Betta pugnax (Cantor, 1850)
Table of Contents

Figure 1. Betta pugnax adult male, collected from Nee Soon Swamp Forest, Singapore. (Photo: Low Bi Wei)
Name
Binomial: Betta pugnax (Cantor, 1850)Vernacular: Forest Betta, Malayan Betta, Penang Betta
Etymology
The species epithet is derived from Latin pugnax, which means 'warlike, ready to fight'. It is probably a reference towards the quarrelsome nature of the species towards conspecifics.Synonyms
Macropodus pugnax Cantor, 1850. Original descriptionBetta anabatoides (non Bleeker, 1851): Bleeker, 1860; Weber & de Beaufort, 1922; Herre & Myers, 1937; Fowler, 1938; Herre, 1940.
Betta picta (non Valenciennes, 1846): Bleeker, 1879; Weber & de Beaufort, 1922; Fowler, 1938; Herre, 1940.
Betta trifasciata (non Bleeker, 1950): Karoli, 1881.
Betta macrophthalma Regan, 1910.
Betta bleekeri Regan, 1910.
Betta brederi Myers, 1935.
Betta fusca (non Regan, 1910): Tweedie, 1936; Fowler, 1938; Herre, 1940.
Betta taeniata (non Regan, 1910): Tweedie, 1936; Herre & Myers, 1937; Herre, 1940.
Betta rubra (non Perugia, 1893): Herre, 1940.
From the re-description of the species by Tan & Tan, 1996.
Figure 2. Betta pugnax juvenile. The holotype for Betta macrophthalma was discovered to be a juvenile Betta pugnax, highlighting the historical confusion over the identity and description of the species. (Photo: Low Bi Wei)
Native Distribution
Betta pugnax can be found across Peninsula Malaysia, in the states of Penang, Kedah, Terengganu, Pahang, Perak, Selangor and Johor, in Singapore, the Anambas Islands, the Riau Archipelago, Lingga Archipelago, and the Riau and Jambi provinces in Sumatra (Figure 3) (Tan & Ng, 2005).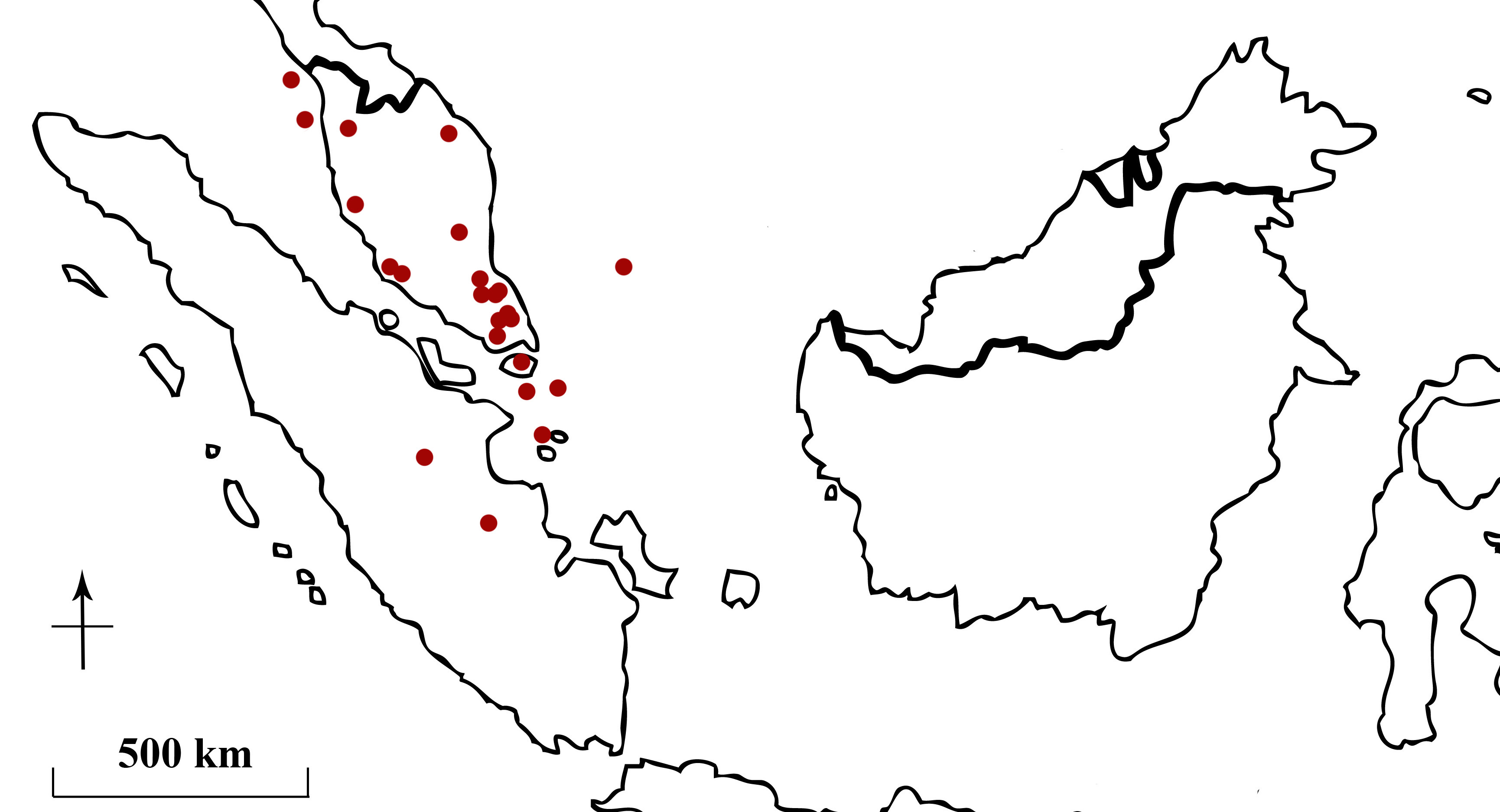
Figure 3. Native distribution of Betta pugnax (red circles).
Habitats
In the type locality (Penang, Malaysia), Betta pugnax inhabits clear, slow to fast flowing hill-streams and foothills, with underlying sandy substrate and rocks, and little or no submerged vegetation. Main habitats were shallow (10-80 cm deep), stagnant areas characterized by overhanging bank vegetation and associated roots, and submerged leaf litter. Waters were neutral to slightly alkaline, pH around 7.4 (Tan & Ng, 2005). Linke (1992) recorded water temperature in collection localities at 26°C, falling to 22°C in the rainy season.In Singapore, Betta pugnax can be found in six undisclosed localities around the Nature Reserves (Tan, 2011, pers. comm.), where it inhabits small, fast flowing hill-streams (Lim & Ng, 1990) and larger lowland streams (Lim, 2011, pers. comm.; Figure 4). Such streams tend to be acidic (Tan & Ng, 2005).

Figure 4. Typical habitats of Betta pugnax in Singapore - larger lowland streams (left, middle) and small, shallow hill-streams (right). (Photos: Kelvin Lim)

Figure 5. Betta pugnax in situ in the Central Catchment Nature Reserve. (Photos: Kelvin Lim)
Diagnosis
Betta pugnax can be differentiated from all other species of the genus Betta by - a relatively large head 27.5-35.2% of standard length (SL), rhombic when viewed from above; green or blue iridescent spots on scales upon a brown body, opercular scales iridescent in males and sometimes extending to the belly; females and juveniles displaying two transverse dark bands converging at a dark spot on the caudal peduncle; a stout body with body depth 24.8-32.1% of SL, juveniles tending to be the slender with proportionately larger eyes; central stripe on head; occasional dark, lower preorbital stripe (chin bar) extending from eye to throat but with no dark marks below central stripe on opercle; mature specimens with pointed, often elongated fins and a broadly lanceolate caudal fin (Figure 6); pelvic fin length 26.6-43.8% of SL; dorsal transverse bars; caudal transverse bars only in males (Figure 7); red subdistal band on anal fin; black edge on anal fin but not caudal fin; 25-28 rays on anal fin; 28-31 lateral scales; 11-12 postdorsal scales; inter-orbital width 32.4-53.0% of head length; lack of broad iridescent patches in iris (Tan & Tan, 1996; Tan & Ng, 2005).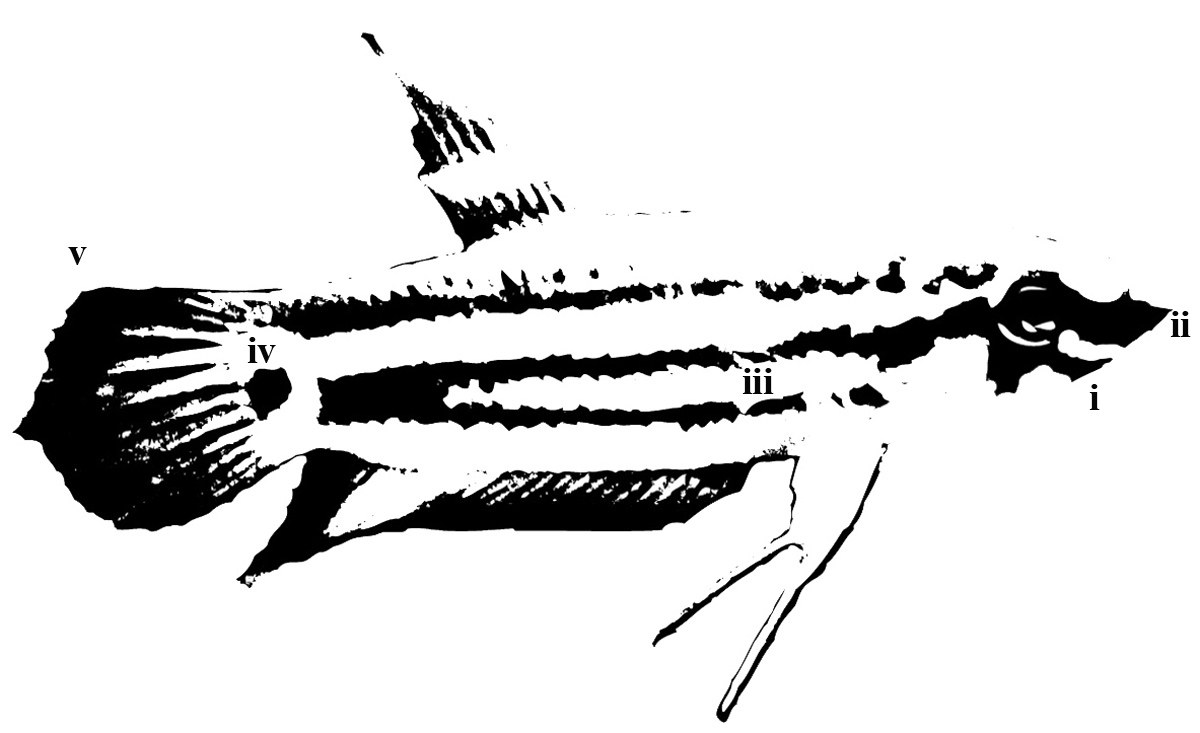
Figure 6. Illustration of a sexually mature female Betta pugnax. Easily discernible diagnostic features are: i. preorbital stripe, ii. central stripe on head, iii. two converging transverse bands, iv. dark spot, v. broadly lanceolate caudal fin. (Illustration: Low Bi Wei)
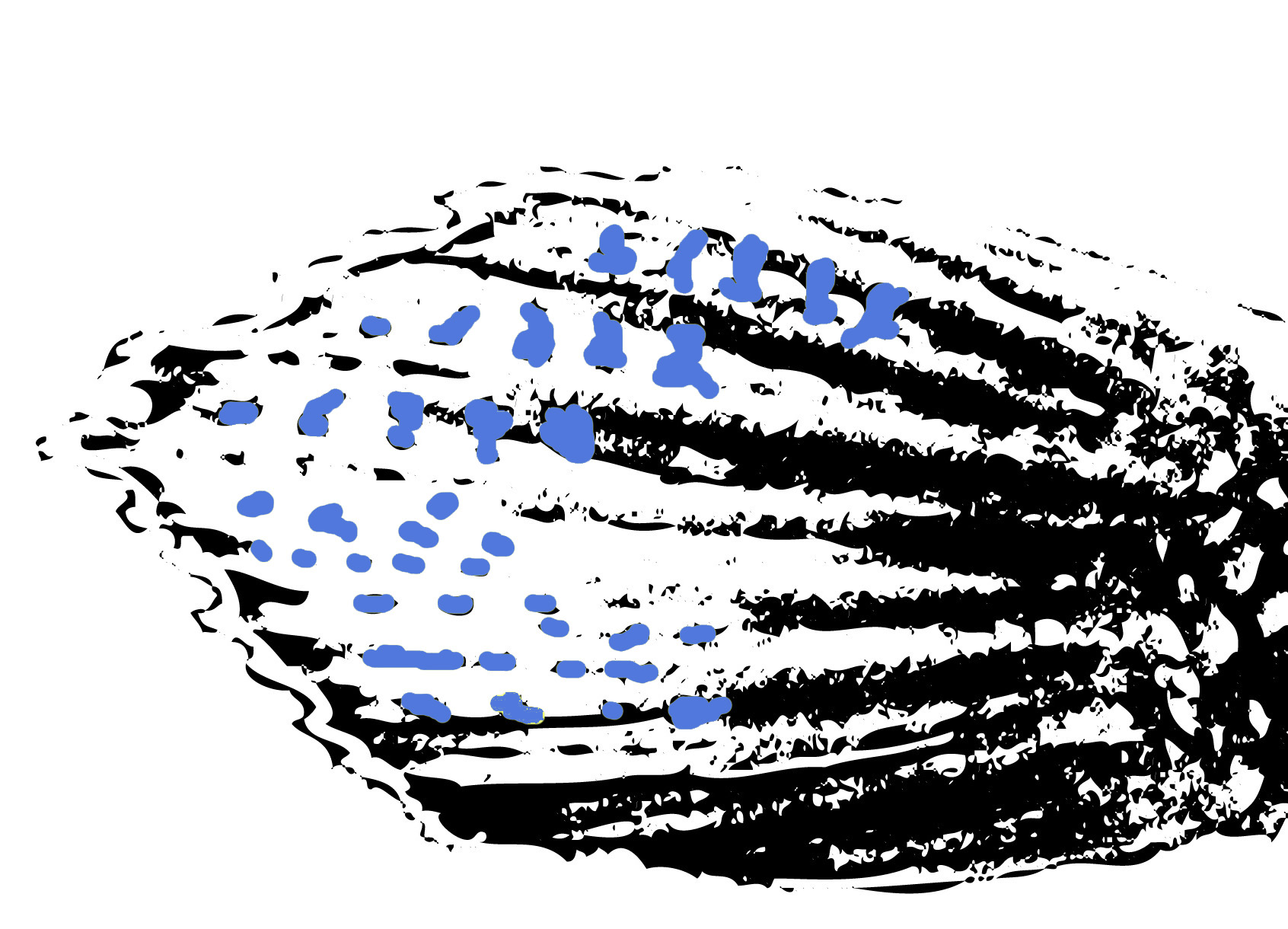
Figure 7. Broadly lanceolate caudal fin with transverse bars (in blue) of male Betta pugnax. (Illustration: Low Bi Wei)
Description
"The head is much depressed, and far broader than the body, which is gradually compressed towards the caudal fin. The profile of the back is slightly arched, the highest part being at the dorsal spine; the abdominal profile is less arched than the former." - Cantor, in his original descriptionThe general body shape and appearance of Betta pugnax (Figure 8) - body slender; caudal fin broadly lanceolate; dorsal fin originating above 13th lateral scale, pointed and posteriorly reaching the anterior third of caudal fin; anal fin originating below 7th lateral scale, posterior extremity pointed and elongated to the midlength of caudal fin.
See here for detailed meristics and morphometrics.
Size
Maximum known size is 67.3 mm SL, and up to 10cm TL (Lim & Ng, 1990).Coloration
Live specimens collected from clear, neutral to slightly alkaline hillstreams (pH 7 and above; Penang; Perak) are light brown with overlying blue iridescence on opercles, and greenish-blue to blue iridescence on body. Specimens from more acidic waters (Singapore) tend to have more green iridescence and less blue.Sexing
Adult males have caudal transverse bars (Figure 7), and iridescent opercles; fins tend to be more elongate. Adult females and juveniles with distinct chin-bar (oblique preorbital stripe), and two visible transverse bands (central and second central stripe) converging on a dark spot at the caudal peduncle (Figures 6,8).From: Tan & Ng, 2005.

Figure 8. Betta pugnax adult male (left) and adult female (right). (Photo: Low Bi Wei)
Biology
Behaviour
Squabbles do occur between conspecifics but not to the extent of the Siamese fighting fish Betta splendens. In captive specimens, it has been observed that fights substantially decrease once a hierarchy has been established amongst a group, and subsequently, serious damage rarely occurs (pers. obs.). When alarmed, these fish tend to scuttle into submerged leaf litter (Tan, 2011, pers. comm.).Figure 9. Betta pugnax tends to hide amongst submerged leaf litter when alarmed. (Photo: Low Bi Wei)
Feeding Habits
Betta pugnax has been observed to feed on aquatic insects, terrestrial insects and spiders in Sungei Gombak, Malaysia (Wootton, 1992), though the ecology of the species in Singapore has not been fully-documented. However, they have been observed to be voracious feeders in the wild (Tan, 2011, pers. comm.) and in captivity, wild-caught specimens readily accept processed food (pers. obs.).Video 1. Captive Betta pugnax feeding.
Reproduction
Betta pugnax is a paternal mouthbrooder. Spawning occurs in a depression in the substrate, where the pair engages in a typical anabantoid embrace where the male entwines his body around the female during fertilization. Eggs are released in batches, exhausted over multiple embraces for up to five hours. The female picks up the eggs and spits them out towards the male for her partner to snap up. Eggs are held by the male in the mouth until the hatching (Figure 10). Incubation takes around two weeks. During this period, the male does not feed and becomes more retiring, staying hidden amongst dense aquatic vegetation (pers. obs.; Linke, 1992; Goldstein, 2001).Specimens from Penang typically reach sexual maturity above 40 mm SL, whereas certain populations from Singapore and Johor were found mouthbrooding and displaying adult coloration at smaller sizes (Tan & Tan, 1996).

Figure 10. A mouthbrooding male Betta pugnax. Note the enlarged buccal cavity (throat). (Photo: Low Bi Wei)
Video 2. A typical anabantoid embrace and spawning rituals as illustrated by a pair of mouthbrooding Betta macrostoma.
Taxonavigation
Ordo: Perciformes Familia: Osphronemidae Genus: Betta| Taxonomic Hierachy |
|
| Kingdom |
Animalia |
| Phylum |
Chordata |
| Subphylum |
Vertebrata |
| Superclass |
Osteichthyes |
| Class |
Actinopterygii |
| Subclass |
Neopterygii |
| Infraclass |
Teleostei |
| Superorder |
Acanthopterygii |
| Order |
Perciformes |
| Suborder |
Anabantoidei |
| Family |
Osphronemidae Bleeker, 1859 |
| Subfamily |
Macropodinae Liem, 1963 |
| Genus |
Betta Bleeker, 1850 |
| Species |
Betta pugnax (Cantor, 1850) |
Phylogeny
The hypothesized evolution of species can be inferred from phylogenetic studies. The partial phylogeny of the genus Betta was derived by Rüber et al. (2004; Figure 11), with the complete molecular systematics of the genus currently in the works (Tan, 2011, pers. comm.).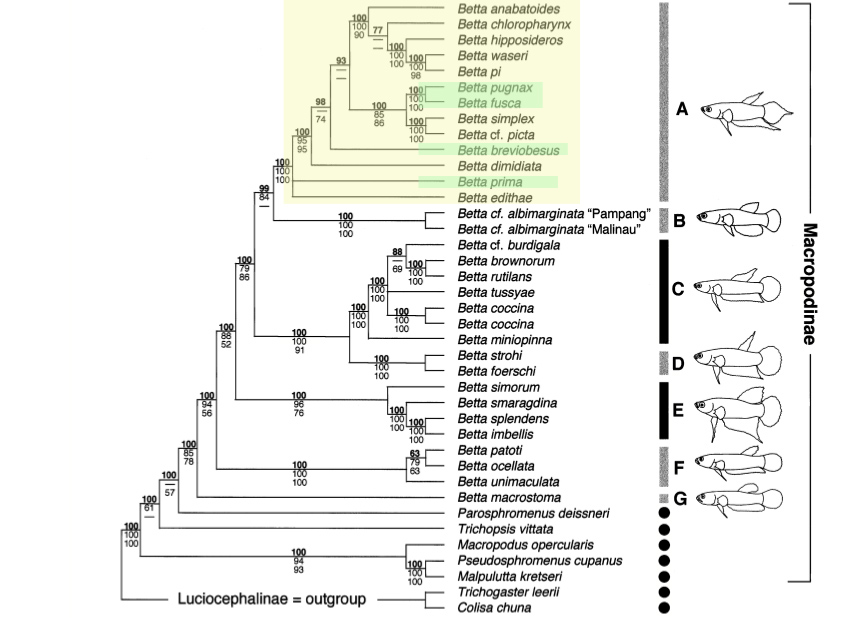
Figure 11. Molecular phylogeny of the genus Betta derived using nuclear (RAG1) and mitochondrial (CytB, 12S rRNA, tRNA Val, 16S rRNA) nucleotide sequence data (4448 base pairs), with two Luciocephalinae taxa used as outgroups. Major Betta clades are denoted by: A - pugnax, B - albimarginata, C - coccina, D - foerschi, E - splendens, F - unimaculata, G - macrostoma. Boxes indicate Betta species, circles non-Betta species; fill colour denotes breeding mode: grey - mouthbrooding, black - bubble-nesting (From: Rüber et al., 2004; permission for use of figure obtained).
Green shading denotes member of the Betta pugnax complex group, and though the molecular systematics of the genus Betta is yet to be fully elucidated (Tan, 2011, pers. comm.), it is clear that the Betta pugnax complex group does not form a monophyletic clade. Rather, as Ruber et al. (2004) denoted, the least inclusive monophyletic clade involving all members of the Betta pugnax complex group (in yellow) also encompass members the Betta anabatoides, Betta waseri, Betta picta, Betta dimidiata and Betta edithae complex groups. The complex groups within the genus Betta should thus not be confused with the concept of species complexes.
The study also hypothesized a model of brood care evolution within the genus. By applying different weights for the postulated directionality of changes (from bubble-nesting to mouthbrooding, or vice versa), based on phenotypic and behavioural differences between the two forms of brood care, the recurrent evolution of mouthbrooding is favoured using parsimony, with bubble-nesting as the plesiomorphic condition (Rüber et al., 2004).
Type Information
A type specimen refers to a specimen or a group of specimens to which a species (scientific) name is officially attached to. It acts as the reference to all defining characteristics of that species.The lectotype for Betta pugnax is stored in the British Museum of Natural History (BMNH 1860.3.19:930; 60.6 mm SL; Pinang, collector Cantor). There are two paralectotypes (BMNH 1860.3.19:317; 51.8 mm SL; BMNH 1860.3.19:318; 66.2 mm SL; Pinang; collector Cantor).
The type locality for Betta pugnax is Penang, Malaysia.
A type was not designated by Cantor when he described the species in 1850, which was based on an unspecified number of specimens. Alfred (1963) designated a complete alcohol-preserved specimen as the lectotype and two formerly dried specimens as paralectotypes. The two paralectotypes are now stored in alcohol, but only the skins on the left side of the specimens remain; the right side of the bodies and vertebrae have been removed, and the whereabouts unknown. Fins of all type specimens are damaged (Tan & Tan, 1996).
Conservation Status
Betta pugnax is not listed in the Singapore Red Data Book, nor the International Union for Conservation of Nature (IUCN) Red List.As Pets
Betta pugnax are generally undemanding in captivity, readily accepting processed food and breeding if optimal conditions are met (pers. obs.). Linke (1992) recommends housing in aquaria upwards of 70 cm long, 40 cm wide and 30 cm high, though they have been known to cope well in smaller tanks. Copious amounts of aquatic vegetation and hiding places should be provided for these generally retiring animals. Though some literature recommend the use of soft, slightly acidic water for this species, they are not fussy with water chemistry, being able to tolerate acidic to slightly alkaline water. Wild-caught fish should, however, be acclimatized carefully, and collection localities recorded. A cover should be fitted as these fish are excellent jumpers. As with all anabantoids, a layer of air must be kept between the water surface and tank cover as these fish are obligate air-breathers.A small group of fishes can be kept together provided ample hiding places are provided. Other suitable tankmates include similar-sized, robust species with a peaceful demeanour, such as rasborines and some of the peaceable barb species . If breeding is to be attempted, it is recommended that a pair be kept alone. As males do not feed while brooding, they should be removed from the spawning tank upon release of fry, to allow for recuperation. Failure to do so may result in overeager females immediately initiating a new breeding cycle, upon which starvation may set in.
Figure 12. Betta pugnax, adult male, collected from Nee Soon Swamp Forest, Singapore. (Photo: Low Bi Wei)
Literature and References
Alfred, E. R., 1963. Notes on a collection of fresh-water fishes from Penang. Bulletin of the Singapore National Museum, 32: 143-153. 2 pl. LinkCantor, T. E., 1850. Catalogue of Malayan fishes. Journal of the Royal Asiatic Society of Bengal, 18: i-xii + 981-1143, pls. 1-14.
Goldstein, R. J., 2001. Bettas: Everything About History, Care, Nutrition, Handling and Behaviour. Barron's Educational Series, New York. 96 pp.
Lim, K. K. P. & P. K. L. Ng, 1990. A Guide to the Freshwater Fishes of Singapore. Singapore Science Centre, Singapore. 160 pp.
Linke, H., 1992. Labyrinth Fish: The Bubble-Nest-Builders. Tetra-Press, Melle. 174 pp.
Rüber, L., R. Britz, H. H. Tan, P. K. L. Ng & R. Zardoya, 2004. Evolution of mouthbrooding and life-history correlates in the fighting fish genus Betta. Evolution, 58(4): 799-813. Link
Tan, H. H. & S. H Tan, 1996. Redescription of the Malayan fighting fish Betta pugnax (Teleostei: Belontiidae), and description of Betta pulchra, new species from Peninsula Malaysia. The Raffles Bulletin of Zoology, 44(2): 419-434. Link
Tan, H. H. & P. K. L. Ng, 2005. The fighting fishes (Teleostei: Osphronemidae: Genus Betta) of Singapore, Malaysia and Brunei. The Raffles Bulletin of Zoology, Supplement No. 13: 43-99.
Wootton, R. J., 1992. Fish ecology. Blackie, Glasgow. 212 pp.
Personal Communications
Lim, Kok Peng Kelvin. Raffles Museum of Biodiversity Research, Department of Biological Sciences, National University of Singapore.Tan, Heok Hui. Raffles Museum of Biodiversity Research, Department of Biological Sciences, National University of Singapore.
Links to other types of species pages
Betta pugnax - International Betta Congress LinkBetta pugnax - Seriously Fish Link
Forest Betta - Ecology Asia Link
Additional Notes
Original Description
Excerpt from T. E. Cantor (1850), Catalogue Of Malayan Fishes:"The head is much depressed, and far broader than the body, which is gradually compressed towards the caudal fin. The profile of the back is slightly arched, the highest part being at the dorsal spine; the abdominal profile is less arched than the former. The length of the head is 1/3, or slightly more, of the length of the body, the caudal not included; the depth at the occiput 2/3 of the length of the head. The eyes are prominent, occupying the second fourth, and bordering on the profile. Their distance across the forehead is nearly double the diameter. The mouth is semicircular, moderate; the angle is in front of the orbit. The posterior opening of the nostrils is situated close to the orbit; the anterior is provided with a small fleshy tube. The tongue is free, fleshy and very pointed. Behind the velvety teeth of the upper jaw appears successively three semicircular membranous folds, of which the posterior is papillular on the margin, which thus appears as if studded with a second series of minute teeth. The head is everywhere covered with large rounded scales like the rest of the body, but the slightly protractile jaws are naked; the posterior part of each branch of the lower jaw is covered by a single large oval scale. The greatest vertical diameter of the body, at the dorsal spine, is in some individuals 3/4 of, in others equal the length of the head. The vertical diameter at the root of the caudal fin varies from 5 1/2 to 1/6 of the length of the body. The dorsal fin commences a little behind the posterior half of the body; the rays gradually increase towards the fifth, the longest; the extent of the base is from 1/8 to 1/9 of the length of the body; the distance from the last ray to the caudal is 1/4 of the length of the body. The caudal is very broadly lanceolate; the two central rays are the longest, in some individuals 1/3 of the entire length of the fish, but frequently less. The length of the anterior filamentous ventral ray rarely exceeds that of the head. The pectorals are rounded. their length but slightly exceeds 1/2 of the head. Opposite their posterior half is situated the anus, immediately behind which the anal fin commences. The rays of the latter gradually increases in length to the twenty-third or fourth, which are sometimes elongated beyond the point of the caudal fin. The extent of the scaly base of the anal equals 1/2 of the length of the body. No lateral line appears, but on the series it would occupy if present, some of the anterior scales have each a central rounded depression, which, however, also appears on every single scales nearer the back. Three series lower down, on the posterior half of the sides, commences sometimes a row of similar depressions, which then continue to the caudal fin. The scales are rather large, higher than long; the anterior margin is straight with 21 striae, the posterior rounded, ciliate; a line from the gill-opening to the caudal fin contains 32; the greatest diameter presents 10. Of the six branchiostegous rays the upper one is longer and broader than the rest; the fifth and six are rounded, setaceous."
Meristics & Morphometrics
Meristics: 29-30 lateral scales; 19-22 predorsal scales; 5-8 subdorsal scales; 11-12 postdorsal scales; dorsal fin rays 0-2 spinous, 7-10 soft branched, segmented (total 8-10); anal fin rays 1-2 spinous, 24-26 soft branched (total 25-28); caudal fin rays 2 unbranched soft, 4+5 branched soft (total 11); pelvic fin rays 1 spinous, 1+1 soft branched (total 3); pectoral fin rays 13 soft branched; 28-31 vertebrae.Morphometrics: Body: Maximum known size 67.3 mm SL (ZRC 8306; Zoological References Collection, Raffles Museum of Biodiversity Research). Total length 130.6-148.1% SL; predorsal length 60.9-73.4% SL; preanal length 40.6-51.2% SL; head length (HL) 27.5-35.2% SL; 41.6-54.3% predorsal length; body depth at dorsal fin origin 24.8-32.1% SL. Fins: Pelvic fin length 26.6-43.8% SL; length of anal fin base 44.2-54.0% SL; length of dorsal fin base 9.9-15.7% SL. Head: Orbital diameter 27.9-38.5% HL; postorbital length 45.0-55.7% HL; interorbital width 32.4-53.0% HL.
| Subject | Author | Replies | Views | Last Message |
|---|---|---|---|---|
| No Comments | ||||