Table of Contents
Introduction
Southeast Asia is a hotspot for palm diversity - this area alone contains almost half of all the palm species identified in the world[1] . In Singapore, there were a total of 54 recorded species of palms, but only 42 species are extant today[2] . The most distinctive of the palms would be Caryota mitis (figure 1) – a fairly common palm in Singapore easily recognized by its “fishtail-like leaflets”. There are a total of 13 species in the Caryota genus , but only one species – Caryota mitis – is found in Singapore[3] .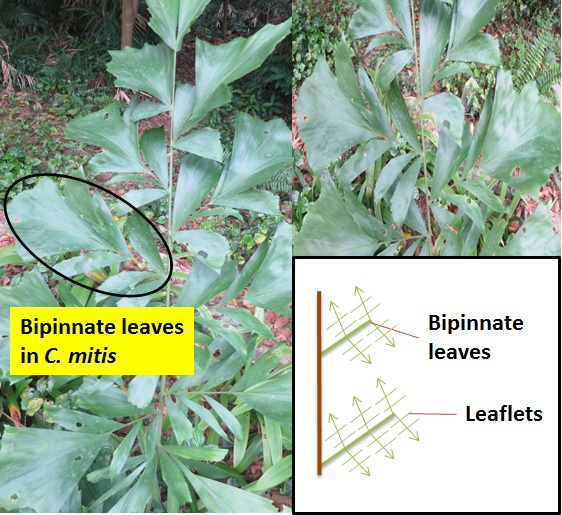 |
| Figure 2. Bipinnate leaves in Caryota mitis. |
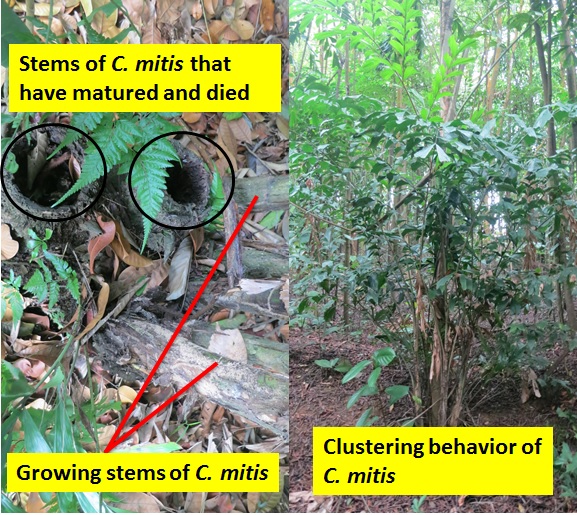
The Caryota genus is unique because they are the only palms with bipinnate (branches twice from the main stem) leaves (figure 2, right, above) and are therefore easily recognized. In addition, all Caryota species, including C. mitis, are monocarpic (syn: semelparous, hapaxanthic). This means they only flower (and set fruit) once in their lifetime before it dies. Fortunately, as the common name of C. mitis suggests, the “Clustering Fishtail Palm” frequently grow in clusters of stems rather than solitary stems and only the flowering stem dies after fruiting (figure 3, left, below). For this reason, C. mitis is often planted as an ornamental palm in Singapore, and around the world[4] . Locally, our National Parks Board (NParks) do plant some species of Caryota as an ornamental plant around Singapore, such as C. mitis and C. no.
Despite C. mitis being common (more than 1000 individuals) in Singapore[5] [6] , little is known about the biology and ecology of C. mitis. In fact, it is common not only on mainland Singapore, but also on Pulau Ubin, where a simple survey along trails found 2657 individuals[7] . That is a staggering number of individuals given the small size of Pulau Ubin! Today, there is ongoing research over at the National University of Singapore (NUS) to find out more about this common but little known palm.
Species description (Field identification)
Extremely common in Singapore, especially around disturbed areas and along roads; abundant on Pulau Ubin, Kent Ridge Park, and secondary forests in Singapore. Often cultivated and seen in residential areas. Widespread over the whole of Singapore, including areas such as East Coast Park, Changi, Pearl Hill Terrace and Bukit Timah Nature Reserve, among other areas.Easily identified by the bipinnate leaves with fishtail-like leaflets (limited to Caryota genus and C. mitis is the only native Caryota to Singapore). While the fishtail-like leaflets is distinctive, it may be confused with palms such as those from the Korthalsia genus(tribe: Calameae) (e.g. K. rigida and K. rostrata) which may also have fishtail-like leaflets. However, as the species epithet suggests, C. mitis is an unarmed palm (figure 4) (as all Caryota are) and does not have spines on its stem, unlike the rattans in the Korthalsia genus. In addition, the leaves of Caryota are bipinnate, while the leaves in all other palms are not.
Medium-sized, clustering palm that grows to a maximum of 8m (generally less than 5m in Singapore[8] ). Inflorescence branched with many rachilla, flowers cream to purple in colour. Fruits round, about 1 cm across. Green when unripe, turns orange, red or even purple when ripe.
For those who are interested in a more complete and detailed description with accompanying pictures, refer to full species description at the end of the page!

Nomenclature
Etymology
Caryota mitis was first described in 1790 by Loureiro in Vietnam[9] . The genus ‘Caryota’ is derived from ‘caryon’, which means a nut in Greek in reference to their fruits. The species epithet ‘mitis’ means unarmed in Latin, referring to the lack of spines in C. mitis (figure 4). Incidentally, all species within the Caryota are unarmed. Refer to this link for more information by NParks about C. mitis!Synonyms
Caryota furfuracea Blume ex Mart (1838), Caryota griffithii Becc. (1871), Caryota javanica Zipp. ex Miq. (1856), Caryota nana Linden (1881), Caryota propinqua Blume ex Mart. (1838), Caryota sobolifera Wall. ex Mart. (1838), Caryota sobolifera Wall. (1848), Caryota speciosa Linden (1881), Drymophloeus zippellii Hassk. (1842) ,Thuessinkia speciosa Korth. (1855).Common names
Fishtail palm, clustering fishtail palm, rabok, tukas.Distribution
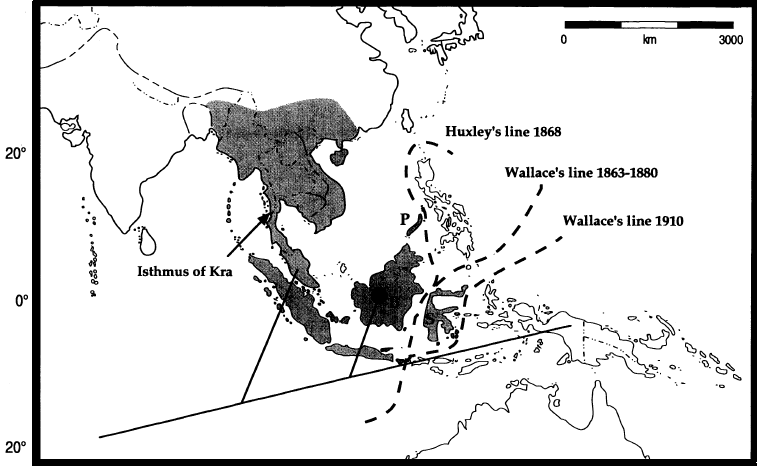
While the Caryota genus spans from India to Northern Australia, the native distribution of C. mitis (figure 5) is limited to the rainforests of Indo-China and Southeast Asia[10] - from the Andaman and Nicobar Islands, to Myanmar, Southeast China, to Southeast Asia and Sulawesi. However, C. mitis has been noted to have been naturalized in Southern Florida, Hawaii and the northern regions of Australia (e.g. Darwin, possibly northern Queensland)[11] [12] .
Flowers and fruits
Male Flowers
The inflorescences in the Caryota genus are bisexual, with separate male and female flowers. The flowers come in triads (one female flower between two male flowers), and are protandrous (meaning the male flowers mature before the female flowers[13] (figure 6) – normally a technique to minimise self-fertilisation)[14] .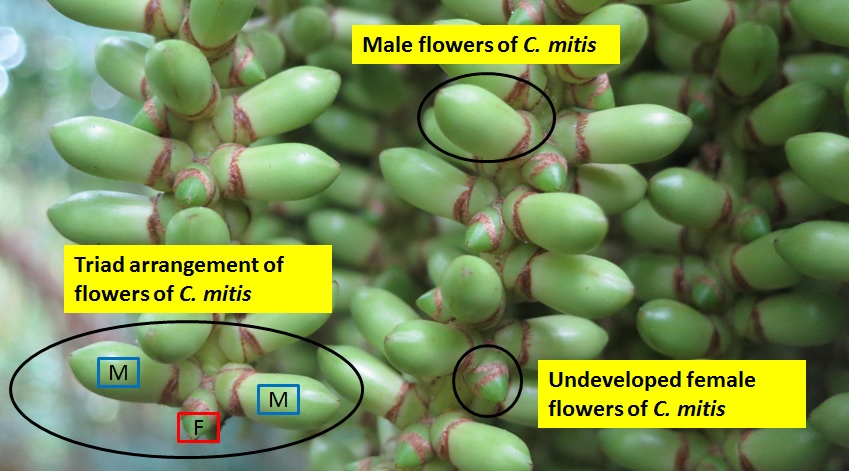
The male flowers are morphologically distinct from female flowers, and are easily recognized. They come in various colours, ranging from cream to dark purple (figure 7). The male flowers are elongated with 3 petals and numerous stamens, and seem to produce copious amounts of pollen. One individual produced so much pollen it appeared that the entire inflorescence was “bathed” in pollen (figure 8). It appears that there is a pattern when it comes to anthesis - only some male flowers will open on the first day (figure 9 - day 1). On the second day, the rest of the flowers will open, while the ones that were previously open the day before would have fallen off (figure 9 - day 2). On the third day, all the flowers would have died and most have fallen off (figure 9 - day 3). This cycle is illustrated in figure 9. The male flowers only last for a day.


Figures 7 (left) & 8 (right) . Variation in flower colour in C. mitis - cream to dark purple (left). Copious amount of pollen produced by C. mitis individual (right).

Figure 9. Flowering of male flowers in C. mitis on a temporal scale.
Female flowers
The female flowers are much less fancy (figure 10). They are globular in shape, with a trilobed stigma and like the male flowers, have 3 petals too. However, do not look as fanciful as the male flowers and no insects have yet been observed to visit the female flowers. Their pollination mechanism is still unknown.
Figure 10. Female flowers of C. mitis - globular shape is clearly distinct from the elongated shape of the male flowers.
Fruits
The fruits of C. mitis are about 1-2 cm in diameter, red to dark purple when ripe and usually has one seed (figure 11). The fruits are eaten by many animals (refer to ecology below).
Figure 11. Fruiting stages of C. mitis.
Ecology
Growth preference - Edge exploitation
Among all the different Caryota species, C. mitis is the only one that is commonly found in the forests where it is distributed[15] . C. mitis is often found in secondary forests, and is especially abundant in disturbed areas[16] . In addition, based on personal observations, C. mitis appears to be much more abundant along forest edges as compared to the interior (once again, research is underway to see if this is true!) (figure 12). This could point towards the ability of C. mitis to thrive along edges, where environmental conditions are harsher and more adverse[17] .
Figure 12. Many C. mitis plants along the edge of a trail in Kent Ridge Park.
Fruits of Caryota mitis - Handle with care!
Curiously, while the fruits contain calcium oxalate crystals that make it difficult to even handle (it causes a severe itching sensation)[18] , much less eat it, many animals have been recorded to eat the fruits of C. mitis. It appears that animals that feed on the fruit are less affected by the calcium oxalate crystals. C. mitis is also an extremely bountiful plant – based on an preliminary estimate, 124 individuals of C. mitis is able to produce up to over 1, 500, 000 fruits on Pulau Ubin[19] ! However, it is important to bear in mind that the length of the branches in different individuals may vary (it can reach up to 1.5m) and this would result in different number of fruiting potential. In addition, not all flowers are bound to set fruit and this may be an overestimation. Nevertheless, there is no doubt that C. mitis serves as an ample food source with its luxuriant “mop” full of fruits! More research is underway for a more comprehensive study on the production of fruits by C. mitis.The popularity of C. mitis fruits may be due two two factors: its year-round abundance and the energy content of the fruits. C. mitis appears to fruit year round as they are not constrained by environmental cues to synchronise their flowering event[20] . Instead, the palm undergoes an initial vegetative stage to accumulate carbohydrate resources, before expending their reserves for flower and fruit production during their reproductive phase[21] [22] . While some botanists argue that that is a simplistic assumption and other factors may trigger flowering instead (such as accumulation of growth-substances and abiotic factors like light availability[23] , it remains without a doubt that that C. mitis is a constant and prolific source of food for animals where it is commonly found. Locally, on Pulau Ubin, out of 2657 individuals, 932 individuals were in their reproductive phase[24] !
In terms of energy content, it appears that C. mitis is fairly nutritious – the average caloric content of ripe C. mitis fruits is 3.36 kcal/g[25] , comparable to that of an apple at 3.59 kcal/g[26] . In fact, palms fruits important food resources in a tropical rainforests[27] , and contributes to a high proportion of energy content within tropical lowland forests[28] . By including the consideration that C. mitis is common in Singapore forests, this confluence of factors appear to contribute to the allure of C. mitis fruits as an important resource in the forests of Singapore.
Consumption of Caryota mitis
Various animals that have been recorded feeding on the fruits on C. mitis: birds, squirrels, civets and jackals[29] . In Singapore, animals such as the long-tailed macaque (Macaca fasicularis)[30] [31] , wild boars (Sus scrofa)[32] , oriental pied hornbill (Anthracoceros albirostris)[33] , and the Asian glossy starling (Alplonis panayensis)[34] have all been recorded feeding on the fruits of C. mitis. Other recorded birds in Ipoh, Perak, Malaysia, that feed on the fruits are the yellow-vented bulbul (Pycnonotus goiavier), the pink-necked green pigeon (Trenon vernans) and the lineated barbet (Megalaima lineata hodgsoni)[35] , all of which can be found locally in Singapore. More importantly, it appears that C. mitis fruits are a significant food resource for the common palm civets (Paradoxus hermaphoditus) on Pulau Ubin[36] .
Figure 13. An Oriental Pied Hornbill (Anthracoceros albirostris) (photo credit: Angie Ng & Hiro Machida) and a Lineated Barbet (Megalaima lineata hodgsoni) (photo credit: Dr. Dato Dr. Amar-Singh HSS) feeding on the fruits of C. mitis.
Furthermore, C. mitis is known to be the host plant for two butterflies in Singapore: the common faun (Faunis canens arcesilas) and the tufted jungle king //(Thauria aliris)[37] . Table 1 summarises the animals that have been recorded eating C. mitis (be it leaves or fruits) in Singapore.
Table 1. List of animals recorded consuming C. mitis in Singapore.
| Common name |
Scientific name |
Part of plant consumed |
| Long-tailed macaque |
Macaca fasicularis |
Fruits |
| Wild boars |
Sus scrofa |
Fruits |
| Oriental pied hornbill |
Anthracoceros albirostris |
Fruits |
| Asian glossy starling |
Alplonis panayensis |
Fruits |
| Common palm civet |
Paradoxus hermaphoditus |
Fruits |
| Common faun |
Faunis canens arcesilas |
Leaves |
| Tufted jungle king |
Thauria aliris |
Leaves |
Pollination
Palms in general area poorly studied group, and little is known about the pollination biology of many palms[38] . However, it has been suggested that the flowers of members in the Caryota genus are entomophilus (attracts insects), and current research has elucidated that point. Both stingless bees and honey bees have been observed visiting the flowers of Caryota mitis in Singapore. More research is underway to determine what other insects visit the flowers of C. mitis for pollen. In addition, no bees thus far have been seen visiting the female flowers of C. mitis. Therefore, the pollination mechanism in C. mitis is still unknown and more research has to be done to determine the pollination syndrome in C. mitis. The bees that visit the flowers of C. mitis are:- Apis cerana (figure 14)
- Tetragonula laeviceps (figure 14)
- Tetrigona apicalis (figure 14)
- Tetragonula pagdeniformis
- Tetragonula geissleri

Figure 14. Some bees that visit C. mitis flowers for pollen. (Photo credit: Chui Shao Xiong)
Human uses
While C. mitis is considered part of the sago palms (as all Caryota are)[39] , it yields little sago and is not very useful for sago production[40] . This is unlike the related species, Caryota urens, also known as the Toddy palm. As the name suggests, the sap of the palm is tapped and used to make palm sugar or fermented to produce toddy.In addition, C. urens is a suitable palm for producing sago from the pith of the trunks of old trees[41] .
In India, there are several uses for the palm – for food, starch is extracted from the stem and the palm heart can be eaten; for construction, the leaves are used for thatching of roofs and can also be weaved into household items, while the fibres from the leaf sheath are used for making ropes; for ornamental purposes, the seeds can be made into beads[42] . The fuzz from young leaves are also used as tinder in some Southeast Asian countries[43] .
The most popular use of this palm today would probably be for cultivation. It is widely cultivated today and due to its clustering growth habit and smaller stature, it is more popular as compared to other palms in the Caryota genus.
Full Species Description of Caryota mitis
Refer to below for a complete description of C. mitis[44] [45] :| Stem |
Leaves |
||
| 5-15cm in diameter; ringed; green, brown or greyish; unarmed (without spines) |
 |
5-12 per stem; spreading; bipinnate (characteristic feature); petiole 50-200cm long; rachis 1-4m long; pinnae 7-23 on each side of rachis, 50-120cm long; 7 – 23 leaflets on each side, 20x13cm in size, broadly triangular, apical margins jaggedly toothed (lending it the fishtail-like form) |
|
| Inflorescences |
Fruits |
||
| 4-7 spadices, initiating at the top and moving down stem (basipetal), interfolia or intrafolia, pendulous, 1-1.5m long; peduncle 30-45cm long; rachis 5-45cm long, rachillae 10-60, 10-65cm long, drooping Male flowers: Very numerous about 1cm long, elongated, light to dark purple in colour, 3 petals, many stamens (yellow) Female flowers: Only develops after the male flowers have fallen off, smaller than males, sepals rounded, petals 3, pink to brownish in colour, yellowish or whitish tips, 3 stigma, ovary roundish ovate |
Multiple inflorescence on a stem  |
1-2cm in diameter, green when unripe, red to purple when ripe, subglobose (round), usually 1 seeded, contains oxalate crystals |
  |
DNA Barcoding
Caryota mitis belongs to the tribe Caryoteae (subfamily Coryphoideae), which includes the following three genera: Caryota, Arenga and Wallichia. Notably, species delimitation in Caryota is difficult due to complex morphological differences in the leaves and inflorescence, which is compounded by the difficulty in collecting specimens due to the large dimensions in many species of the genus (C. no, C. urens, C. gigas etc.)[46] . The notoriety of Caryota stems from the relative scarcity of materials in herbaria, making traditional alphataxonomy difficult.In a relatively recent experiment (2011), it appears that DNA barcoding is a useful tool for palm taxonomists with reference to the Caryoteae tribe. Within the Caryoteae tribe, to augment species identification accuracy, it appears that three DNA markers: matK, rbcL and nrITS2 are especially useful - it has a 92% species discrimination rate. This is considered high for a barcoding experiment[47] . While Caryota mitis may not be a difficult species to identify in Singapore (it is the only Caryota species), it has a wide geographical distribution, therefore it may be misidentified as Caryota monostachya (also a clumping species distributed from China to Vietnam). The key distinction between the two species if that C. monostachya forms unbranched inflorescences (figure 15), while that of C. mitis is branched (figure 9 or full species description). Therefore, DNA barcoding may still be useful for species identification when no reproductive organs are available for analysis.

Molecular Systematics of Caryota
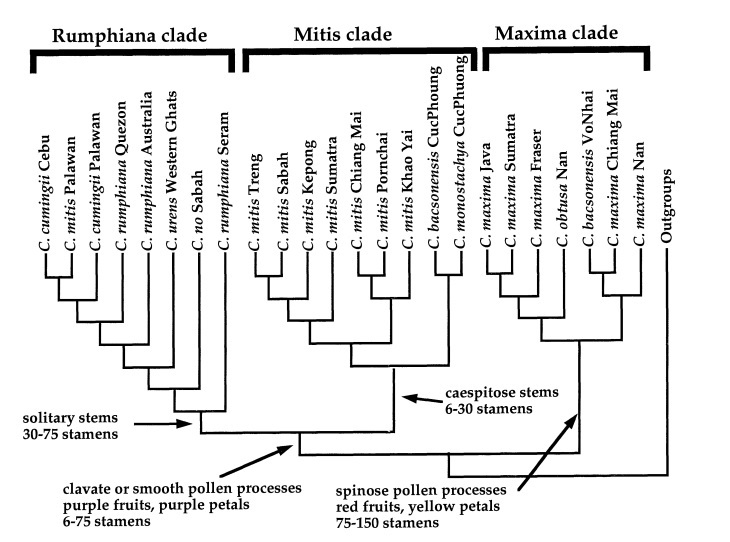
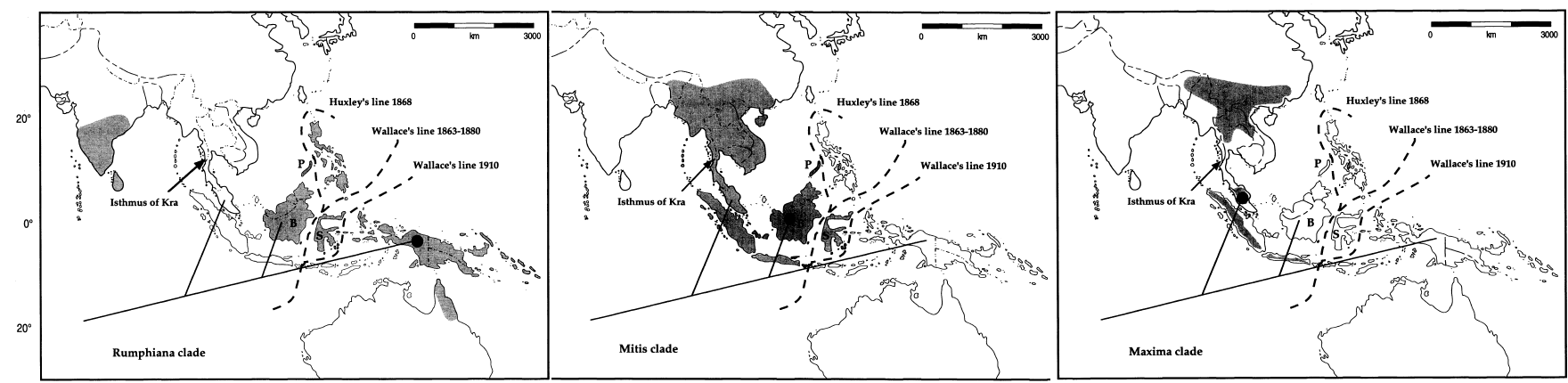
Figure 16. Summary phyloheny for the species of Caryota (9 out of 13 species), with geographic distributions indicated. The tree is rooted with the other genera in the Caryoteae tribe - Arenga and Wallichia. The three major clades (Rumphiana clade, Mitis clade and Maxima clade) are identified based on molecular characters but are consistent with morphological features. (Image take from Hahn and Sytsma, 1999 - Molecular systematics and biogeography of the Southeast Asian genus Caryota (Palmae) in Systematic Botany 24(4): 558-580).
Based on cpDNA, Hahn and Sytsma (1999)[48] identified three principle clades: the Rumphiana clade, the Mitits clade and the Maxima clade (figure 16-17).
The first clade, the Maxima clade, consists of C. maxima, C. obtusa and C. bacsonensis. These three species are similar morphologically: they have solitary growth habits (does not cluster), yellow petals (versus purple petals in C. mitis), red fruits and spinose (spiny) pollen. Their distribution are limited to the Asian continent and Sumatra and Java.
The second clade, the Mitis clade, consists of most sampled C. mitis, one C. monostachya and one C. bacsonensis. Considering that C. bacsonensis was from Cuc Phuong, Vietnam, along with C. monostachya, it could be that the C. bacsonensis was a result of the hybridization with C. monostachya. C. mitis and C. monostachya are fairly similar morphologically, as previously discussed: they have clustering growth habits, purple petals and maroon or purple fruits.
The third clade, the Rumphiana clade, consists of C. cumingii, C. no, C. rumphiana and one C. mitis. Besides the C. mitis (likely the result of hybridization), all these species have solitary stem growth habit, purple petals and fruits, and clavate (club-like) pollen processes.
The pairing of the Mitis and Rumphiana clades are consistent with morphological characters (pollen morphology – clavate, petal and fruit pigmentation – purple and stamen number – 6 to 75), despite not having strong support based on the shortest equally and differentially-weighted Wagner parsimony analyses and minimum evolution analyses and a Dollo parsimony analysis nullifies this relationship. Therefore, Hahn and Sytsma (1999) considers it the best supported intrageneric relationship topology
.
Figure 17. Distribution of the three clades - Maxima clade, Mitis clade and Rumphiana clade. (Image take from Hahn and Sytsma, 1999 - Molecular systematics and biogeography of the Southeast Asian genus Caryota (Palmae) in Systematic Botany 24(4): 558-580).
Summary model of evolution in Caryota
In Southeast Asian biogeography, two interpretations predominate the discussion of biological distribution – vicariance due to tectonic events or sea level changes or dispersal events that helped extend the distribution of the species in question. In Caryota, it is likely that a both mechanisms were invoked concurrently based on the three clades. The distribution of the three clades correlates well with vicariance and the geologic history of SE Asia. However, a full account of the distribution and evolutionary history of Caryota requires the consideration of dispersal to explain the deviation from the correlation between vicariance and geologic history with the relationship between the three clades.The model of evolution as proposed by Hahn and Sytsma (1999) is as follows: there was an original, common ancestral species resembling that of the Maxima clade on mainland SE Asia. This was followed by two separate episodes of cladogenesis, based on habitat preference shifts, morphological evolution, and range expansion due to vicariance and dispersal.
Considering that C. maxima is a species that is limited to higher elevations, the first cladogenic event be the invasion of the lowlands and shifts in floral and fruiting biology as per the Mitis and Rumphiana clade. The clustering growth form may also be indicative of the colonization of the understory by the Mitis clade.
The second cladogenic event would be the evolution of the Rumphiana clade, a different track from that of the clustering growth habit of the Mitis clade. Given the uncharacteristically wide distribution of C. rumphiana, it is probable that dispersal was a critical factor in the evolution of the Rumphiana clade. The exception of this clade lies with C. no, an endemic species of Borneo. It is more likely the result of speciation and cladogenesis due to vicariance of Borneo and Sulaweisi, and less likely due to dispersal.
Hahn, W. J. & K. J. Sytsma, 1999. Molecular systematics and biogeography of the Southeast Asian genus Caryota (Palmae). Systematic Botany, 24(4): 558-580.
Krempin, J., 1995. Popular Palms. Krempin Books, Queensland. 96 pp.
Hsuan, K., S. C. Chin & H. T. W. Tan. 1998. The Concise Flora of Singapore, Volume II: Monocotyledons. Singapore University Press, Singapore. 215 pp.
Loo, A. H. B., W. F. Ang, W. J. Baker & H. T. W. Tan, 2014. A Guide to the Native Palms of Singapore. Singapore Science Center, Singapore. 174 pp.
Loureiro, J. D., 1970. Flora cochinchinensis : sistens plantas in regno Cochinchina nascentes. Quibus accedunt aliae observatae in Sinensi imperio, Africa Orientali, Indiaeque locis variis. Omnes dispositae secundum systema sexuale Linnaeanum. Labore. URL: http://www.biodiversitylibrary.org/page/654262#page/215/mode/1up (Accessed on 04 Nov 2014). DOI: http://dx.doi.org/10.5962/bhl.title.560
Henderson, A., 2009. Palms of Southern Asia. Princeton University Press, New Jersey. 199 pp.
Uhl, N. W. & J. Dransfield, 1987. Genera Palmarum: A classification of palms based on the world of Harold E. Moore, Jr., L. H. Bailey Hortorium And The International Palm Society, Allen Press, Kansas. 610 pp.
Corner, E. J. H., 1966. The Natural History of Palms. Weidenfeld and Nicolson, London. 393 pp.
Snyder, D. S., G. M. Hatfield & K. F. Lampe, 1979. Examination of the Itch Response from the Raphides of the Fishtail Palm Caryota mitis. Toxicology and Applied Pharmocology, 48: 287–292.
Rathcke B. & E. P. Lacey, 1985. Phenological patterns of terrestrial plants. Annual Review of Ecology and Systematics, 16: 179–214.
Omar, L. 2014. Resource Availability of the Clumping Fishtail Palm, Caryota mitis, for the Common Palm Civet (Paradoxus hermaphroditus) Along the Trail Edges of Pulau Ubin, Singapore. In Undergraduate Research Opportunities in Sciences, Department of Biological Science, National University of Singapore.
Corner, E. J. H., 1966. The Natural History of Palms. Weidenfeld and Nicolson, London. 393 pp.
Loo, A. H. B., W. F. Ang, W. J. Baker & H. T. W. Tan, 2014. A Guide to the Native Palms of Singapore. Singapore Science Center, Singapore. 174 pp.
Henderson A., 1986. A review of pollination studies in the Palmae. Botanical Review, 52: 221 – 259.
Kiew, R. 1977. Taxonomy, ecology and biology of sago palms in Malaya and Sarawak. In: Tan, K. (ed.), Sago-76: Papers of the first international sago symposium. Kemajuan Kanji, Kuala Lumpur. Pp. 147 – 154.
Johnson, D. V., 1995. Tropical Palms. FAO Corporate Document Repository. URL: http://www.fao.org/docrep/x0451e/x0451e00.HTM. (Accessed 05 Nov 2014)
Johnson, D. V., 1995. Tropical Palms. FAO Corporate Document Repository. URL: http://www.fao.org/docrep/x0451e/x0451e00.HTM. (Accessed 05 Nov 2014)
Hodel, P. R. &P. Vatcharakom, 1998. The palms of Thailand. In: Hodel, D. R. (ed.), The Palms and Cycads of Thailand. Kampon Tanasacha, Nong Nooch Tropical Garden, Thailand. Pp. 1–177.
Jeanson, M. L., J.-N. Labat & D. P. Little, 2011. DNA barcoding: a new tool for palm taxonomists? Annals of Botany, 108: 1445–1451.
Jeanson, M. L., J.-N. Labat & D. P. Little, 2011. DNA barcoding: a new tool for palm taxonomists? Annals of Botany, 108: 1445–1451.
Hahn, W. J. & K. J. Sytsma, 1999. Molecular systematics and biogeography of the Southeast Asian genus Caryota (Palmae). Systematic Botany, 24(4): 558-580.