiberblocks or cotton containing the powerful male attractant methyl-eugenol, and the sterile insect (sterile male) technique. Steiner traps baited with a lure and toxicant are also used to monitor the presence and control of the flies. In 1995, Oriental fruit fly established near Cairns and cost $33.5 million and took 4 years to eradicate [11].4. Distribution
4.1. Worldwide distribution
Native to the tropical Asian region, the fruit flies have spread throughout the world and become a prominent pest species across multiple continents. The fly is believed to be introduced through the import of infected fruits from native regions in Asia.4.2. Asian distribution
Bactrocera dorsalis is native to tropical Asian countries such as Singapore, India, Malaysia and Bangladesh. In countries such as Cambodia and China, B. dorsalis is regarded as an invasive species [30].4.3. African distribution
First introduced to Africa through Kenya in 2003. Since then, most countries within sub-Saharan Africa have faced B. dorsalis infestations [29].4.4. Pacific Islands distribution
Bactrocera dorsalis was accidentally introduced in the United States between 1944 to 1945 and is now present and a major threat on all major Hawaiian islands [19]Not shown on map: Bactrocera dorsalis has also been reported in Queensland, Australia [30].
5. Description
5.1. Adult
The adult, which is noticeably larger than a house fly, has a body length of about 7.0-8.0 mm; the wing is about 7.3 mm in length. The color of the fly is very variable, but there are prominant yellow and dark brown to black markings on the thorax (a). Generally, the abdomen has two horizontal black stripes (b) and a longitudinal median stripe extending from the base of the third segment to the apex of the abdomen (c). These markings may form a T-shaped pattern, but the pattern varies considerably. The wings are clear (d). In females, the ovipositor is very slender and sharply pointed (e) [11].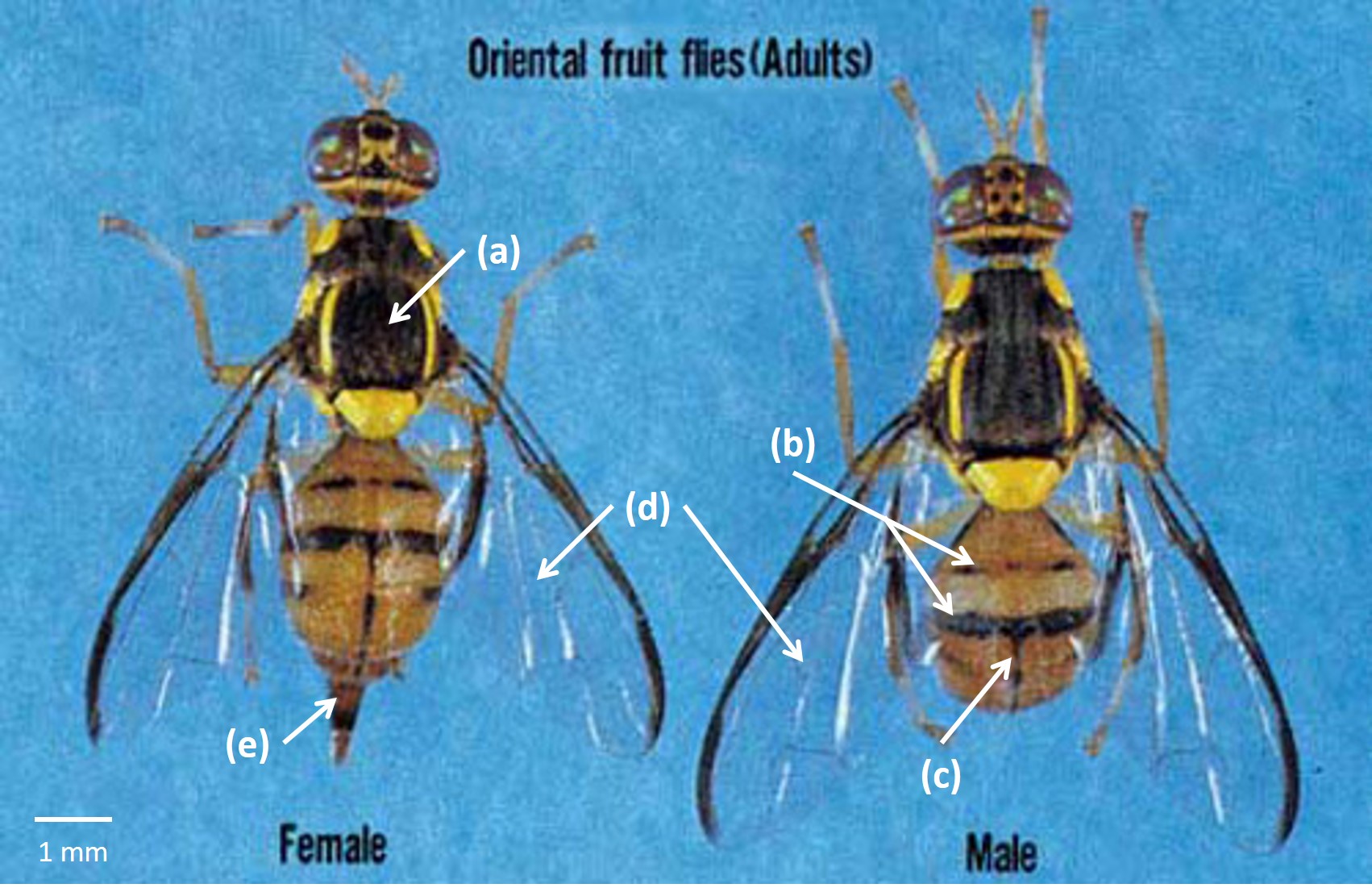 |
| Figure 15: Adults of the Oriental fruit fly. Image adapted from Okinawa Prefectural Fruit Fly Eradication Project Office [11]. |
5.2. Larva
The third-instar, which has a typical maggot appearance, is about 7.5-10.0 mm long and 1.5-2.0 mm wide and creamy white coloured [33]. The only band of spinules encircling the body is found on the first segment. The external part of the anterior respiratory organs, the spiracles, located one on each side of the pointed or head end of the larva, has an exaggerated and deflexed lobe at each side and bears many small tubercles. The caudal segment is very smooth. The posterior spiracles are located in the dorsal third of the segment as viewed from the rear of the larva [11].The mature larva emerges from the fruit, drops to the ground, and forms a tan to dark brown puparium about 4.9 mm in length.
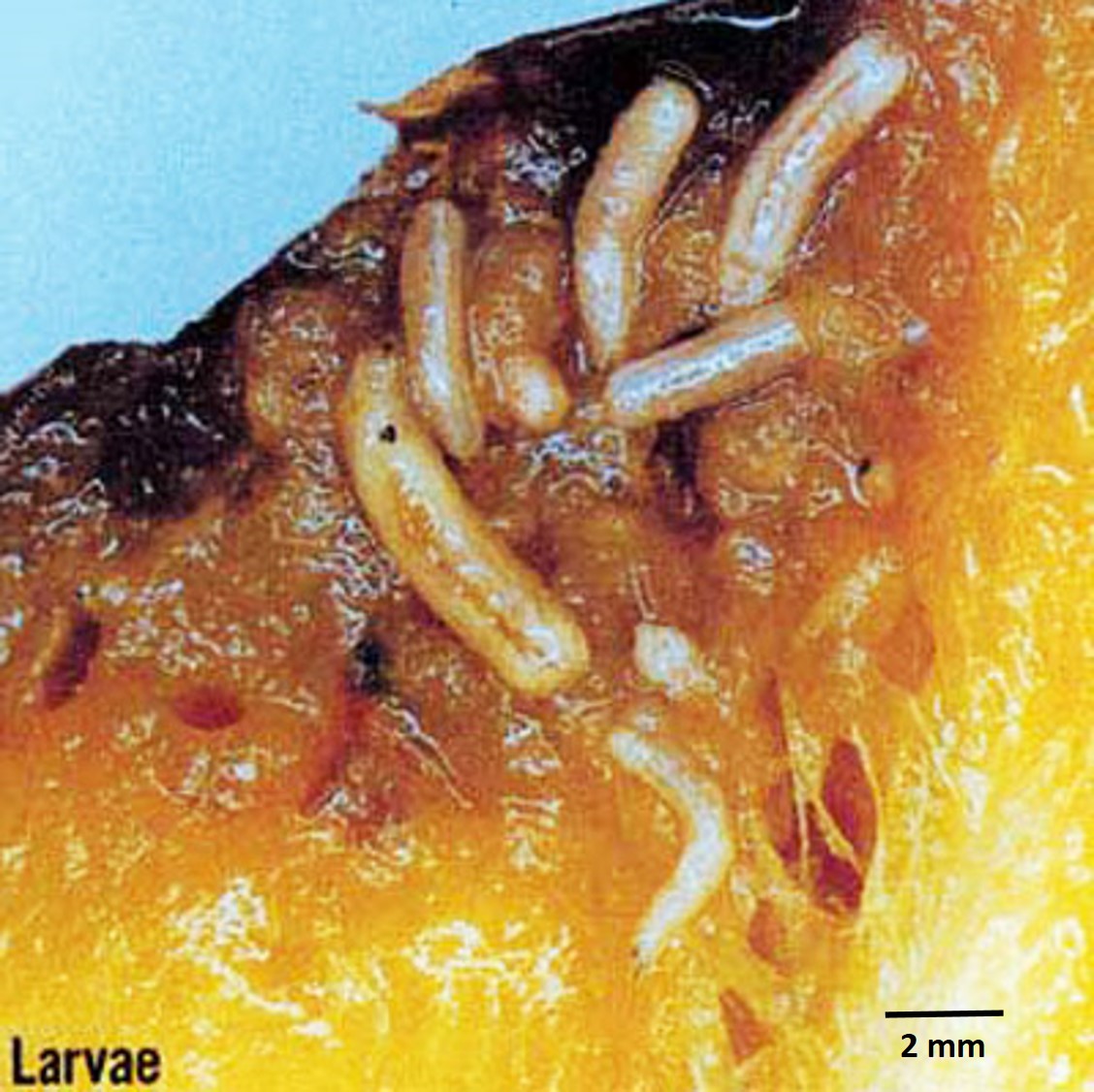 |
| Figure 16: Larvae of the Oriental fruit fly. Image adapted from Okinawa Prefectural Fruit Fly Eradication Project Office [11]. |
5.3. Puparium
Barrel-shaped with most larval features unrecognisable. White to yellow-brown. Usually approximately 60-80% the length of the larva [33].5.4. Egg
The white, elongate and elliptical egg measures about 1.17 x 0.21 mm and has a chorion without sculpturing.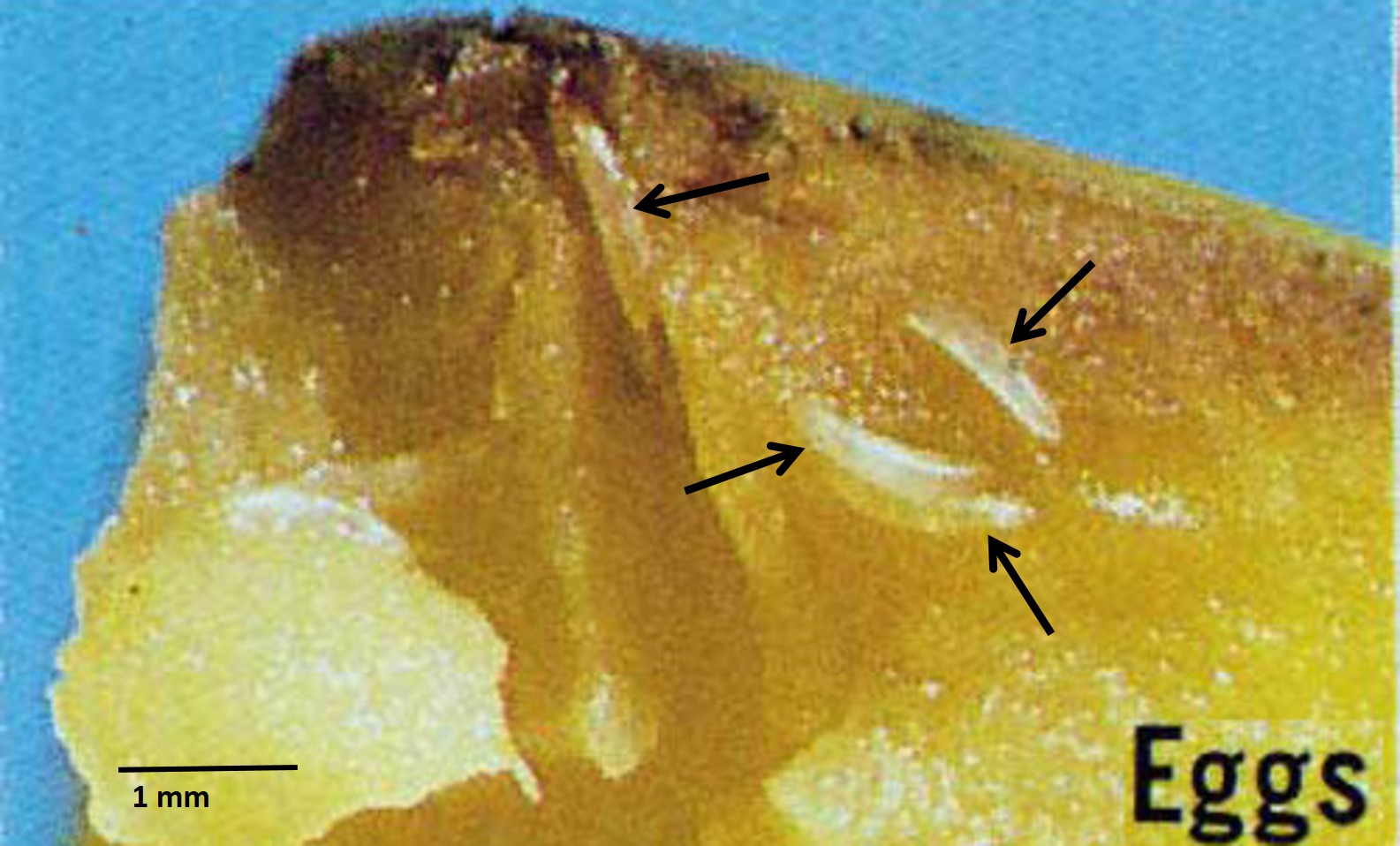 |
| Figure 17: Eggs of the Oriental fruit fly. Image adapted from Okinawa Prefectural Fruit Fly Eradication Project Office [11]. |
5.5. Official Diagnosis
First flagellomere shorter than ptilinal fissure, face with a black spot in each antennal furrow. Tomentum pattern without longitudinal gap in the middle of prescutum. Scutum colour (other than vittae) red-brown to black. Lateral vittae of scutum present, parallel-sided, yellow, ending at or just behind intra-alar seta. Medial vitta of scutum absent. Scutellum largely yellow. Setae: anterior supra-alar present, prescutellar present, scutellar one pair. Anepisternal stripe not extended forward. Wing costal band width from vein subcostal to slightly below vein R4+5 at wing apex; confluent with vein R2+3 in depth. Cell basal costal without microtrichia. Cell costal with microtrichia in the anterodistal corner only. Cell basal radial with microtrichae at the base. Wing length: 5.4–6.9mm. Foretibia pale fuscous to dark fuscous, mid-tibia pale fuscous to fuscous, hind tibia fuscous to dark fuscous. Femora largely fulvous. Abdominal terga free, except I and II. Terga markings: terga III–V withmedial longitudinal black band, tergum III with a basal narrow transverse black band, terga IV and V with black triangular lateral markings, sometimes longitudinal black bands on lateral margins and sometimes without any mark. Tergum III (males) with pecten. Sternum V (males) with V-shaped notch. Posterior surstylus lobe short. Males attracted to methyl eugenol [34].6. Taxonomy & Systematics
6.1. Original Description
Bactrocera dorsalis was first described by Friedrich Hendel as Dacus dorsalis in the "Entomologischen Mitteilungen" or "Entomological Messages" in 1912. |
| Figure 18: Frist Description of the oriental fruit fly in the "Entomologischen Mitteilungen" or "Entomological Messages" in 1912. Image credit: Biodiversity Heritage Library [35] |
6.2. Type Information
The holotype of Dacus ferrugineus Fabricius is currently located in the Natural History Museum of Denmark. although the specimen is nearly entirely destroyed, the taxonomically informative ‘red-brown’ colour of the thorax is still evident (See Fig.19) [31]. |
| Figure 19: Holotype of Dacus ferrugineus Fabricius located in the Natural History Museum of Denmark. Photo credit: Verner Michelsen [31]. |
6.3. Common Names
English: Oriental fruit flyMalay: lalat buah
Spanish: mosca oriental de la fruta
French: mouche de fruits asiatique; mouche orientale des arbres fruitiers
Portuguese: la mosca oriental das frutas
Germany: Orientalische Fruchtfliege
Japan: mikan-ko-mibae
Netherlands: mangga-vlieg
6.4. Scientific Name & Synonyms
Scientific Name: Bactrocera dorsalis (Hendel, 1912)Originally described by Hendel in 1912 as Dacus dorsalis, the Oriental fruit fly has since been redescribed, reclassified and renamed multiple times by numerous authors internationally. One major cause was the large variability in colouration and patterning, between the otherwise morphologically and genetically similar pest species within the Bactrocera dorsalis species complex.This resulted in the hindrance of the development of reliable diagnostic characters to distinguish between intra- and inter-specific variation. The prolonged difficulties in differentiating putative species, coupled with the highly disjunct geographic distribution of closely-related species within the Bactrocera dorsalis species complex, has resulted in grouping errors in the form of separate populations of B. dorsalis worldwide being described as new separate Bactroceran species [34].
6.4.1. Case study: Bactrocera invadens
One example of a grouping error was a group of B. dorsalis specimens detected in Kenya in 2003. After comparison with B. dorsalis specimens from the purported native range of Sri Lanka, the Kenyan material was described as a new species, Bactrocera invadens [36]. This was in spite of the considerably similar morphology of Kenyan specimens to the B. dorsalis specimens obtained from Sri Lanka [37]. What led to this decision was in particular, the scutum colour of the Kenyan specimens which ranged "from pale red-brown to black with the existence of variable lanceolate-patterned intermediates". This was in contrast with the B. dorsalis description in which the scutum had been defined as "strictly black" [4]. As a result of the confusion over the unreliable discrimination between B. dorsalis and B. invadens, significant problems with African horticulture and food security emerged, especially with regards to quarantine and pest management policies [7].6.4.2. Final resolution
Fortunately, in 2009, a major international collaboration was established by the United Nations Food and Agriculture Organization (UN-FAO) and the International Atomic Energy Agency (IAEA) with the aim of resolving biological species limits among several highly morphologically and genetically similar species: Bactrocera papayae Drew & Hancock, Bactrocera philippinensis Drew & Hancock, Bactrocera carambolae Drew & Hancock, Bactrocera invadens Drew, Tsuruta & White and Bactrocera dorsalis (Hendel, 1912). The study, published in 2014, resolved the problem by using multidisciplinary evidence (morphological, molecular, cytogenetic, behavioural and chemoecological data) to synonymise B. dorsalis, B. invadens and B. papayae. Bactrocera dorsalis (Hendel) was subsequently declared a senior synonym and a more comprehensive re-description was published to include intra-specific colour variations. The following are the synonyms of Bactrocera dorsalis (Hendel, 1912) listed in the paper [34].6.4.3. Other synonyms
| Synonyms |
Author, Year |
Remarks |
| Musca ferruginea |
Fabricius, 1794 |
Preoccupied by Musca ferruginea Scopoli, 17634 |
| Dacus ferrugineus |
Fabricius, 1805 |
|
| Dacus dorsalis |
Hendel, 1912 |
Lectotype ♀ in The Natural History Museum, London, U.K. |
| Bactrocera ferruginea |
Bezzi, 1913 |
|
| Chaetodacus ferrugineus var. dorsalis |
Hendel, 1915 |
|
| Chaetodacus ferrugineus |
Bezzi, 1916 |
|
| Chaetodacus ferrugineus dorsalis |
Bezzi, 1916 |
|
| Chaetodacus ferrugineus var. okinawanus |
Shiraki, 1933; Hardy & Adachi, 1956; Hardy, 1969 |
|
| Dacus (Strumeta) dorsalis |
Hardy & Adachi, 1956; Hardy, 1969; 1973; 1974 |
|
| Strumeta dorsalis |
Hering, 1956 |
|
| Strumeta ferruginea |
Hering, 1956 |
|
| Strumeta dorsalis okinawa |
Shiraki, 1968 |
|
| Dacus (Bactrocera) dorsalis |
Hardy, 1977; Drew, 1982; 1989 |
|
| Dacus (Bactrocera) semifemoralis |
Tseng et al., 1992 |
synonymized by Drew & Romig, 2013 |
| Bactrocera (Bactrocera) dorsalis |
Drew & Hancock, 1994; Norrbom et al., 1998; Mahmood & Hasan, 2005; White, 2006; Drew et al., 2007 |
Lectotype designation by Drew & Hancock, 1994 |
| Bactrocera (Bactrocera) variabilis |
Lin & Wang, 2011 in Lin et al., 2011 |
Holotype in HEIQ; synonymized by Drew & Romig, 2013: 76 |
| Bactrocera (Bactrocera) philippinensis |
Drew & Hancock, 1994 |
synonymized with B. papayae by Drew & Romig, 2013 |
| Bactrocera (Bactrocera) papayae |
Drew & Hancock, 1994 |
syn.n. |
| Bactrocera (Bactrocera) invadens |
Drew et al., 2005 |
syn.n. |
6.5. Taxonomic Hierarchy
The classification below reflects above species-level rankings (from Kingdom to Species) for B. dorsalis:| Figure 21: Taxonomic hierarchy of B. dorsalis (Hendel, 1912). Data obtained from UniProt & ITIS [38, 39] |
7. Phylogeny
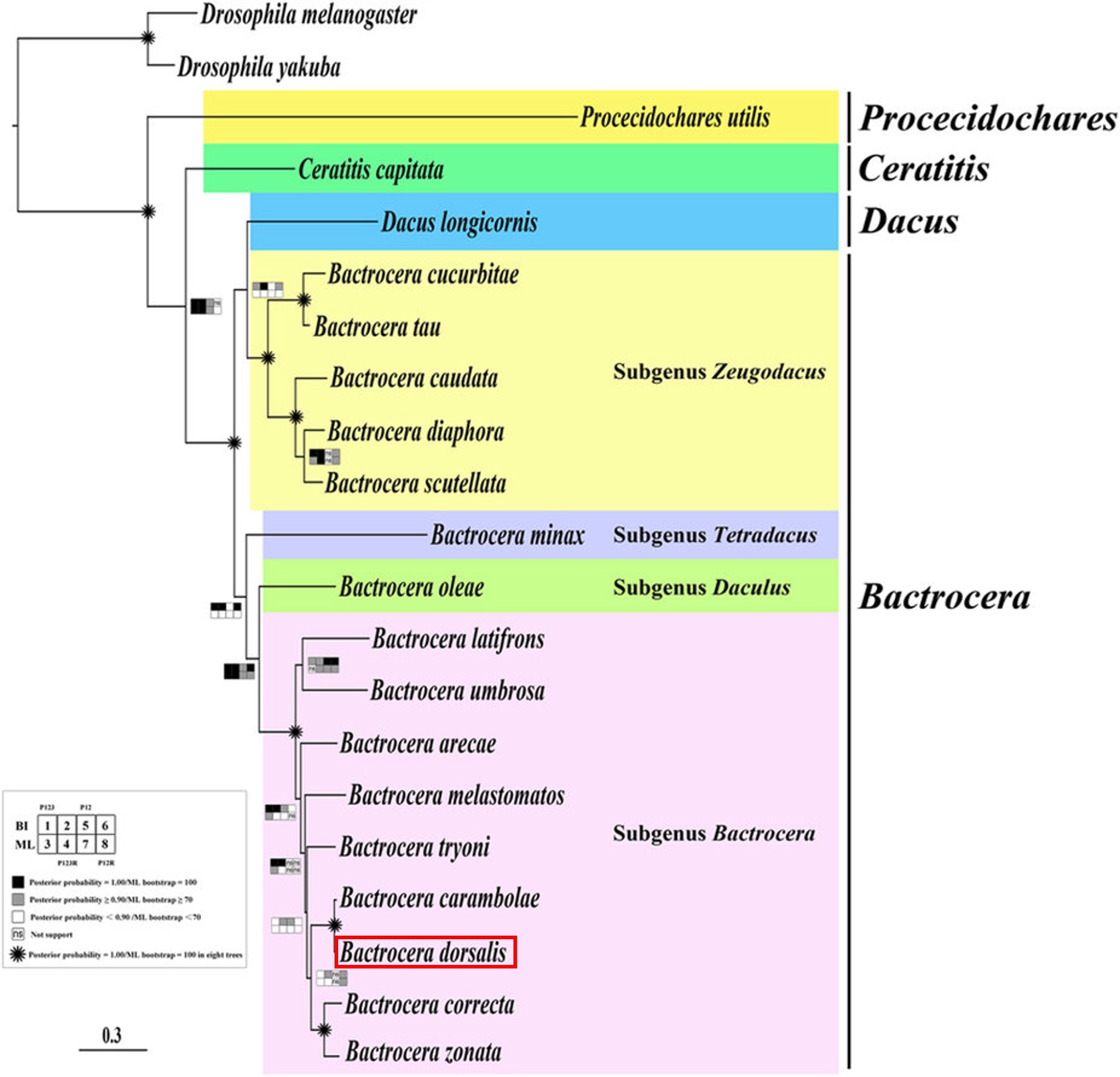
The position of the Tephritidae family within Diptera: Schizophora can be observed in Fig. 3, a subsection of the combined molecular phylogenetic tree for Diptera. The combined phylogenetic tree was produced through partitioned Maximum Likelihood (ML) analysis of data calculated in RAxML. Molecular and morphological data from 149 of 157 dipteran families, including 30 kb from 14 nuclear loci and complete mitochondrial genomes as well as 371 morphological characters were used in this analysis[10].Within the family Tephritidae, the following phylogenetic tree has been obtained (Fig. 21) using Bayesian and ML analysis of mitochondrial DNA sequences obtained through next-generation sequencing (NGS) [40]. The tree is fairly stable with 8 out of 16 nodes within the Tephritidae tree being well supported (Bayesian posterior probabilities = 1.00 or ML bootstrap = 100 and 4 out of the remaining nodes having majority relatively high support (posterior probabilities ≥ 0.90 or ML bootstrap ≥ 70). Results show that both the family Tephritidae and the tribe Dacini (represented in the figure by genera Dacus and Bactrocera) are monophyletic. The genus Bactrocera, on the other hand is not monophyletic.
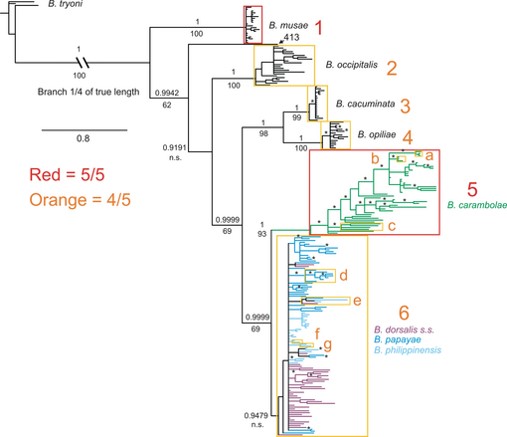
Within the Bactrocera dorsalis species complex, a separate phylogenetic study was conducted in 2013 (before B. dorsalis was synonymised with B. invadens, B. papayae and B. philippinensis [34]) to study the phylogeny of several Bactrocera species. A molecular phylogenetic tree was build with molecular data obtained from six loci (cox1, nad4-3′, CAD, period, ITS1, ITS2) for approximately 20 individuals from each of 16 sample sites. Specimens used were morphoogically sorted into the "ingroups" B. dorsalis, B. papayae, B. philippinensis and B. carambolae. Several "outgroup species" were also used in this study, some being species within the B. dorsalis species complex (namely the Australian species B. cacuminata and B. opiliae, and the Philippine species B. occipitalis (which occurs sympatrically with B. philippinensis) and some not being within the species complex (namely B. musae and B. tryoni). The resultant tree obtained using Bayesian and ML analysis showed the non-monophyly of B. dorsalis, B. papayae and B. philippinensis and supports the more recent synonymisation of the three species as a single species under B. dorsalis (Box 6 in Fig. 22). The tree also strongly supported the monophyly of B. carambolae and its' distinction from the remaining 3 ingroup species (Box 5 in Fig 22).
Thank you for reading! :)
8. References
[1] “Oriental fruit fly (Bactrocera dorsalis) (Hendel, 1912)”, by Florida Division of Plant Industry, Florida Department of Agriculture and Consumer Services, Bugwood.org. URL: http://www.invasive.org (accessed on 15 Nov 2016).[2] Headrick, D. H. & R. D. Goeden, 1998. The biology of nonfrugivorous tephritid fruit flies. Annual Review of Entomology, 43: 217-241.
[3] White, I. M. & M. M. Elson-Harris, 1992. Fruit flies of economic significance: their identification and bionomics. C.A.B International in association with ACIAR, Wallingford, Oxon.
[4] Drew R. A. I. & D. L. Hancock, 1994. The Bactrocera dorsalis complex of fruit flies in Asia. Bulletin of Entomological Research (Suppl. 2), CAB International, Wallingford, UK.
[5] Drew, R.A.I.& M.C. Romig, 2013. Tropical fruit flies of South-east Asia. CAB International, Wallingford, UK.
[6] Clarke, A.R., Armstrong, K.F., Carmichael, A.E. et al., 2005. Invasive phytophagous pests arising through a recent tropical evolutionary radiation: the Bactrocera dorsalis complex of fruit flies. Annual Review of Entomology, 50: 293–319.
[7] Khamis, F.M., D.K. Masiga, S.A. Mohamed et al., 2012. Taxonomic identity of the invasive fruit fly pest, Bactrocera invadens: concordance in morphometry and DNA barcoding. PLoS One, 7, e44862.
[8] “Drosophila melanogaster,” by Conrad Miller. Animal Diversity Web. URL: http://animaldiversity.org/accounts/Drosophila_melanogaster/ (accessed on 14 Nov 2016).
[9] “Oriental fruit fly complex – Fact sheet,” by Plant Health Australia. URL: http://www.planthealthaustralia.com.au/wp-content/uploads/2013/09/Oriental-fruit-fly-complex-FS-Papaya.pdf (accessed on 14 Nov 2016).
[10] Wiegmann, B. N., M. D. Trautwein, I. S. Winkler et al., 2011. Episodic radiations in the fly tree of life. Proceedings of the National Academy of Sciences. Available from: https://www.researchgate.net/50395098_fig1_Fig-1-Combined-molecular-phylogenetic-tree-for-Diptera-Partitioned-ML-analysis-of (accessed on 22 Nov 2016).
[11] “oriental fruit fly,” by H.V. Weems, J.B. Heppner, James L. Nation & Gary Steck. University of Florida, Florida Department of Agriculture and Consumer Services, Aug 2016. URL: http://entnemdept.ufl.edu/creatures/fruit/tropical/oriental_fruit_fly.htm (accessed on 14 Nov 2016).
[12] “Oriental fruit fly,” byDepartment of Agriculture and Fisheries, Queensland Government, 19 Feb 2015. URL: https://www.daf.qld.gov.au/plants/health-pests-diseases/a-z-significant/oriental-fruit-fly (accessed on 14 Nov 2016).
[13] Daniel, C. & J.Grunder, 2012. Integrated Management of European Cherry Fruit Fly Rhagoletis cerasi (L.): Situation in Switzerland and Europe, “Pupae of R. cerasi © 2012”. Insects, 3(4): 956-988 URL: http://www.mdpi.com/2075-4450/3/4/956/htm(accessed on 22 Nov 2016).
[14] “A female Oriental fruit fly (Bactrocera dorsalis) lays eggs by inserting her ovipositor in the skin of a papaya on Dec. 27, 2011,” U.S. Department of Agriculture (USDA) photo by Scott Bauer. Flickr.com, 27 Dec 2011. URL: https://flic.kr/p/dQtRto (accessed on 14 Nov 2016). (permission pending)
[15] “Oriental fruit fly larvae” U.S. Department of Agriculture (USDA) photo by Scott Bauer. Flickr.com, 05 March 2012. URL: https://flic.kr/p/bu2Xpw (accessed on 14 Nov 2016). (permission pending)
[16] “Bactrocera zonata eggs” by Russell IPM. URL: http://russellipm-agriculture.com/en/insect/bactrocera-zonata (accessed on 22 Nov 2016).
[17] USDA, 22 July 2016. A Review of Recorded Host Plants of Oriental Fruit Fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Version 2.1 A Product of the USDA Compendium of Fruit Fly Host Information (CoFFHI), a Farm Bill Project.
[18] "Oriental Fruit Fly FACT SHEET," by California Department of Food & Agriculture. CDFA, 25 Aug 2008. URL: https://www.cdfa.ca.gov/plant/factsheets/OFF_FactSheet.pdf (accessed on 15 Nov 2016).
[19] “Bactrocera dorsalis (Hendel),“ by Ronald F.L. Mau, Jayma L. Matin & J. M. Diez. Crop Knowledge Master, Apr 2007, URL: http://www.extento.hawaii.edu/Kbase/Crop/Type/bactro_d.htm (accessed on 15 Nov 2016).
[20] Steiner, L. F., 1957. Field Evaluation of Oriental Fruit Fly Insecticides in Hawaii. Journal of Economic Entomology, 50: 16-24.
[21] Mahmood, K., 2004. Identification of Pest Species in Oriental Fruit Fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) Species Complex. Pakistan Journal of Zoology, 36(3): 219-230.
[22] Bhagat, D., Samanta, S. K. & Bhattacharya, S., 2013. Efficient Management of Fruit Pests by Pheromone Nanogels. Scientific Reports 3, 1294. doi:10.1038/srep01294
[23] Singh, R.B., 1991. Significance of fruit flies in fruit and vegetable production in the Asia-Pacific region. Proceedings First International Symposium on Fruit flies in the Tropics (eds. S. Vijaysegaran and A.G. Ibrahim), pp. 11- 29. Kuala Lumpur, 1988. Malaysian Agricultural Research and Development Institute, Kuala Lumpur.
[24] “Florida growers, Adam Putnam debate aerial pest spray for invasive Oriental fruit fly,” by Alex Harris. Miami Herald, 21 Sep 2015. Hoested on MiamiHerald.com: http://www.miamiherald.com/news/local/environment/article35949096.html(accessed on 21 Nov 2016).
[25] "Fruit flies, Oriental Mindoro, Philippines," by warrenlaurde. YouTube, 06 Jun 2011. Gif made using gifyoutube.com.
[26] “Jumping Tephritidae fruit fly maggots,” by hillbournesian. Youtube, 05 Oct 2011.
[27] “Mpumalanga citrus farmers hit hard by Oriental Fruit Flies,” by SABC Digital News. SABC YouTube Channel, 08 Apr 2016.
[28] “Alerta Verde: La Mosca Oriental de la Fruta,” by DaniSilva TV. Youtube, 21 Sep 2015. Gif made using gifyoutube.com.
[29] Goergen, G., J. F. Vayssières, D. Gnanvossou & M. Tindo, 2011. Bactrocera invadens (Diptera: Tephritidae), a new invasive fruit fly pest for the Afrotropical Region: host plant range and distribution in West and Central Africa. Environmental Entomology. 40: 844-854
[30] EPPO (2015) PQR - EPPO database on quarantine pests (available online). http://www.eppo.int
[31] “Holotype of Dacus ferrugineus Fabricius located in the Natural History Museum of Denmark,” by Verner Michelsen, Oct 2014. One and the same: Integrative taxonomic evidence that Bactrocera invadens (Diptera: Tephritidae) is the same species as the Oriental fruit fly Bactrocera dorsalis - Scientific Figure on ResearchGate. URL: https://www.researchgate.net/267761592_fig3_Figure-7-Holotype-of-Dacus-ferrugineus-Fabricius-located-in-the-Natural-History-Museum (accessed on 23 Nov 2016).
[32] Bactrocera dorsalis (Oriental fruit fly) Datasheet,” by Centre for Agriculture and Biosciences International. CABI.org, 23 Sep 2016. URL: http://www.cabi.org/isc/datasheet/17685 (accessed 14 Nov 2016).
[33] White, I.M., Elson-Harris, M.M., 1994. Fruit Flies of Economic Significance. Their Identification and Bionomics. Wallingford, UK: CAB International.
[34] Schutze, M. K., N. Aketarawong, W. Amornsak et al., 2014. Synonymization of key pest species within the Bactrocera dorsalis species complex (Diptera: Tephritidae): Taxonomic changes based on a review of 20 years of integrative morphological, molecular, cytogenetic, behavioural and chemoecological data. Systematic Entomology, DOI: 10.1111/syen.12113.
[35] “Original description of Dacus dorsalis inEntomologischen Mitteilungen,” byFriedrich Hendel, 1912. Image obtained from Biodiversity Heritage Library (BHL). URL: http://biodiversitylibrary.org/page/10424191 (accessed 14 Nov 2016).
[36] Lux, S.A., R.S. Copeland, I.M. White et al., 2003. A new invasive fruit fly species from the Bactrocera dorsalis (Hendel) group detected in East Africa. Insect Science and its Application, 23, 355–361.
[37] Drew, R.A.I., K. Tsuruta & I.M. White, 2005 A new species of pest fruit fly (Diptera: Tephritidae: Dacinae) from Sri Lanka and Africa. African Entomology, 13, 149–154.
[38] Retrieved [14 Nov 2016], from the UniProt Consortium, UniProt: a hub for protein information, Nucleic Acids Res, http://www.uniprot.org/taxonomy/27457.
[39] Retrieved [14 Nov 2016], from the Integrated Taxonomic Information System on-line database, http://www.itis.gov.
[40] Jiang F., X. Pan, X. Li et al., 2016. The first complete mitochondrial genome of Dacus longicornis (Diptera: Tephritidae) using next-generation sequencing and mitochondrial genome phylogeny of Dacini tribe. Scientific Reports, 6.
[41] Boykin, L. M., M. K. Schutze, M. N. Krosch et al., 2014. Multi-gene phylogenetic analysis of south-east Asian pest members of the Bactrocera dorsalis species complex (Diptera: Tephritidae) does not support current taxonomy. Journal of Applied Entomology, 138: 235–253. doi:10.1111/jen.12047
[42] Hardy, D.E., 1969. Taxonomy and distribution of the Oriental fruit fly and related species (Tephritidae-Diptera). Proceedings of the Hawaiian Entomological Society, 20, 395–428.