Carcinoscorpius rotundicauda (Latreille, 1802)
 |
| "In this ancient ritual that predates the dinosaurs, thousands of horseshoe crabs emerge from the water to spawn in the pre-dawn light." Photo taken by Steve Greer at Delaware Bay, USA (pending approval). |
|| Introduction || Name || Taxonavigation || Type Information || Description || Diagnosis
|| Distribution|| Biology|| Human Uses|| Threats ||Phylogeny ||References ||

Back to Top
Horseshoe crabs are marine chelicerates closely related to arachnids [1] . There exist four extant species - Limulus polyphemus (Linnaeus, 1758), Tachypleusgigas (Müller, 1758), Tachypleus tridentatus (Leach, 1819) and Carcinoscorpius rotundicauda (Latreille, 1802). L. polyphemus is distributed along the eastern coast of North America whereas the three remaining species inhabit shallower waters in the Indo-Pacific. In fact, horseshoe crabs are commonly referred to as ‘living fossils’ as their external morphology have remained remarkably unchanged over the past 150 My or more [2] . This lack of morphological disaparity over time is accompanied by exceptionally low rates of diversification throughout their entire evolutionary history [3] [4]
Of particular interest is C. rotundicauda, a data-deficient, endangered and vulnerable species [5] .

Back to Top
 |
| An individual found on the mudflats at Mandai Mangroves, Singapore. Picture taken by Rachel Oh. |
Binomial
Carcinoscorpius rotundicauda (Latreille, 1802)Vernacular
Mangrove Horseshoe Cran [6] ; Japanese Horseshoe Crab, Chinese Horseshoe Crab [7]Etymology
The common name is derived from the shape of their carapace (i.e. the chitin armour that covers the anterior body parts) [8] .Original Description
The original description of Carcinoscorpius rotundicauda was retrieved from the Biodiversity Heritage Library on 22 October 2011 (http://www.biodiversitylibrary.org/bibliography/15764). The collection is titled “Histoire naturelle, générale et particulière des crustacés et des insectes: ouvrage faisant suite aux oeuvres de Leclerc de Buffon, et partie du cours complet d'histoire naturelle rédigé par C. S. Sonnini / par P. A. Latreille.” Specifically, the description can be found in Volume 4, on pages 98 - 100.
Back to Top
Order: Xiphosura
- Familia:Limulidae
- Genus: Carcinoscorpius
Species Authority: Latreille, 1802


Back to Top
The type information functions as a reference as to when a species was first described and named. It is essential in determining the appropriate taxonomical position of the species. However, given inadequate catalogue protocols in the past, most type specimens have either been lost or misplaced. Hence, the type information for C. rotundicauda could not be traced although it was mentioned by Latreille that he "found this species in the collection Museum of Natural History" as translated from the original description shown on the right.

Back to Top
Anatomy
1) Body Divisions
There are three divisions to the anatomy of a horseshoe crab – (1) the prosoma, (2) the opisthosoma and (3) the telson (Figure 1). These are sometimes referred to as the cephalothorax, the abdomen and the tail.The prosoma encloses the following:
- - The intestinal tract with an esophagus and proventriculus (necessary for grinding food)
- - A nervous system concentrated into a bulbous brain
- - A heart
- - Excretory glands at the bases of the walking legs
- - Connective tissue
- - Cartilaginous plates
The opithosoma contains namely the musculature necessary for functioning of the book gills and telson.
The telson is used to right itself when overturned although many hold the misconception that it is used as a defence weapon.
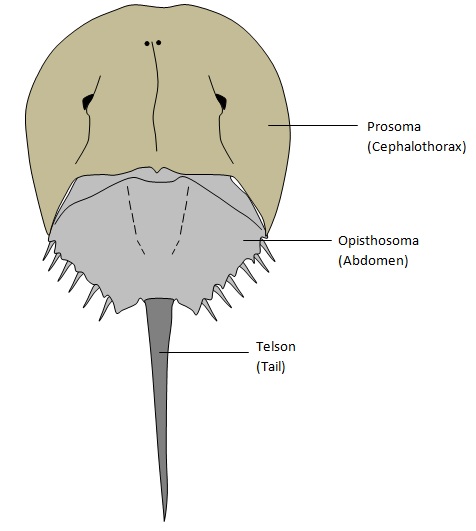 |
| Figure 1: The three main divisions to the anatomy of a horseshoe crab – the prosoma (brown), opisthosoma (grey) and telson (dark grey). Illustrations by Rachel Oh. |
2) Dorsal View
Figure 2 illustrates a typical dorsal view. Of particular significance are the compound eyes and the hinge. The horseshoe crab has two compound lateral eyes, used primarily for finding mates. It also has a hinge which spans the width of the body, functioning as a mobile joint between the head and segmented abdomen. This allows the animal to rapidly jack-knife rhythmically to and fro when burying itself into the seabed.
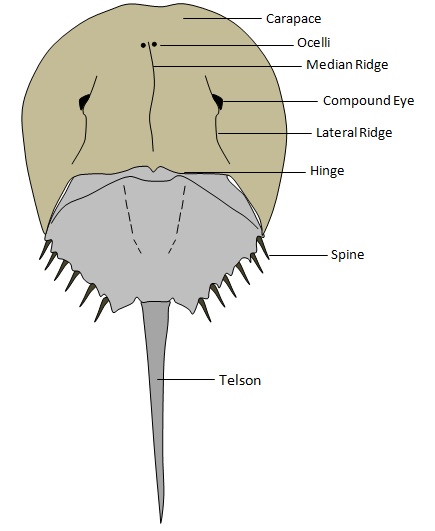 |
| Figure 2: Dorsal view of the horseshoe crab. Illustrations by Rachel Oh. |
3) Ventral view
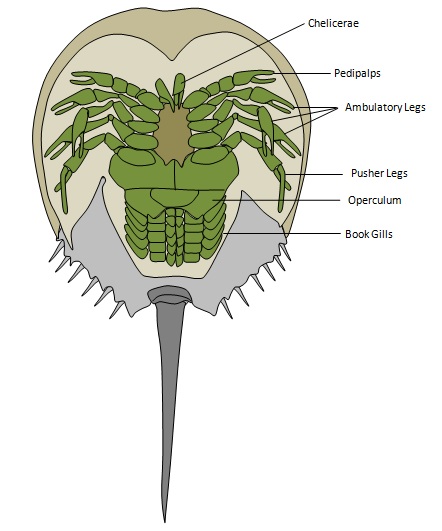 |
| Figure 3: An ventral view of the horseshoe crab. Illustrations by Rachel Oh. |
There are six paired appendages on the underside of the horseshoe crab. The first pair, called the chelicerae, is used to place food in its mouth. The next pair is the pedipalps, also known as the first ambulatory legs. In adult males, the tarsus of these legs is modified into a grasping appendage such that the male can clasp onto the female during spawning. The final pair is the ‘pusher’ legs which are necessary for locomotion. In fact, for maximum stability and efficiency, the animal generally swims upside down. To stop swimming, it settles on the seabed inverted and subsequently uses its long telson to right itself.
Towards the telson is six pair of book gills. The first pair is called the operculum and it functions to cover the subsequent five pair of book gills (which function as respiratory organs). The operculum also houses the opening of the genital pores through which gametes are released. Similar to gills in fishes, the respiratory book gills are a membrane that allows oxygen to pass through whilst keeping water out. For an illustration of the ventral view, please refer to Figure 3.
For those who require an even detailed anatomical description, please refer to this link: http://www.horseshoecrab.org/anat/anat3.html
Vision
Unique only to horseshoe crabs, they are the only living chelicerates that have compound eyes. Each individual has two compound lateral eyes, used primarily for finding mates. They have 5 additional eyes on the top if its carapace (two median eyes, two rudimentary lateral eyes and one endoparietal eye) that function as light-sensing organs. On the underside, the horseshoe crab has two ventral eyes that may aid orientation of the animal when swimming.Digestive Tract
Digestion begins with the bristly mouth, continuing into a cuticle-lined esophagus and the proventriculus. The proventriculus composes of a 'crop' and a 'gizzard'. The former can expand considerably as it functions to accommodate ingested food. Food is then passed into the gizzard for reduction into a pulp. Undigestible pieces such as fish bones or large shell pieces are regurgitated. Digestion then occurs in the midgut and waste disposed of via the hindgut (rectum). |
| A small individual found on the mudflats of Mandai Mangrove, Singapore. Picture taken by Rachel Oh. |
Adult
Generally, an adult can grow up to 40cm in length (inclusive of telson) [9] . Also, there exists sexual dimorphism with respect to size with males being generally smaller compared to females [10] [11] [12]Larva
The larvae hatched closely resemble the adults [13] with the exceptions that they are transparent, appear bluish green because of their blood and lack the telson [14] . In fact, biologists call these “trilobite” larvae as they closely resemble the long-extinct trilobites. As they grow, the larvae undergo ecdysis (moulting) and this is associated with increase in size and weight.
Back to Top
As abovementioned, C. rotundicauda share similar ecological, morphological and serological traits with the other three extant horseshoe crab species. Nonetheless, it can be distinguished from the other three species via multiple morphological features (Table 1).C. rotundicauda is noticeably smaller in size compared to other extant horseshoe crab species. The carapace is more rounded [15] . Taking a frontal view of the prosoma of an adult male, one can identify the ventral arch, a morphological feature that aids fitting of males over the female’s mid-piece during mating. For C. rotundicauda, the ventral arch is considerably flatter. Also, the cross-section of the telson for C. rotundicauda is cylindrical whereas the other three species are triangulate and spike-like. It is not crested on top and lacks a grooved underside.
The second and third pairs of prosomal appendages in males are modified into complete chleate claspers with two long, slender fingers. C. rotundicauda lacks a spur on the apex of the fourth segment of the last appendage of the prosoma. Additionally, C. rotundicauda have comparatively short lateral, moveable spines bordering the opisthosoma spines in both sexes. The spines decrease in length from front to back with the second and third spines generally being the longest [16] . Lastly, the genital operculum of C. rotundicauda lacks the small pair of terminal flaps present in Limulus and is somewhat triangulate.
To differentiate between species for both male and female specimens, one can refer morphological characteristics numbered 5 to 8. Conversely, characteristics 1 – 4 can be used if the specimen is a male.
Table 1: Distinguishing morphological characteristics of the four species of extant horseshoe crabs (modified from Sekiguchi and Nakamura, 1979).
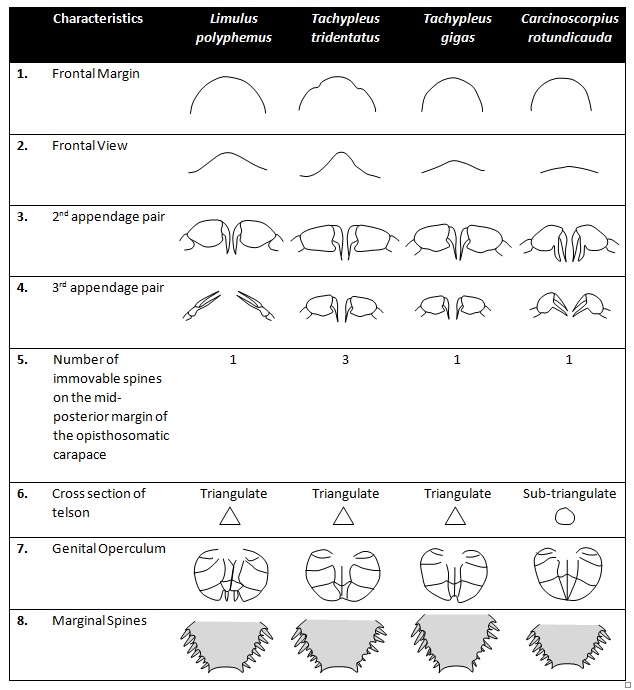

Back to Top
C. rotundicauda occurs only in Asia: around India; Indonesia; Malaysia; Philippines; Singapore; Thailand and Hong Kong [17] . It generally prefers habitat in muddy rivers, swampy estuaries and mangroves [18] , and is common in brackish waters [19] ). On the main island of Singapore, a study conducted by Cartwright-Taylor et al. [20] found C. rotundicauda at multiple locations – Changi Point, Pasir Ris, Lower Seletar, Mandai (near Kranji), Lim Chu Kang, Sarimbun North, Sarimbun South and Pandan Reservoir (Figure 4). Unlike other species, they do not return to the sea with the receding tide. Adults can be found buried in the wet mud at a depth of 2 – 3 cm whereas sub-adults and juveniles remain on the surface [21] .
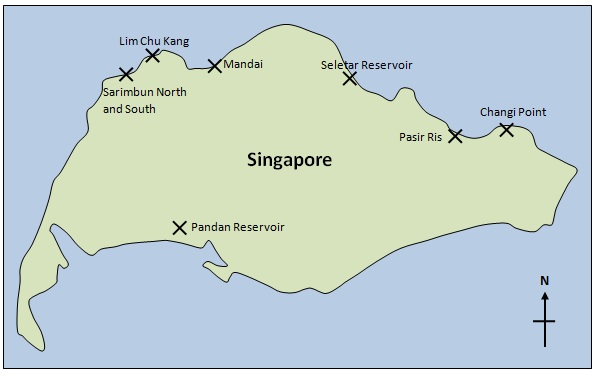 |
| Figure 4: Diagrammatic representation (for illustration purposes only) of the sites on the main island of Singapore at which C. rotundicauda can be found. |
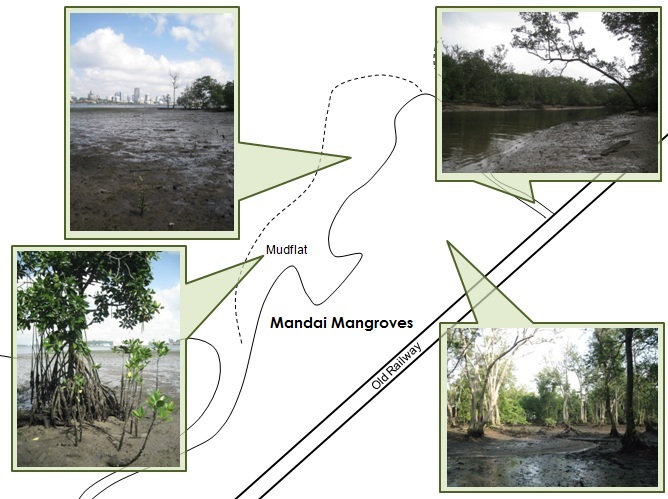 |
| Images of a typical mangrove forest and mudflat. Pictures were taken at Mandai Mangroves by Rachel Oh. |

Back to Top
Feeding habits
C. rotundicauda are generally scavengers although they do also feed on bivalves [22] and worms [23] . |
| Male individual on top of female individual. Picture courtesy of Ria Tan from WildSingapore. |
Reproduction
Spawning behaviour seem to exhibit some form of plasticity given variations in spawning season at different geographical locations. In Thailand, a continuous breeding season is suggested [24] . In India [25] and Malaysia [26] , spawning has been reported to occur during spring tides throughout the year. However, in countries such as Borneo and China, spawning appears to occur seasonally during March/April to June/July [27] .Yet, a study by Cartwright-Taylor et al. [28] conducted in Singapore suggests a lack of seasonal or continuous breeding behaviour. A higher frequency of larger individuals observed in some months with a continuous presence of small individuals throughout the year suggest that C. rotundicauda has a short rest period with low or no breeding activity. Moreover, there was never a time when sex ratios were heavily male-dominated, a feature characteristic of spawning seasons of L. polyphemus in eastern USA [29] [30] . Lastly, attached pairs were present all year round.
To mate, an amplexus (i.e. partnership) is forged when the male grabs onto the opisthosoma of the female using its chleate claspers. If successful, the female digs a nest approximately 2 – 5 cm deep in locations such as the mud of a seashore or river bed. Eggs are then deposited in clusters of 80 – 150 per nest although females are known to carry approximately 10 000 eggs, each with a diameter of approximately 2.0 mm [31] . The male then deposits its sperm for external fertilization to occur. Hatching occurs after 32 – 35 days.

Back to Top
Food
The unlaid eggs are cooked and eaten by some. |
| The unlaid eggs are cooked and eaten. This dish (apparently) costs RM8. Pictures were taken in Malaysia by Yeoh Kean Nam in 2010. |
Chitin
Chitin from the horseshoe crabs are used in the manufacturing of chitin-coated filament for suturing and chitin-coated wound dressing for burn victims as it is capable for reducing healing time by 35 – 50%.Blood
In the biomedical industry, blood from the horseshoe crab is drawn. Amebocytes are then separated from the hemolymph and lysed in distilled water to produce Limulus Amoebocyte Lysate (LAL). LAL is essential in detecting Gram-neagtive bacterial endotoxins as clots formation occurs immediate upon contact with minute quantities of endotoxins [32] . It has applications in disease detection and maintaining high standards of cleanliness of equipment [33] . In fact, LAL has a high commercial value whereby 2.5 ml is estimated to cost about US $110 in the international market [34] . Fortunately, the proteins responsible for this clotting activity have been cloned by a team of researchers from the National University of Singapore, hence, taking the pressure of the wild animals.Also, LAL has applications in the pharmaceutical and food industry, the clinical laboratory and in monitoring the environment. Table 1 describes a summary of such applications.
Table 2: Other Applications of LAL
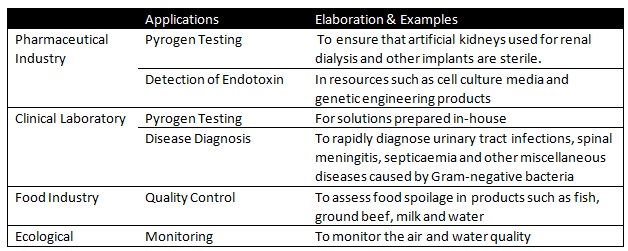
Additionally, other reagents and medically useful compounds have been discovered in the blood of the horseshoe crab. These include:
- G test - a new test for fungal infections
- An endotoxin-neutralizing protein (ENP) which holds potential as an antibiotic as well as an alternative endotoxin assay
- Other proteins that demonstrate anti-viral and anti-cancer
Biological Indicator
They are excellent indicators for the health of mangroves [35] .
Back to Top
Globally, two main threats faced by C. rotundicauda are (1) loss of habitat reclamation and development and (2), pollution. In Singapore, loss of habitat remains the main threat as areas of coastal land have been used for development, land reclamation and coastal development [36] . For example, mangrove forest area have declined from 70 km2 in 1819 to 6.59 km2 today, most of which is limited to the north-west coast of mainland Singapore [37] .Additionally, they are also trapped in fishing nets and commercially harvest as food. In Thailand and Malaysia, C. rotundicauda is consumed as an exotic delicacy [38] . In India, collection of C. rotundicauda for pharmaceutical research continues and is in fact, increasing, despite protection regulations.

Back to Top
As mentioned in the Introduction, horseshoe crabs have exhibited exceptionally low rates of diversification throughout their entire evolutionary history and are thus often referred to as ‘living fossils’.
Despite the wide range of non-morphological data studied to date (see Avise et al., 1994 for more details), only one consensus in which the Atlantic species (L. polyphemus) is a sister taxon to the three Indo-Pacific species has been reached. This consensus lies in accordance with the current taxonomy in which Limulus is in the subfamily Limulinae and the three other extant species in Tachypleinae, with all four species of Limulidae as sole living representatives of the Merostomata [39] .
To date, the phylogenetic relationship between the three Indo-Pacific species remains unresolved despite the multitude of analyses conducted as summarized in Table 3. Results were highly conflicted, leading to some authors concluding that the three species constitute a phylogenetically irresolvable trichotomy which could (possibly) have resulted from all three Indo-Pacific species forming within a short geological time [40] .
Table 3: Summary of analyses conducted in attempts that try to resolve the phylogenetic relationship between T. gigas, T. tridentatus and C. rotundicauda.
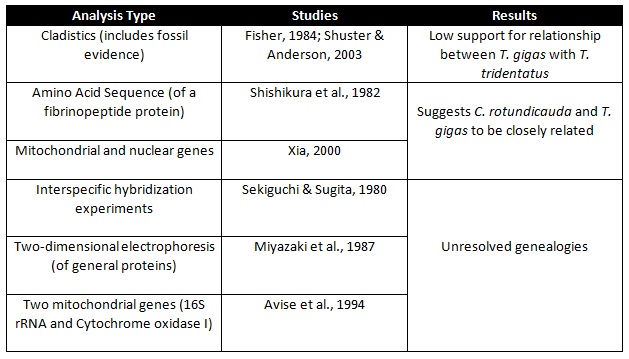
Nonetheless, the phylogenetic relationships were re-examined by Obst et al. [41] using an intra-specific analysis. It involved 18S rDNA, 28S rDNA and mitochondrial gene cytochrome c oxidase I (COI) and results suggest the following:
- 1) Strong support for a monophyletic genus Tachypleus and a diversification of the three Asian species during the Paleogene period. Speciation events were temporally well-separated by several million years.
- 2) Tree topology suggests that the “three Asian species originated in central South East Asia from a marine stem group that inhabited the shallow coastal waters between the Andaman Sea, Vietnam, and Borneo”. In fact, “C. rotundicauda probably separated from the Tachypleus stem group by invading estuarine habitats, while T. tridentatus most likely migrated northeast along the Southern coast of China and towards Japan”.

HTML Comment Box is loading comments...
References
Back to Top- ^ Masta, S.E., Longhorn, S.J. & Boore, J.L., 2009. Arachnid relationships based on mitochondrial genomes: asymmetric nucleotide and amino acid bias affects phylogenetic analyses. Molecular Phylogenetics and Evolution, 50: 117–128.
- ^ Fisher, D.C., 1984. The Xiphosurida: archetypes or bradytely? In: Eldredge, N., Stanley, S.M. (Eds.), Living Fossils. Springer, Berlin. Pp. 106–212.
- ^ Störmer, L., 1952. Phylogeny and taxonomy of fossil horseshoe crabs. Journal of Paleontology. 26(4): 630–639.
- ^ Störmer, L., 1955. Merostomata. In: Kaesler, R.L. (Ed.), Treatise of Invertebrate Paleontology – Part P: Arthropoda 2. University of Kansas and Geological Society of America, Lawrence, Kansas, USA. Pp. 4–41.
- ^ World Conservation Monitoring Centre, 1996. Carcinoscorpius rotundicauda. In: IUCN 2011. IUCN Red List of Threatened Species. Version 2011.1. <www.iucnredlist.org>. [Accessed 02/10/11]
- ^ Ng, P. K. L. & N. Sivasothi (eds.), 1999. A Guide to the Mangroves of Singapore II (Animal Diversity). Singapore Science Centre, Singapore. 168 pp.
- ^ Tree of Life Web Project, 2008. Carcinoscorpius rotundicauda. Japanese Horseshoe Crab, Chinese Horseshoe Crab. Version 05 November 2008 (temporary).(http://tolweb.org/Carcinoscorpius_rotundicauda/14743/2008.11.05) in The Tree of Life Web Project, (http://tolweb.org/)
- ^ Ng, P. K. L., L. K. Wang & K. K. P. Lim (eds.), 2008. Private Lives: an Exposé of Singapore's Mangroves. The Raffles Museum of Biodiversity Research, Singapore. 249 pp.
- ^ Ng, P. K. L. & N. Sivasothi (eds.), 1999. A Guide to the Mangroves of Singapore II (Animal Diversity). Singapore Science Centre, Singapore. 168 pp.
- ^ Ng, P. K. L. & N. Sivasothi (eds.), 1999. A Guide to the Mangroves of Singapore II (Animal Diversity). Singapore Science Centre, Singapore. 168 pp.
- ^ Cartwright-Taylor, L., J. Lee & C.C. Hsu, 2008. Population structure and breeding pattern of the mangrove horseshoe crab Carcinoscorpius rotundicauda in Singapore. Aquatic Biology, 8: 61-69.
- ^ Hong R.F., 2004. Population and distribution of horseshoe crab Carcinoscorpius rotundicauda at the Kranji Nature Trail estuaries, Western Johor Straits. The National University of Singapore, Department of Biological Sciences, Project report, 49.
- ^ Ng, P. K. L. & N. Sivasothi (eds.), 1999. A Guide to the Mangroves of Singapore II (Animal Diversity). Singapore Science Centre, Singapore. 168 pp.
- ^ Ng, P. K. L. & N. Sivasothi (eds.), 1999. A Guide to the Mangroves of Singapore II (Animal Diversity). Singapore Science Centre, Singapore. 168 pp.
- ^ Mikkelsen, T., 1988. The secret in the blue blood. Science Press, Beijing, China.
- ^ Mikkelsen, T., 1988. The secret in the blue blood. Science Press, Beijing, China.
- ^ World Conservation Monitoring Centre, 1996. Carcinoscorpius rotundicauda. In: IUCN 2011. IUCN Red List of Threatened Species. Version 2011.1. <www.iucnredlist.org>. Downloaded on 02 October 2011.
- ^ Mikkelsen, T., 1988. The secret in the blue blood. Science Press, Beijing, China.
- ^ Chatterji, A. & Abidi, S. A. H. J., 1993. The Indian horseshoe crab: a living fossil. Journal of Indian Ocean Studies, 1:43 – 48. <ref>Davidson, G.W.H., Ng, P.K.L. & Ho, H.C (Eds.). 2008. The Singapore Red Data Book (2nd Edition). Singapore: Nature Society (Singapore). 285pp.
- ^ Cartwright-Taylor, L., V.B. Yap, H.C. Chi & L.S. Tee, 2011. Distribution and abundance of horseshoe crabs Tachypleus gigas and Carcinoscorpius rotundicauda around the main island of Singapore. Aquatic Biology, 13: 127-136.
- ^ Cartwright-Taylor, L., V.B. Yap, H.C. Chi & L.S. Tee, 2011. Distribution and abundance of horseshoe crabs Tachypleus gigas and Carcinoscorpius rotundicauda around the main island of Singapore. Aquatic Biology, 13: 127-136.
- ^ Ng, P. K. L. & N. Sivasothi (eds.), 1999. A Guide to the Mangroves of Singapore II (Animal Diversity). Singapore Science Centre, Singapore. 168 pp.
- ^ Ng, P. K. L., L. K. Wang & K. K. P. Lim (eds.), 2008. Private Lives: an Exposé of Singapore's Mangroves. The Raffles Museum of Biodiversity Research, Singapore. 249 pp.
- ^ Mikkelsen, T., 1988. The secret in the blue blood. Science Press, Beijing, China.
- ^ Chatterji, A. & Abidi, S. A. H. J., 1993. The Indian horseshoe crab: a living fossil. Journal of Indian Ocean Studies, 1:43 – 48.
- ^ Cartwright-Taylor, L., V.B. Yap, H.C. Chi & L.S. Tee, 2011. Distribution and abundance of horseshoe crabs Tachypleus gigas and Carcinoscorpius rotundicauda around the main island of Singapore. Aquatic Biology, 13: 127-136.
- ^ Mikkelsen, T., 1988. The secret in the blue blood. Science Press, Beijing, China.
- ^ Cartwright-Taylor, L., J. Lee & C.C. Hsu, 2009. Population structure and breeding pattern of the mangrove horseshoe crab Carcinoscorpius rotundicauda in Singapore. Aquatic Biology, 8: 61-69.
- ^ Carmichael, R. H., Rutecki D. & Valiela I., 2003. Abundance and population structure of the Atlantic horseshoe crab Limulus polyphemus in Pleasant Bay, Cape Cod. Marine Ecology Progress Series, 246:225–239.
- ^ James-Pirri, M. J., Tuxbury, K., Marino, S. & Koch, S., 2005. Spawning densities, egg densities, size structure, and movement patterns of spawning horseshoe crabs, Limulus polyphemus, within four coastal embayments on Cape Cod, Massachusetts. Estuaries, 28:296–313.
- ^ Mikkelsen, T., 1988. The secret in the blue blood. Science Press, Beijing, China.
- ^ Tanacredi, J. T., 2002. Limulus in the limelight: a species 250 million years in the making and in peril? Kluwer Academic Publishers, New York. Pp. 79 – 84.
- ^ Ng, P. K. L. & N. Sivasothi (eds.), 1999. A Guide to the Mangroves of Singapore II (Animal Diversity). Singapore Science Centre, Singapore. 168 pp.
- ^ Sahoo, D., & Pandey, P. C., 2002. Advances in marine and Antarctic science. S. B. Nangia and A. P. H. Publishing Corporation, New Delhi. 14 pp.
- ^ Ng, P. K. L., L. K. Wang & K. K. P. Lim (eds.), 2008. Private Lives: an Exposé of Singapore's Mangroves. The Raffles Museum of Biodiversity Research, Singapore. 249 pp.
- ^ Davison, G.W.H., Ng, P.K.L. & Ho, H.C (Eds.). 2008. The Singapore Red Data Book (2nd Edition). Singapore: Nature Society (Singapore). 285pp.
- ^ Yee, A.T.K., W.F. Ang, S. Teo, S.C. Liew & H.T.W. Tan, 2010. The present extent of mangrove forests in Singapore. Nature in Singapore, 3: 139-145.
- ^ Christianus A. & Saad C. R., 2007. Horseshoe crabs in Malaysia and the world. FishMail, 16:8–9.
- ^ Yamasaki, T., 1988. Taxonomy. In: K. Sekiguchi, ed. Biology of horseshoe crabs. Science House, Tokyo. Pp. 10 – 21.
- ^ Avise, J.C., Nelson, W.S. & Sugita, H., 1994. A speciational history of living fossils – molecular evolutionary patterns in horseshoe crabs. Evolution, 48:1986–2001.
- ^ Obst, M., Faurby, S., Bussarawit, S. & Funch, P., in press. Molecular phylogeny of extant horsecrabs (Xiphosura, Limulidae) indicates Paleogene diversification of Asian species. Molecular Phylogenetics and Evolution, DOI:10.1016/j.ympev.2011.08.025