Chremistica umbrosa (Hemiptera: Cicadidae)
 |
| Picture 1. Picture of Chremistica umbrosa. Permission for use granted by Dr Yaakop. |
Table of Contents
Cicadas
Cicadas are synonymous with the noises that they produce. Literally.The term 'cicada' in Latin means 'buzzer', or a tree cricket. It is no wonder how this name is derived, given that one would naturally associate cicadas to the sounds that immerse them in a forest. Indeed, cicadas are one of the primary perpetrators of forest noises, making one of the loudest songs (recorded to be up to 120dB in certain species-cite) among hexapods. Yet more often than not, they are heard but not seen. Not many of us in Singapore had seen a cicada in the wild before. Their short aboveground lifespan, in addition to their close to impeccable camouflage, allow these creatures to evade our detection. There have been interesting accounts of cicada life histories- them spending 17 years underground before emerging from the ground for a short few weeks to mate, and then die. While this romanticised account are certainly true for one taxa of cicadas (Magicicadas sp.), this should not be generalised to all cicadas. What many fail to understand is the wide diversity of this taxa, Cicadoidea.
Video 1: Magicicada sp. is the species featured within the clip (Life in the Undergrowth, by BBC wildlife).
Cicada species are classified by many different factors: Geography, habitat, season, diurnal patterns of reproductive activity and songs (Moore, 1993). There is an estimated of 3000 species of cicadas worldwide (Cooley et al., 2012). One of the most well documented and researched cicada taxa is the Magicicada sp. These are one of the most charismatic groups, in terms of cicada life history due to their periodicity. Primarily found in North America species in this taxa have particularly long life histories They spend their nymphal years underground, then emerge synchronously from the ground every 13 or 17 years, depending on individual species. Another cicada species, found in India from the genus Chremistica, is known to emerge every four years (Mozgai, 2012), in synchrony with the FIFA World Cup (yes, even to the month) . These above-mentioned cicadas are termed as 'periodic cicadas', in contrast to 'annual cicadas' that emerge yearly. In terms of size, they vary widely too. The largest cicada, Megapomponia imperatoria in the below picture, has a body length of about 7.5cm (AGR, 2010), while Pacuella puella spans less than 2cm.
|
|
Why do cicadas sing?
Since most of us have experienced their loud shrill songs, we sometimes question: what are their songs for?There are a few types of cicada songs: the free song (produced by undisturbed, free living males), protest song (produced when males are disturbed, injured or caught) and for some species, the courtship song (produced only in the presence of females) (Simmons & Young, 1978). The song that is normally encountered by people in the forests is the free song, or the courtship song. Male cicadas sing to attract females to mate. And because of that, the females don't sing.
Video 2: Protest calls of a captured cicada. In this video, the protest call resulted in its liberation! Video: sbtmko, Youtube.
Some cicada songs
Because cicada songs are species specific, one can usually identify cicadas to the species level just by listening to their songs. Even species within the same genus would have distinctly different songs. To faciliate comparisons between Chremistica umbrosa songs with other cicada songs, a few links are included below:| Purana usnani, singing probably the most familiar cicada song in Singapore. |
Audio: Dr Leong T.M, from youtube. Click here for the full video. |
| Dundubia vaginata Dominant frequency: 3.94kHz (Leong et al., 2011b) |
(Permission for use granted by Dr Leong T.M) This recording was taken in Singapore. |
| Huechys fasca Frequency range: 7.8kHz -11.9kHz. Peak intensity: 9.8kHz (Leong et al.,2011c) |
(Permission for use granted by Dr Leong T.M) This recording was taken in Singapore. |
| Chremistica umbrosa Frequency range: 4.09kHz - 5.46kHz. Peak intensity: 4.81kHz (Leong et al., 2011a) |
This recording was taken in Singapore. Recording: Edwin Khoo |
| Chremistica guamusangensis Dominant frequency: Broad band: 3.3kHz, Narrow band: 2.75kHz (Gogola & Trilar, 2004) |
Click here to hear the song! (Gogola and Trilar, 2004) |
| Chremistica pontianaka Dominant frequency: 2.85kHz - 2.95kHz (Gogola & Trilar, 2004) |
Click here to hear the song!(Gogola and Trilar, 2004) |
How do cicadas sing?
In general
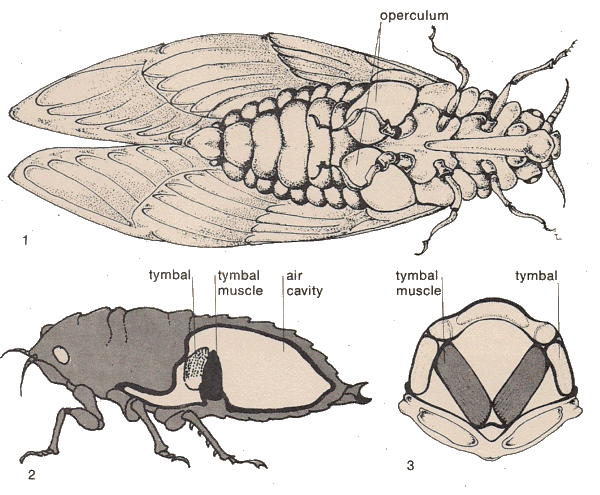
Sound producing system in cicadas consists of two coupled resonators: Firstly, the sound producing tymbal, and secondly, the Helmholtz resonator that consists of the abdominal air sac and tympana (Young & Bennet-Clark, 1995). The tymbals produce the sounds, the resonator radiates and amplifies the sounds produced. This is why sometimes, the singing of the cicadas is also known as tymbalisation.
1. Ventral view of cicada. 2. Lateral cross section of cicada. 3. Antero-ventral section of of tymbal area. Note that there is one pair of tymbals. For more information, refer to The Robinson Library here. |
In detailThe pair of tymbals of cicadas, located dorsolaterally on each side of the first abdominal segment, are responsible for the sounds they produce. Tymbals are cuticular membranes that are strengthened by sclerotised, dorsoventrally oriented ribs (Claridge, 1985). A tymbal muscle is attached to the inner face of the tymbal. The contraction of the tymbal muscle buckles the tymbal, causing a loud click that makes up the basic unit of cicada calls. Most cicadas have synchronous tymbal muscles, meaning that they require a separate nervous stimulation for every contraction, as opposed to asynchronous muscles that respond to previous cycles of nervous stimulations (Claridge, 1985). Because of this, the frequency tymbal muscle contraction is limited, and rarely reaching observed calling frequencies. Frequency multiplication takes place to reconcile muscle contraction oscillation to observed calling frequencies (Michelsen & Young, 1974) : for a call which peaks at 4kHz (in Cyclochila), the oscillation of the tymbal muscles happens at a rate of only about 120Hz (Young & Bennet-Clark, 1995). This frequency multiplication is carried out by the tymbal ribs for Cyclochila, having the ribs vibrate at 4000Hz when tymbal muscle contraction oscillation is 120Hz.This is one way for the cicada to carry out modulation of their songs, which is to vary the pitch, timing, or amplitude. Another muscle, the tensor muscle, anterior to the tymbal, serves to modify the elastic and acoustic properties of the tymbal, which could also help in song modulation (Claridge, 1985). Abdomen positioning is yet another means of song modulation- some cicadas deliberately raise their abdomen during song making to increase the gap between its operculum and the abdomen, allowing for louder acoustics (Claridge, 1985). This could explain why some cicadas adopt special 'song making' postures during tymbalisation. |
Video 3. Cicada tymbalisation, by Magicicada sp. Video: Acejackalope, Youtube. The sound is made by the tymbal of the cicada, and is the protest song from this species. The vibrating part that can be seen Note that the mechanism of cicada calls (tymbalisations) is different from that used by most orthopterans, (stridulation), as tymbalisation does not involve the rubbing of one surface against another surface.
Why don't cicadas go deaf?
Interestingly, the tympana, which are used by the males as resonators for their song, are also used to detect sounds, both in the males and females. So, if the organ that is exposed to the loud and shrill songs, propagated through a forest, is also the organ that is supposed to receive sounds, could all male cicadas be deaf by the time they sing?Hennig et al. in 1994 found that there were folds on the male tympana when cicadas were singing, but were not present when they were inactive. The folds conferred protection from 'deafness' caused by mechanical overstimulation of the tympana when cicadas were singing, reducing the cicada's sensitivity to noise by up to 20dB. A more recent study by Sueur et al. (2008) found that male cicadas of his study species had high sensitivity towards only selective frequencies, which was lower than their dominant calling frequency. Also known as frequency detuning, this allows male cicadas certain amount of sensitivity towards auditory signals, and at the same time prevent deafening. On the other hand, they found that the female tympanum was tuned very precisely to the species' dominant calling frequency, allowing optimal detection and recognition of the species specific song sung by the males. More studies could be conducted to determine if these findings can be generalised to all cicadas.
Song making posture and song modulation
For C.umbrosa, their songs are very distinct and unmistakable. Able to be heard from a few hundred meters away, their songs are shrill and continuous, punctuated short periods of by coarse-sounding portions in between. To create the coarse sounding portions, C.umbrosa seems to change the position of its abdomen, as shown in the below video.Video 4. C.umbrosa song modulation through repositioning of its abdomen. Note that whenever the 'coarse' region of the song is sung, this male moves its abdomen downards, towards the tree branch. Video: Edwin Khoo.
When C.umbrosa is singing, it adopt a singing posture, forming a curved abdomen. This posture could possibly function to serve as protection against over-stimulation of its tympana and to increase the amplitude of its songs when it tymbalises.
 |
| Picture 4. C.umbrosa when it is at rest and NOT singing. Note the abdomen. |
 |
| Picture 5. C.umbrosa when it is singing. Note the curved abdomen, and the downward-opened operculum. |
Life stages
Being hemimatabulous, all cicadas go through three main stages in their life cycle, comprising of the egg, nymph and adult.| Life stage |
How does it look like? |
Description |
||
| Egg |
|
A female cicada after mating, would deposit eggs, through its ovipositor, into small slits made by itself on the bark of a tree. |
||
| Nymph |
|
Cicada nymphs have body forms that look similar to the adult stage, only without wings. Upon hatching, the nymphs fall from the bark onto the ground. Using their fossorial front legs, the nymphs dig into the ground where they would feed and grow. They derive their nutrition from piercing their rostrum (their feeding mouthparts) into the xylem of roots, and taking in plant sap. As the nymph grows, ecdysis takes place multiple times. |
||
| Adult |
|
The last nymphal instar digs out of the soil at night and makes its way onto a vertical surface, which usually are tree barks. There, it carries out its last moult. Emerging as a winged adult, it leaves its moulted exoskeleton (exuvia) behind on the bark. Adults, too, feed on plant fluids through the xylem vessels. |
Diet
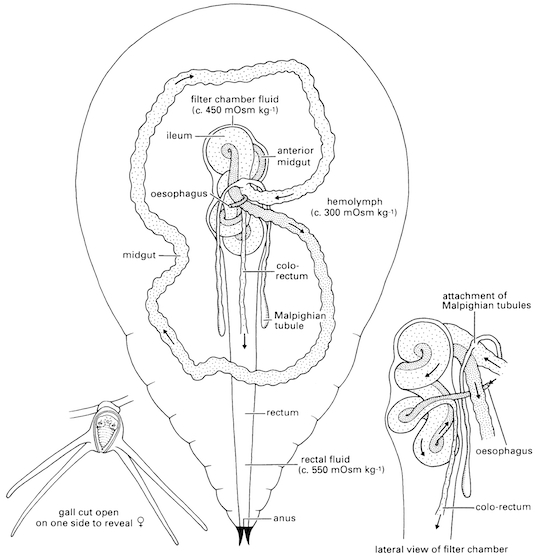
C.umbrosa, like other cicadas, feed on xylem sap. Their distant relatives from the taxon Sternorrhyncha specialise on getting fluids from the phloem. The mouthparts comprise mandibles and maxillae modified to become needle-like structures, lying in a beak-like labium, forming a rostrum. These are adapted such that the cicada would be able to pierce through bark, into the xylem vessels to take up fluids. Xylem sap from contains a large proportion of water, and ions of potassium, sodium, calcium, magnesium, chlorides and phosphates (Cheung & Marshall, 1973). Trace amounts of sucrose and amino acids are also present. To derive sufficient nutrients from xylem sap, the cicada has to intake a high volume of xylem fluid. To avoid osmotic dilution throught the high amount of water intake, cicadas employ certain physiological adaptations:| Presence of specialised structures known as filter chambers Uptake of xylem fluid --> Fluid transported to filter chamber via the esophagus --> Water is removed rapidly from the fluids in filter chamber --> Removed water is directly transported to ileum --> Remaining fluids get channeled to mid gut, where absorption of solutes occur. The filter chamber allows water to directly take a shorter route through the digestive system of the cicada, reducing osmotic dilution, and to be excreted rapidly. Details of the mechanism of this process can be obtained in the paper written by Cheung and Marshall, 1973. |
|
||
| Rapid discharge of excess water as urine According to the rate of uptake of xylem fluids, cicadas also have to excrete excess fluids in order to continue uptake at the given rate. On a day where high amounts of feeding take place, urine is quickly discharged as cicadas feed. Components in the urine are in about the same proportions as the xylem sap (Cheung & Marshall, 1973), but in lower quantities. By gently tapping its abdomen, manual discharge of cicada urine can be simulated. Urine is discharged from the anus as fine, discontinuous squirt . WARNING: 'Cicada rain' can occur as a result of numerous cicadas feeding simultaneously atop a tree. By having every individual discharging urine at intervals, the cumulative effect is that which is similar to rain. (It is therefore advisable not to be over-relieved when it suddenly rains under a tree on a warm day.) |
Video 4. Chremistica umbrosa discharging urine atop a tree. Location: Labrador Park, Singapore. Video: Dr Leong, T.M. |
Chremistica umbrosa life history
As with all cicadas, C.umbrosa is hemimetabulous. Yet, not much is known about the life history of C.umbrosa currently. While it has been observed that there have been mass emergence events during late March and early November for a few years since 2010 (Leong et al., 2011), more studies have to be carried out to better understand the periodicity of this species. While the periodicity of this species cannot be confirmed at the moment, there is speculation that this species is likely to be an annual or bi-annual cicada. There are also some suspicions that the abundance peak during the March mass emergence event is slightly higher than the November peak (Leong, unpublished). The populations of C.umbrosa decline about 20-30 days after the mass emergence events (Leong et al., 2011). Hence, the aboveground lifespan of the adult C.umbrosa is likely to be about one month. Long term observational studies are required to further ascertain this postulation.Chremistica umbrosa last moult
Materials extracted from Leong et al. (2011), unless otherwise stated. Permissions for use granted by Dr Leong T.M.Cicadas moult (carry out ecdysis) throughout their nymphal stages. Moulting is the shedding of the exoskeleton, required such that the cicada body size can increase. The number of nymphal instars for C.umbrosa has yet to be determined, and could be a potential area of study. The below pictures document various stages of the final nymphal moult for C.umbrosa to reach adulthood (the imago) (Leong et al., 2011). The pictures were taken on 29 March, 2011, at Labrador Park. In this observation, it was noted that the whole moulting process (from the second picture onwards) took about 30 minutes.
|
|
|
||||||
|
|
|
||||||
|
|
|
||||||
|
|
|
Video 5. Cicada moulting. Even though the cicada in the video is not C.umbrosa, one can easily notice the similarities in moulting chronology between this species of cicada and C.umbrosa. Video: Robert Vingsnes.
Mating
This mating event of Chremistica umbrosa was witnessed and documented first hand in Labrador Park, Singapore. The male had been tymbalising discontinuously through for at least an hour, alone. At around 12.53pm, his persistence was rewarded. |
| 12.43pm: The tymbalisation of this male fills the air. |
|
|
|
||||||
 |
 |
|
||||||
|
 |
 |
||||||
|
|
 |
 |
| Chremistica umbrosa mating- posterior view. Photo: Edwin Khoo. |
 |
| Chremistica umbrosa mating- lateral view. Photo: Edwin Khoo |
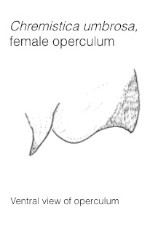
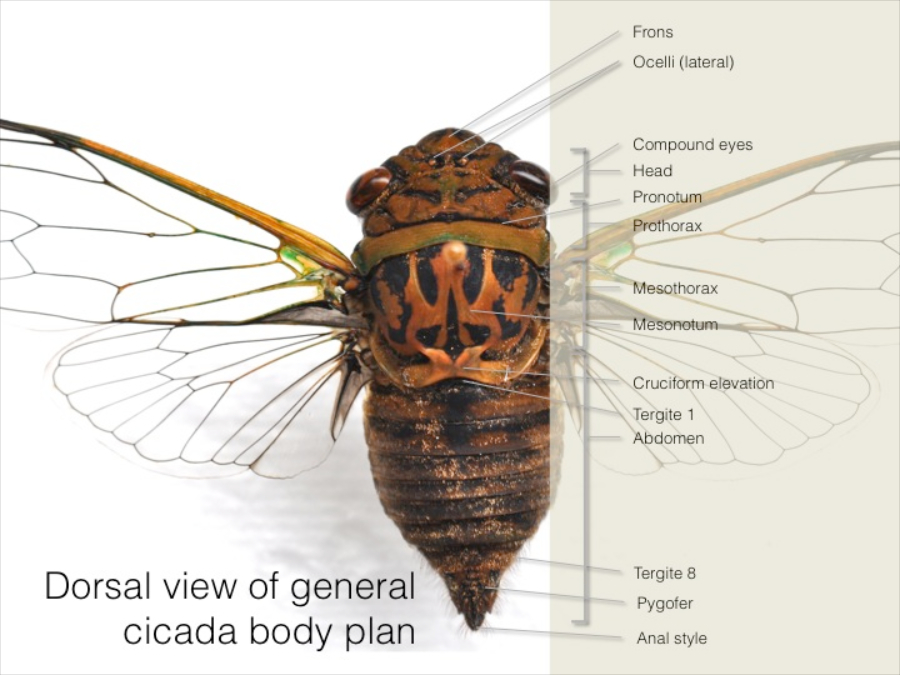
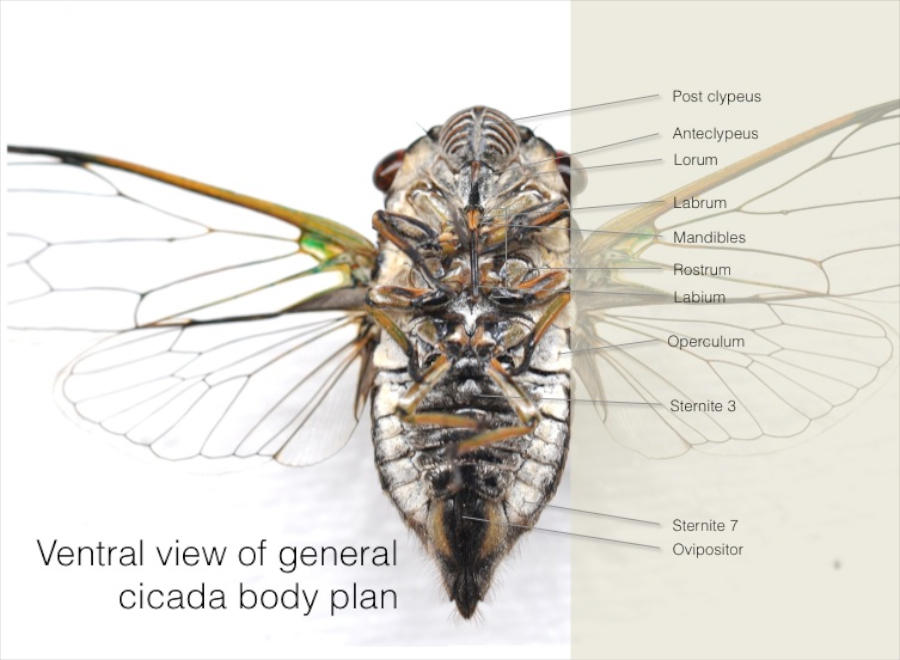
Generalised external anatomy of cicadas
 |
| Female Chremistica umbrosa. Photo: Edwin Khoo. |
 |
| Female Chremistica umbrosa. Photo: Edwin Khoo |
Chremistica umbrosa (Distant, 1904)
Taxonomy
Animalia
- Arthropoda
- Insecta
- Hemiptera
- Cicadomorpha
- Cicadoidea
- Cicadallidae
- Cryptotympanini Boulard, 1979
- ChremisticaStål, 1870
- Tridentigera (Bregman 1985)
- Chremistica umbrosa Distant 1904
- Tridentigera (Bregman 1985)
- ChremisticaStål, 1870
- Cryptotympanini Boulard, 1979
- Cicadallidae
- Cicadoidea
- Cicadomorpha
- Hemiptera
- Insecta
Synonyms
Cicada umbrosa Distant, 1904
Rihana umbrosa Distant, 1906
Rihana pisanga Moulton, 1923
Chremistica pisanga Metcalf, 1963
Geographic distribution
Chremistica Stål, 1870
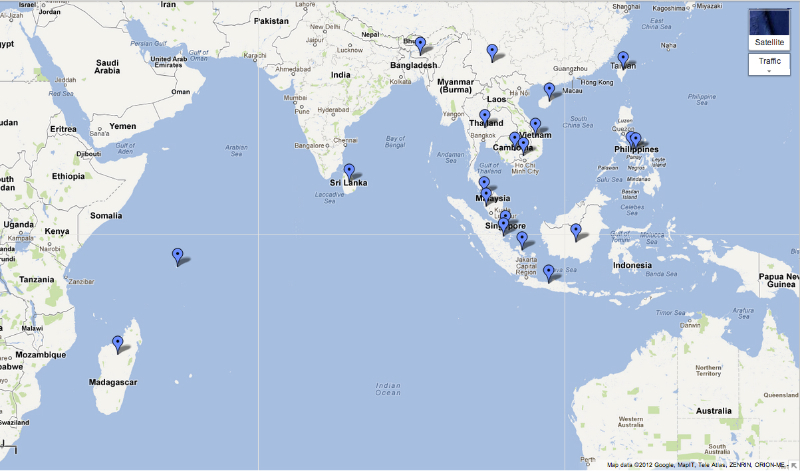 |
Chremistica Stål, 1870, is a genus containing 49 extant species occurring throughout the Oriental Region (Southeast Asia, China, Taiwan, Sri Lanka) and 1 species from Madagascar. (Yaakop et al., 2005). Blue waypoints indicate the following regions: Vietnam, Philippines, Kedah, Meghalaya, Taiwan, Kedah, Java, Borneo, Sumatra, Thailand, Singapore, Sri Lanka, Vietnam, China (Hainan, Yun Nan), Cambodia, Seychelles Island, Madagascar, Bangka Island. At least 3 Chremistica species are found in Singapore: Chremistica nesiotes Breddin, 1905, Chremistica pontianaka (Distant, 1888) Chremistica umbrosa (Distant, 1904) |
Chremistica umbrosa (Distant 1904)
Worldwide:
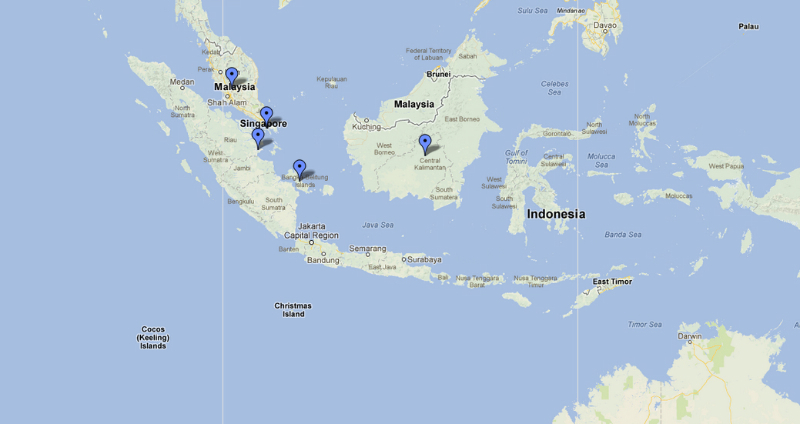 |
Occurrence of this species has been recorded in southern part of the Malayan Peninsula, the eastern coasts of Sumatra, Banka Island, Borneo, and other islands in the southern part of the Strait of Malacca (including Singapore). (Yaakop et al., 2005; Leong et al., 2011; Salmah et al., 2005) |
In Singapore:
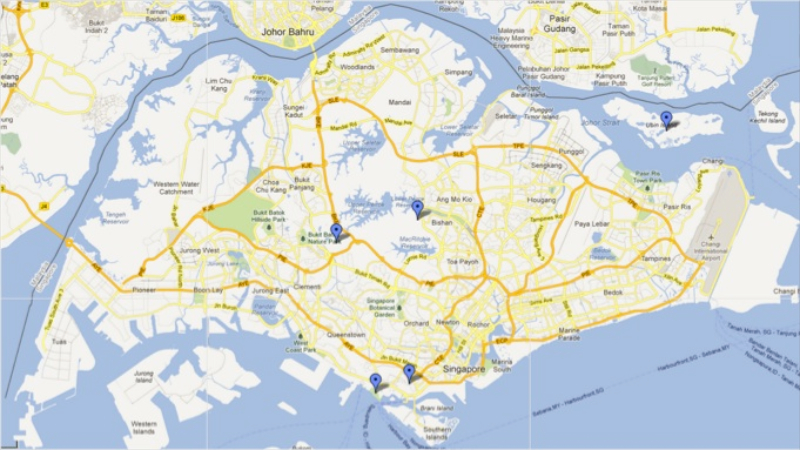 |
Individuals of this species have definitively been observed at least in Bukit Timah Nature Reserve, Labrador Park and Pulau Ubin (Leong et al., 2011)[4] , Mount Faber (Leong, unpublished) and Venus Drive. |
Description / Morphology

Picture 21. Lateral view of C.umbrosa. Permission granted by Dr Leong, T.M., taken from Leong et al., 2011.

Diagnosis
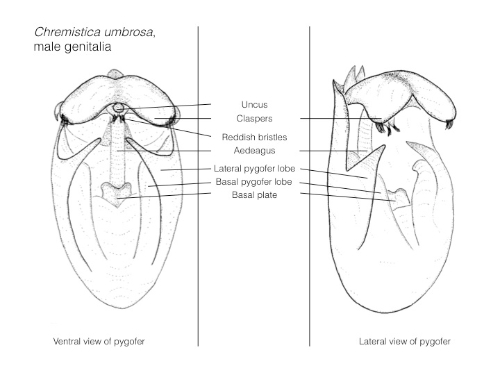
- Male genitalia As described in the genitalia portion.
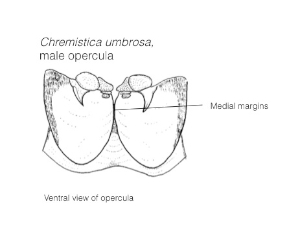
- Strongly diverging medial margins in the male opercula As illustrated in the opercula portion.
- Patterns of spots on mesonotum Patterns of the paramedial and lateral obconical fields.
- Dense white pilosity on lateral sides of ventral surface Very apparent from a lateral view.
- Calling song This is the best way to distinguish this species from other species, because of its distinct calling songs.
Distinguishing males from females
|
|
|
Type information
The holotype of species is the single physical example of an organism that is used when the species was originally formally described. The first formally described Chremistica umbrosa (at that point known as Cicada umbrosa) is now stored in the British Museum of National History (Yaakop et al., 2005).Phylogeny
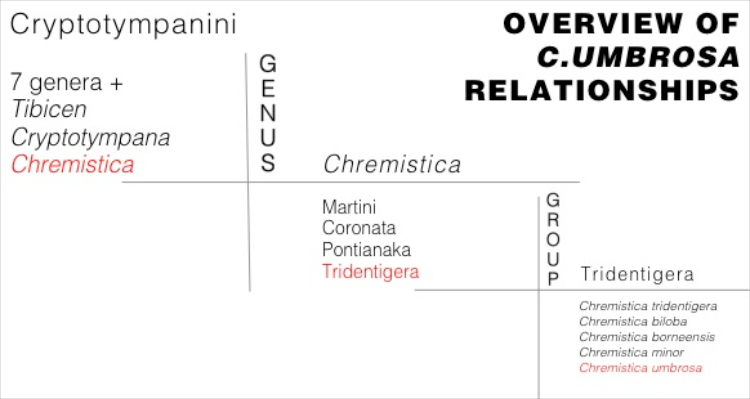 |
| Illustration 6. An overview of taxa that C.umbrosa is nested within, adapted from Yaakop et al. (2005) and Hayashi (1987). Illustration: Edwin Khoo. |
Why is Chremistica umbrosa nested within these groups?
From Yaakop et al. (2005), unless otherwise stated.Species nested within Crytotympanini usually have large tymbal covers that reach laterally to the operculum, and anteriorly to the metathorax. Also, males usually have greatly enlarged tergites 2 and 3 which are bent to the posterior at the sides of the abdomen.
Chremistica sp. can be differentiated from species within the abovementioned taxa by its triangular head, prominent post-clypeus, and well developed claspers with lateral and medial lobes.
Species placed within the Tridentigera group are characterised by arc-shaped claspers, long lateral pygofer lobes, and trilobate median black markings on the vertex connected with the black colouration around the eyes.
Cicada higher classification
Based mainly on Australian cicadas, Moulds (2005) carried out an phylogenetic analysis to review the taxonomy of cicada taxa. Cladistic parsimony is used as a tree search criterion to determine the monophyly of cicada taxa, using 117 morphological characters, accounting for 290 states. These morphological characters are identified from both the internal and external anatomy of the tested cicadas. Even though C.umbrosa was not included in this study, some useful insights about its higher classification can be inferred from the produced tree. The area highlighted in grey indicates the tribe that C.umbrosa is nested within, and the species highlighted in green is nested within a sister genus of Chremistica.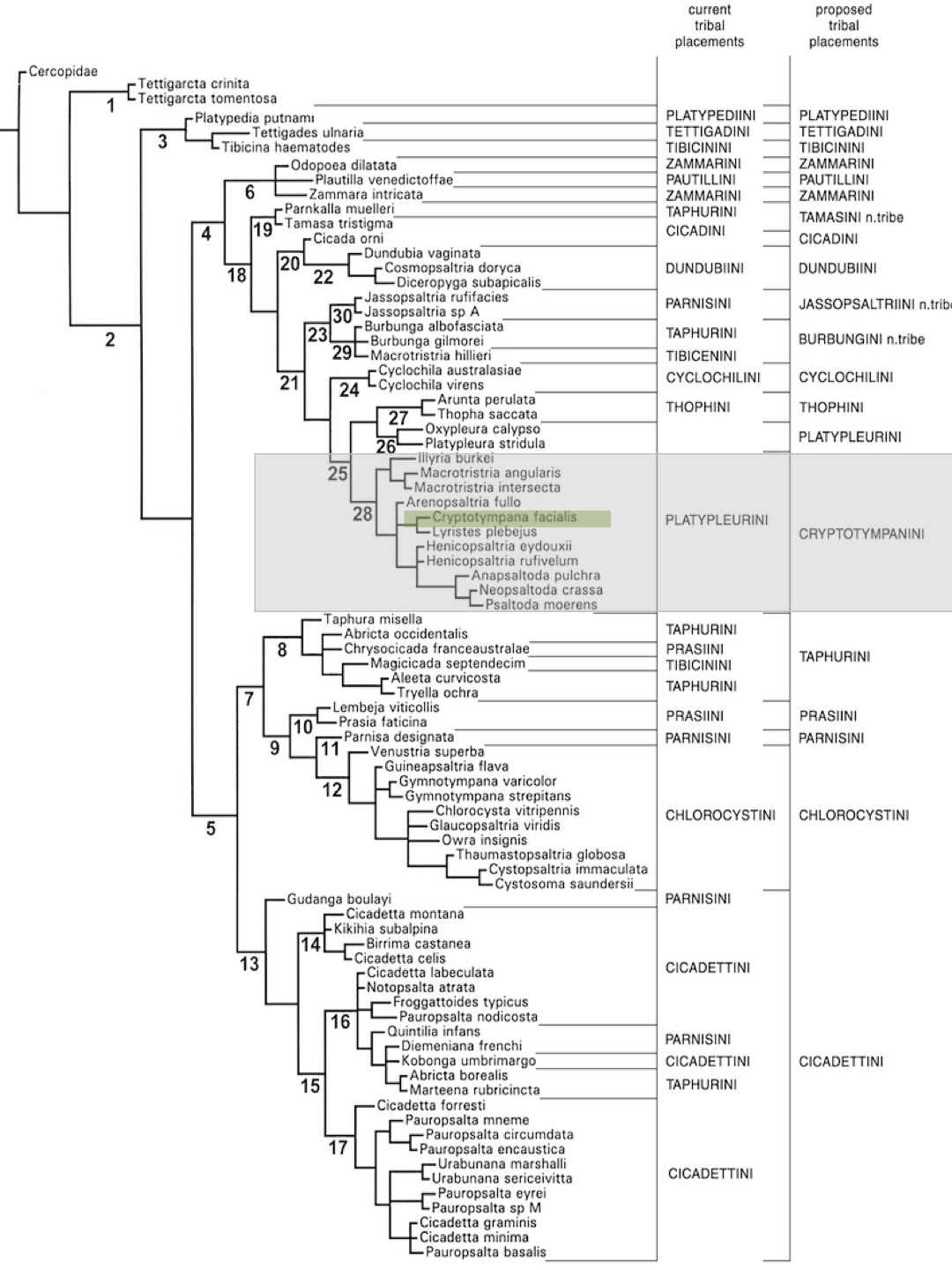 |
| Illustration 7. Strict consensus tree showing current tribal placements for selected species, together with their proposed tribal placements under the revised classifications derived from this study (Moulds, 2005). Illustration: Permission for use granted by Dr Max Moulds. |
How to spot cicadas in Singapore
Cicadas are more heard than spotted. So if you really want to see one, here are a few tips that would increase your cicada spotting chances
- Bring binoculars, or cameras with telephoto lenses attached.
- Go around the late morning or afternoon, in very warm weather!
- Listen out to get an indication of their presence. If there are no cicada songs on a warm afternoon, they are probably not around.
- If you hear them, use their songs to get to a more precise location --> walk towards the source of the songs
- Narrow the source down to one or two trees. If singing stops when you're under a tree, remain quiet and still. The cicadas know you're there. So wait until they start singing again.
- Start by looking at the tree trunks for odd protrusions, or peculiar wing like structures --> these could be resting females!
- If possible, use your hearing to triangulate to a more precise location of the cicada on top of the tree, and use the binoculars to start searching.
- If all these fail, try to locate the cicadas through cicada rain --> stand against the wind direction and look out for the fine purges of cicada urine. Where the cicada rain comes from, there the cicadas are.
Discussion
If you spot any interesting cicadas, or wish to add to or modify anything in this wiki, please feel free to share it with us in this comment area. Your comments are much appreciated! Alternatively, you send an email to elekhoo@gmail.com to do the same.Acknowledgements
Many thanks to the people who had made this wiki possible, both by willingly providing materials and by providing valuable advice. Special thanks to Dr Leong Tzi Ming, for the short but very productive interview, sharing his field experiences with Chremistica umbrosa. My sincere gratitude extends also to Dr Salmah Yaakop, Dr Max Moulds, Wiley-Blackwell, The Robinson Library, Paul Lenhart- these people made the experience of creating of this wiki page very enjoyable!
References
AGR, 2010. Introduction. In Cicada. Retrieved Nov 20, 2012, from http://www4.agr.gc.ca/AAFC-AAC/display-afficher.do?id=1229028581727&lang=eng#img2.
Cheung, W.W.K. & Marshall, A.T., 1973. Water and ion regulation in cicadas in relation to xylem feeding. Journal of Insect Physiology, 19: 1801 - 1816.
Claridge, M.F., 1985. Acoustic signals in the Homoptera: Behavior, Taxonomy, and Evolution. Ann. Rev. Entomol., 30: 297 - 317.
Cooley, J., Marshall, D. & O'Brien, M., 2012. What is a periodical cicada? In Periodical cicada page. Retrieved Nov 20, 2012, from
http://insects.ummz.lsa.umich.edu/fauna/michigan_cicadas/Periodical/
Gogolar, M. & Trilar, T., 2004. Biodiversity of cicadas in Malaysia- A bioacoustic approach. Serangga, 9, 1-2: 63 - 81.
Gullan, P.J. & Cranston, P.S., 2010. The gut, digestion, and nutrition. In The insects: an outline of entomology (4th ed.). UK: Wiley- Blackwell.
Hayashi, M., 1987. A revision of the Genus Cryptotympana (Homoptera, Cicadidae) Part I. Bull. Kitakyushu Mus. Nat. Hist., 6: 119 - 212.
Hennig, R.M., Weber, T., Huber, F., Kleindeinst, H.-U., Moore, T.E. & Popov. A.V., 1994. Auditory threshold change in singing cicadas. The Journal of Experimental Biology, 187: 45 - 55.
Leong, T.M., Aminurashid & Lee, B.P.Y-H, 2011a. Records of the cicada, Chremistica umbrosa (Distant, 1904) in Singapore, with accounts of its mass emergence (Homoptera: Cicadidae: Cicadinae). Nature in Singapore, 4: 163 - 175.
Leong, T.M., Shunari, M., Aminurashid & Harvey-Samuel, T.D., 2011b. The jade-green cicada, Dudubia vaginata (Fabricus, 1787) in Singapore, with notes on emergence, bioacoustics and mating (Homoptera: Cicadidae: Cicadinae). Nature in Singapore, 4: 193 - 202.
Leong, T.M., Shunari, M., Leong, L.Y.K. & Foo, S.K., 2011c. Records of the black and golden cicada, Huechys Fusca Distant, 1892 in Singapore, with natural history observations (Homoptera: Cicadidae: Cicadettinae). Nature in Singapore, 4: 203 - 211.
Michelsen, A. & Nocke, H., 1974. Biophysical aspect of sound production in insects. Adv. Insect Physiol., 10: 247 - 296.
Moore, T.E., 1993. Acoustic signals and speciation in cicadas (Insecta: Homoptera: Cicadidae). In Evolution Patterns and Processes, Linnean Society Symposium No. 14, D.R. Lees & D. Edwards (eds.). London: Academic Press.
Moulds, M.S., 2005. An appraisal of the higher classification of cicadas (Hemiptera: Cicadoidea) with special reference to the Australian fauna. Records of the Australian Museum, 57: 375 - 446.
Mozgai, D., 2012. Cicadas and prime numbers. In Cicada Mania. Retrieved Nov 20, 2012, from http://www.cicadamania.com/cicadas/cicadas-and-prime-numbers/
Salmah, Y., Duffels, J.P. & Zaidi, M.I., 2005. Taxonomic notes of the Malaysian species of Chremistica Stal (Homoptera: Cicadidae). Journal of Asia-Pacific Entomology, 8, 1: 15 - 2.
Simmons, P. & Young, D., 1978. The tymbal mechanism and song patterns of the bladder cicada, Cystosoma saundersii. The Journal of Experimental Biology, 76, 27 - 45.
Sueur, J., Windmill, J.F.C. & Robert, D., 2008. Sexual dimorphism in auditory mechanics: tympanal vibrations of Cicada omi. Journal of Experimental Biology, 211: 2379 - 2387.
Yaakop, S., Duffels, J.P. & Visser, H., 2005. The cicada Genus Chremistica Stal (Hemiptera: Cicadidae) in Sundaland. Tijdschrift vooe Entomologie, 148: 247 - 306.
Young, D. & Bennet-Clark, H.C., 1995. The role of the tymbal in cicada sound production. The Journal of Experimental Biology, 198: 1000 - 1019.