Table of Contents
Clibanarius infraspinatus (Hilgendorf, 1869)
 |
  |
| Orange-striped hermit crab, one of the most common hermit crabs found on the inter-tidal shores and mangroves of Singapore. This animal is almost always spotted using various gastropod shells as their home. Picture by: Ria Tan (top-right) and Tay Yuke Ling (2017). |
|
What is special about them?
- They have a soft and vulnerable abdomen and hence are always found using a gastropod shell as their home. They are not quite picky with their shells and can be found in many different shell types!
- They have bright orange stripes and claws that will greet you when they emerge from their shell. You can easily differentiate them by their unique coloration!
- The males are slightly larger in size than the females during to a difference in energy expenditure. Females spends more energy on reproduction than on growth!
- They live together with many other animals in the same shell. Some are beneficial while some are detrimental to them!
- They are first described using species collected off Singapore. Hilgendorf first described this native species in 1869 using those found on our shores!
Where are they usually found?
In Singapore
This large hermit crab is relatively common on many of our shores, in sandy or silty areas and among seagrasses. Such places includes the seagrass flats of Changi Beach, sand bars of Chek Jawa at Pulau Ubin and at the sinking core of Cyrene Reef. Those present on the Northern shores are seen to be generally larger. Apart from so, they can also be found near river mouths on fine sand or sand-mud bottoms in mangrove areas.
In the map below, reports of Clibanarius infraspinatus sightings and geotagged pictures by Ria Tan of WildSingapore can be seen.
On a global scale
Beyond Singapore, Clibanarius infraspinatus have also been previously reported from the Red Sea, Indian Ocean, Indonesia, North of Australia, Philippine Islands, Vietnam, Philippine Islands, Japan, Taiwan and Thailand [1, 2].
Read on to understand more about this interesting species! Be sure to check out the glossary section below in learning more about certain technical terms.
1. Introduction
1.1 Name
Binomial name: Clibanarius infraspinatusVernacular (common) names: Orange-striped hermit crab, striped hermit crab, 下棘細螯寄居蟹, コブヨコバサミ ('Kobuyokobasami')
1.2 Etymology
Clibanarius infraspinatusThe genus epithet of Clibanarius is said to have derived from latin roots, meaning a solider clad in mail or amour, likely alluding to the gastropod (snail) shell that the hermit crab uses as its home.
The species epithet of infraspinatus derives from latin roots with infra as 'below or underneath' and spinatus from the word spina as 'thorn', possibly referring to the strong lower spine it has.
Orange-striped hermit crabs
Why are hermit crabs called hermit crabs? Do they shy away from others and live in recluse like a hermit? To call these animals a 'hermit' is quite a misnomer when these hermit crabs are anything but hermits! They on the contrary, are very social animals which are often seen in large aggregate colonies or clusters.The term 'hermit' is derived from the act of it carrying its home, often an empty gastropod shell, everywhere that it goes. And as with its vernacular name suggests, this species of hermit crab usually have striking orange stripes lining its walking legs, making it easily recognizable from other hermit crabs.
2. Description
These crab-like animals are not the usual 'true' Branchyuran crabs that we do know, which typically have a reduced abdomen that is folded up and tucked completely under their thorax and are protected by a calcified exoskeleton. Hermit crabs like Clibanarius infraspinatus belongs instead to a subgroup called Anomura in the Order Decapod and have a soft abdomen, absent of any calcified exoskeleton. In order to protect their vulnerable abdomen for protection against predator or to buffer against changes in the external environment (e.g. desiccation risk during drier seasons) [3].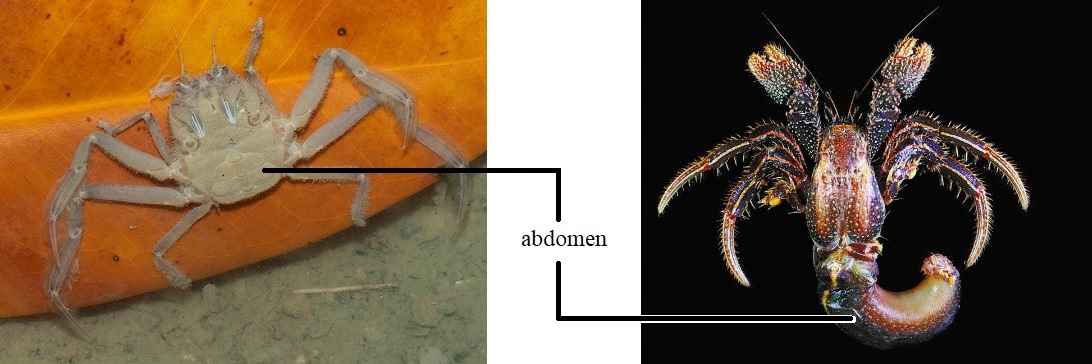 |
| The leaf porter crab (left), a true crab of typically a short projecting "tail" (abdomen) that folds and tucks in completely under its thorax. The Clibanarius infraspinatus, orange striped hermit crab (right), differs from the Brachyuran crab by having an uncalcified abdomen, usually tucked inside a gastropod shell for protection. Photo: Ria Tan (2004) (left) and Teng-Wei Wang (2010) CC BY-NC (right). |
2.1 Morphology
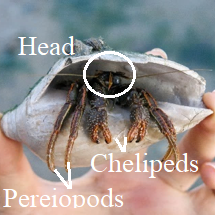
General Body Plan
- When viewing an emerging C. infraspinatus, one would usually observe its cheliped (claws), 2 pairs of pereiopods (legs) and its head.
- The cheliped of the hermit crab is the first pereiopod pair out of the 5 pairs that Decapoda have. These claws or pincers are almost equal in sizes and are used for a range of functions including climbing, foraging and for defense.
- The next 2 pairs of pereiopods are the 2nd and 3rd pair of appendages of the hermit crab, known as the "walking legs".
- The head consists of its compound eyes held out by eye-stalks, as well as antennae and antennules located beside its eyes and and maxillipeds located near its mouth. The top of the head is protected by its shield.
- When viewing an C. infraspinatus without its shell, the rest of its cephalothorax (head-body) region, 2 pairs of pereiopods and abdomen are seen.

Attention: DO NOT attempt to forcefully pull out the hermit crab as
this may rip their appendages or tear their delicate abdomen!
- The head fuses with the body to form the cephalothorax and is covered in calcium carbonate exoskeleton, known as the carapace.
- The 4th and 5th pereiopods of the hermit crab are greatly reduced to aid in gripping of the shell and for maneuvering during movement.
- The soft abdomen that is not protected by an exoskeleton is seen below the cephalothorax. Its abdomen has a distinct curvature, bent like a corkscrew in order to fit into the spirals of the gastropod shells. The abdominal muscles are strong to help grip onto its shell.
- The abdomen ends with an appendage called 'uropod', capable of securing the shell and with a tip bearing its anus, known as the 'telson'.
- An adult usually has a body of 3 to 5 cm long.
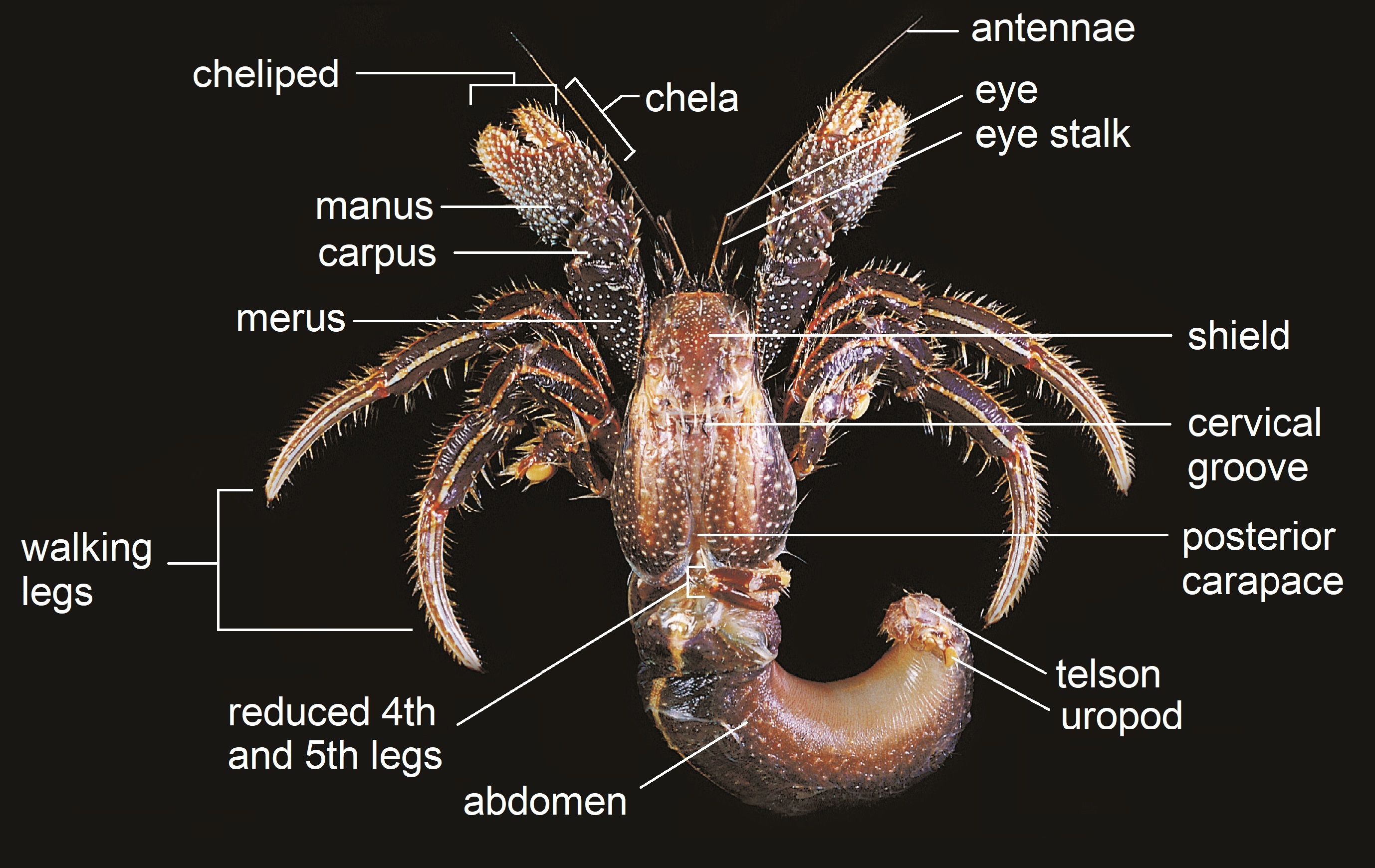 |
 |
| Labelled body plan of an adult Clibanarius infraspinatus, pictured without its shell. Picture: Adapted from Teng-Wei Wang (2010) CC BY-NC |
A naked Clibanarius infraspinatus, making a quick dash into a new shell, fearful of potential predation that may otherwise prove to be fatal. Picture: Ria Tan (2013) |
Colouration
|
 |
|
|
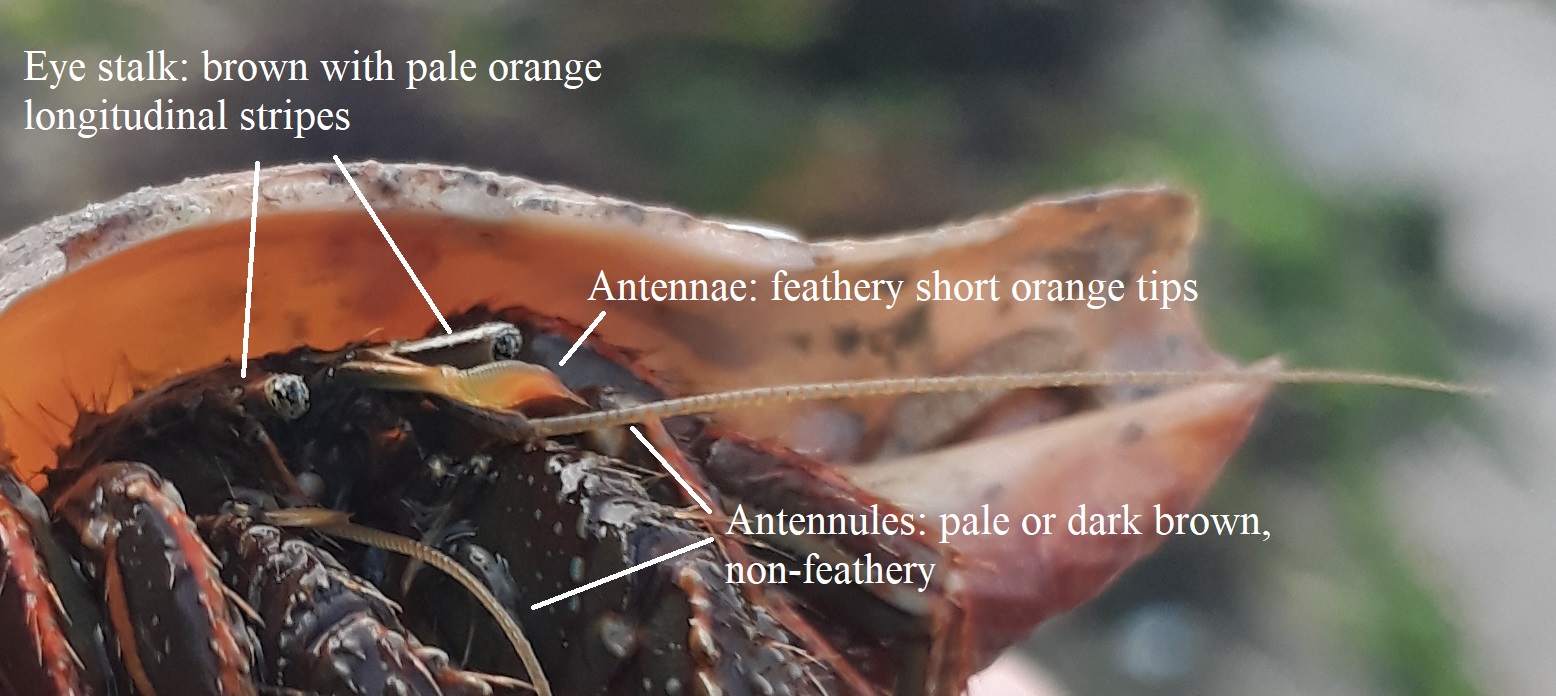 |
Shell
The selection of shell by hermit crabs may depend on a range of different factors, such as shell availability, size or shape and even gender of the hermit crab.
- In looking at the shape it provides, highly spiraled, elongated but yet thinner shells are known to be able to conserve more water and prevent desiccation risk while less spiraled but often tougher shells have thicker walls for greater protection from predators.
- In looking at the gender of hermit crab, ovigerous (egg-bearing) females are predicted to select a larger shell than the optimal shell size [5].
 |
| Different gastropod shells are being used as hermit crab homes, including the spiral melongena (left), noble volute (middle) and pearl conch (right). Picture: Tay Yuke Ling (2017) |
2.2 Diagnosis
Step 1: Differentiating from other genus of hermit crabs (e.g. Diogenes and Dardanus)
| Clibanarius infraspinatus has distinctive almost equal-sized chelipeds and pincers. |
 Picture: Tay Yuke Ling (2017) |
| Diogenes and Dardanus have elongated left chelipeds [4, 7, 8]. |
 Tidal hermit crab (Diogenes sp.), with a larger left cheliped than its right. Picture: Ria Tan (2005)  |
Step 2: Differentiating from other Clibanarius species that are common on our shores
Clibanarius infraspinatus has tell-tale signs of:
Note: since the colouration of hermit crabs of genus Clibanarius is said to remain unchanged throughout their life span [1], it can be used as a reliable character for species differentiation. |
 |
The tawny hermit crab has:
|
 Tawny hermit crab (C. sp.) Picture: Ria Tan (2014) |
The gold-spotted hermit crab has:
|
 Gold-spotted hermit crab (C. cruentatus) Picture: Ria Tan (2012) |
The all-black hermit crab:
|
 All-black hermit crab (C. sp.) Picture: Ria Tan (2010) |
The blue-striped hermit crab has:
|
 Blue-striped hermit crab (C. longitarsus) Picture: Ria Tan (2012) |
In examining the hermit crab closer, observing for presence of strong tooth available on the merus of cheliped, along its ventral margin, can also be used as another distinguishing feature [9].
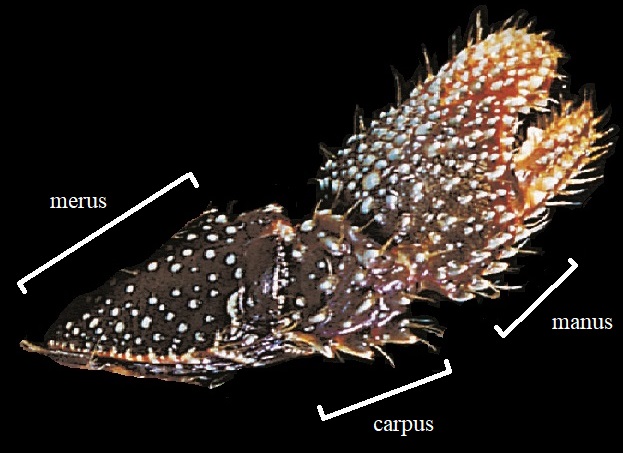 |
| Presence of strong tooth present along the on the cheliped of C. infraspinatus. Picture: Nirmal (2015) Figure 1.1b |
2.3 Sexual dimorphism
Based on a direct visual observation, the chances of telling apart a male from a female can be difficult as there are no distinct colouration differences between the two.Generally, when comparing adults males and females of the same biological age (which can be determined by its shield length), males are usually larger than females in terms of their cheliped lengths and chelae widths [9]. This could be possibly due to their differences in energy expenditure [4, 9].
- Females generally spend more energy investing in ovaries and egg production than males' testes at an expense of its growth. They might also select lighter shells during periods of egg production so that the effect shell weight has on their energy consumption is minimised.
- Males utilises more energy for growth to a larger body size to help play a potential advantage in antagonistic counters against competitors, such as during mating or for shell competition. This may further drive a female preference for a bigger-sized male as it may reflects the male strength, adding a selection pressure for greater-sized males.
Some ovigerous hermit crabs have been noted to be spotted only with Thais lacera and Murex occa shells [3] but this shell type distinction still remains vague. Other factors in shell selection may also come into play in the selection of shell types for both genders.
3. Biology Behaviour
3.1 Reproduction & Life cycle
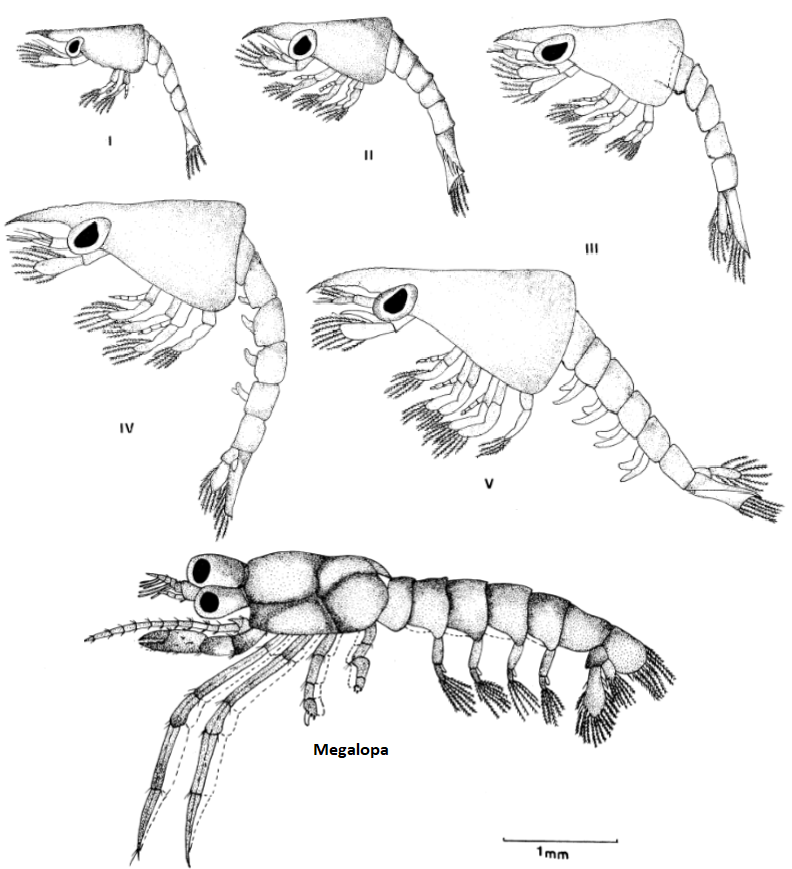
Clibanarius infraspinatus reproduce throughout the entire year. In order to mate, hermit crabs of both genders partially emerge from their shells to allow for transfer of spermatophore from the males to the females. Pre-copulation, the male of C.infrapsinatus may perform tapping and rotating of the female in its gastropod shell into position ideal for the transfer, as seen in a closely related species C. viattus [10]. During copulation, the pereiopods of both genders are often intertwined [10], or in certain cases, the larger male may grasp onto the female's shell using its chelipeds [11]. Spermatorphic masses are attached to the the left side of the females' abdomen, where her eggs are stored and carried. The female carries the eggs as it undergoes the first two larval stages of nauplius and protozoea. As the larvae grows and develop into the third larval stage of zoea, the eggs are ready to be hatched. In order to release the hatching larvae, the female extends her body out of the shell as far as possible and uses her claws to fan them out into the surrounding water. These zoea larvae are planktonic and undergoes four to five stages of growth [12] until it reaches the final post-larval stage of glaucathoe or megalopa. At this stage, the hermit crab appears as a miniature version of an adult C. infraspinatus. During or immediately after this stage, the larvae reduces its swimming activity, settles to the bottom and focuses on finding an appropriate shell to protect its uncalcified abdomen [13]. The larvae spends up to two weeks before it metamorphises into the juvenile C. infraspinatus.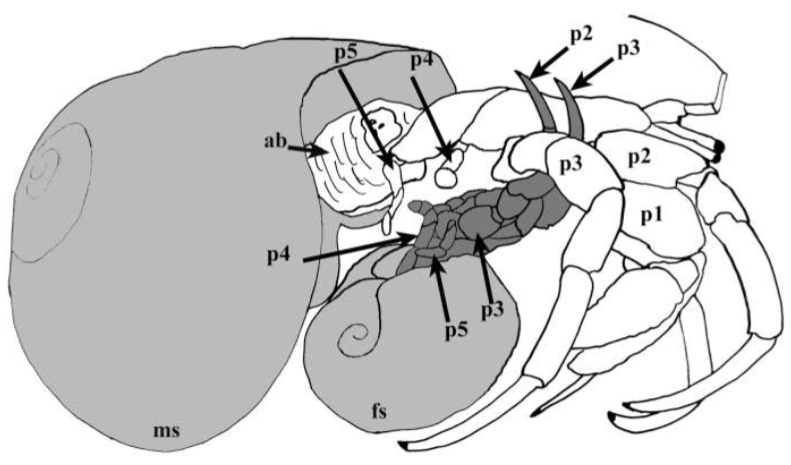 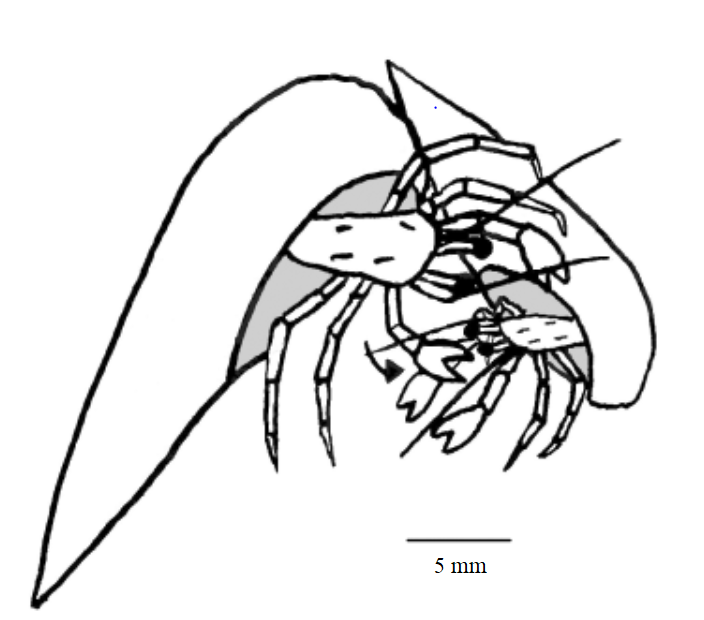 |
 |
| Schematic diagrams of copulatory behaviour in inter-tidal hermit crabs to allow the transfer of spermatophore from the male to the female. The above picture shows the intertwining of both pereiopods (p = pereiopod; ab = abdomen; ms = male shell; fs = female shell) while the below picture shows the grasping of the female's shell by the larger male individual. Picture: Hess & Bauer (2002) Figure 4 (top); Turra (2005) Figure 1 (bottom). |
Illustrations of the dorsal view of different zoeal stages common of a Clibanarius species, which may have either 4 to 5 stages. Following which, a miniature version of the adult hermit crab is seen in its megalopa structure, which must quickly find itself a shell to protect its abdomen. Picture: adapted from Brossi-Garcia (1987): Figures 2 and 12. |
As the hermit crab grows, it undergoes moulting, which is the shedding of its old exoskeleton to grow a new one. With growth in size of the hermit crab, a change into a bigger shell is also necessary because a shell too small would be ineffective in providing protection from predators since they are unable to retreat deep into the shell [4]. A hermit crab does not necessarily always use the same kind of shell. The hermit crab is said to have attained sexual maturity after its puberty moult, whereby the necessary pereiopod and structures needed for reproduction are fully developed.
While the lifespan of a C. infraspinatus has yet to be made known, the lifespan of other Clibanarius in the wild is expected to range from 42 to 47 months [14].
 |
 |
| Moulted exoskeleton left beside an individual after moulting. Moults are recognisable by having 'transparent' eye instead of the usual black eye colouration. Picture: Ria Tan (2009) |
The shell has to big enough for it to retreat and hide deep inside for better protection against predators. Here is a curious one emerging to have a peek! PIcture: Tay Yuke Ling (2017) |
3.2 Movement
The daily movement of this animal depends greatly on the tidal regime, predominantly active at low tides [15]. Larvae C. infraspinatus are free-swimming drifters in the water column while adult C. infraspinatus are mobile hermit crabs, which relies on its cheliped and 2 pairs of pereiopods for locomotion. During movement, the reduced 4th and 5th pereiopods and its abdominal muscles are responsible for grasping tightly to the shells, allowing them to carry their shell wherever they go. Interestingly, they walk 'backwards' as seen in the video below, with their eyes facing the opposite where it is moving towards.3.3 Feeding and diet
Like all other hermit crabs, C. infraspinatus are omnivorous detritivores which scavenge the benthos for food [15]. They rely on their keen sense of smell to locate their food, consuming everything and anything edible ranging from detritus, barnacles and even to carrions (dead animals) [16]. Feeding is done by scooping food using its claws and passing to its maxillipeds. Prior to metamorphisis into post-larval stages, a larval C. infraspinatus filter feeds actively in the water column by constructively creating water current through high speed movements of their maxillipeds [13]. |
| A party of C. infraspinatus and other animals crowding to feast on a rare carrion. Picture: Ria Tan (2010) |
3.4 Social behaviour
Clibanarius infraspinatus are social creatures, preferring to live and forage together in groups. Being in groups not only provides more chances for finding a partner to mate, but also provides opportunity for these animals to spot a more 'desirable' shell and allow attempts for them to steal it from other hermit crabs. While hermit crabs may fight over shells that are more desirable, it is interesting to note that hermit crabs would never kill the original occupant of the shell. |
| An empty big shell draws a big crowd of C. infraspinatus, all ready to fight for a new home. Picture: Ria Tan (2010) |
3.5 Role in ecosystem
Clibanarius infraspinatus plays an indispensable role in the ecosystem, being a non-fussy detritivore responsible for driving nutrient cycling and recycling dead matter quickly on the shore. These animals are in turn eaten by many animals higher up in the food chains, such as fishes, birds or even bigger crabs which can pry them out of their shells. As such, it establishes the important trophic role of hermit crabs as intermediaries linking primary producers to predatory fishes in the estuarine food web and more [4].
Apart from so, C. infraspinatus also plays an important role of providing many animals a home to stay in. In marine habitats where hard substrates such as rocks that are ideal for settlement are hard to come by, shells are often an option for many epibiotic and endolithic animals. Without the active unearthing of gastropod shells played by hermit crabs, shells are often buried in sand by wave action [15]. As mentioned above, there can often be frequent spotting of hitchhikers on the shells of hermit crabs. These animals not only enjoy having a stable substrate to live in, they also enjoy the constant flow of oxygenated water generated from the movement and locomotion of the hermit crab. And to that, hitchhikers also have a reduction in desiccation risk during periods of low tides as the hermit crab would hide in areas which is safe and wet.
 |
 |
 |
| On the shells of hermit crabs, different hitch-hikers exist, such as drill snails (top), sea anemones (middle), and slipper snails (bottom). Picture: Tay Yuke Ling (2017) (left), Ria Tan (2007, 2005) (middle and right) |
||
The benefit of the C. infraspinatus however, is a complex issue because different organisms have a different impact on the hermit crab. In the case of slipper snails, when there are too many of them on the shell of the hermit crab, the added weight slows down the hermit crab and causes a greater exertion of energy in carrying it [15, 17]. Sea anemones on the other hand, while it can be advantageous for the hermit crab to scare away potential predators, may also have a negative impact on the reproductive output of the C. infraspinatus [17]. In another note, C. infrapspinatus have also been noted to be host to a range of parasites such as the Pseudione kensleyi [18] and more.
3.6 Habitat
Clibanarius infraspinatus can be found at estuarine zone, both in the mangrove forests (fine sand or mud-bottom areas) and on the shores (particularly on the lower to mid-littoral zone of sandy or silt areas and among seagrass meadows). Clibanarius species can tolerate low salinities present at such areas better than other Paguroidea, owing to its specialised osmoregulatory mechanisms [7]. These hermit crabs are generally found in the tropices, where the temperature is generally high at 30 to 35 degree Celsius [18], an important factor in deciding the development and metarmorphosis of a Clibanarius larvae [12]. |
| C. infraspinatus can be found at the lower to mid-littoral zones of sandy silty areas on the seashores, commonly around seagrass meadows. Picture: Tay Yuke Ling (2017) |
4. Value to humans
4.1 Economic value
In Singapore, while only land hermit crabs (Coenobita rugosus) are sold in licensed pet stores, aquatic hermit crabs like C. infraspinatus are not fortunately not licensed to be sold. However, there is still an evident interest in its collection among hobbyist and exotic pet collectors that may drive illegal catching of such individuals from the wild and selling them.4.2 Threat status
C. infraspinatus is not listed on the IUCN Red list or Singapore Red List and its threat status is yet to be evaluated. Like other inter-tidal animals, the livelihood of these animals are increasingly threatened by anthropogenic activities such as over-collection of individuals for the pet trade and by hobbyists, over-collection of shells from the beach, by reclamation and pollution.5. Taxonomy & Systematics
Clibanarius infraspinatus was first described in 1869 by Franz Martin Hilgendorf as Pagurus (Clibanarius) infraspinatus. It was published in the chapter of 'Crustaceen' in the book publication of 'Reisen in Ost-Afrika in dem Jahren 1859-1965' by Baron Carl Claus von der Deckens, on page 97 as a footnote [19].| Excerpt taken from 'Reisen in Ost-Afrika in dem Jahren 1859 - 1965' by Baron Cal Calus von der Deckens, containing the original description of Pagurus (Clibanarius) infraspinatus by F. M. Hilgendorf in 1869 (in German). |
- *) Pagurus (Clibanar.) infraspinatus, n. Eyestalk shorter than the frontal width, much shorter than the inner ones, little longer than the outer autennensticle; The end bristle of the outer antennae is not joined to the posterior margin of the cephalothorax. Middle front tooth with a strip-like projection to the rear. Right scissors larger, a strong spine on the underside of the branchial chamber. Carpus above with three dorsals as at P. Clibanarius. Tarsen of the third pair of feet longer than the second, both with a very strong Crista. Singapore, three males and one female. (Berl. Mus. 1600.)
Today, this species is referred to as Clibanarius infraspinatus as it fulfilled Dana's (1852) Clibanarius genus criterion of having near-equal sizes of claws that opens horizontally and by having a small rostrum or triangular median point to the front [20].
5.1 Type information
LocalitySpecimens used by Hilgendorf were collected from Singapore, with three males and one female [19, 21]. Clibanarius infraspinatus are possibly native species of Singapore.
Present deposition
There were no holotypes designated by Hilgendorf in describing C. infraspinatus, although the Singapore specimens were noted to be deposited in the Humboldt Museum in Berlin (Museum fr Naturkundeder Humboldt-Universitt or ZMB) [19, 21]. The digitised catalogue of ZMB presently shows catalogues of C. infraspinatus under the collections of 'GBIF Crustacea- ZMB Berlin', with specimens collected from Singapore by both Andreas Fedor Jagor (catalogue numbers: 1600) and Eduard Karl von Martens (catalogue numbers: 3041, 3417). Considering that Jagor and von Martens are major collectors chiefly responsible for the Southeast Asia specimens that reached Berlin at that time [22, 23], those few specimens may hint at being the specimens used by Hilgendorf.
Syntypes however, are positively deposited at the ZMB [21].
5.2 Synonyms
- Pagurus (Clibanarius) infraspinatus Hilgendorf, 1869: 97
- Clibanarius vulgaris De Man, 1890 (in Notes Leyden Mus. XII., p. 112)
Clbanarius infraspinatus is said to be highly similar to Clibanarius vulgaris in terms of morphological features, where Hilgendorf only distinguishes between the two using the 'arms of the anterior legs not being armed with a spiniform tubercle at the proximal end of the inner margin of the under surface' for C. vulgaris [21].
5.3 Taxonomic hierarchy
The taxonomic navigation of C. infraspinatus seen below can be obtained from WoRMS.| Kingdom |
Animalia |
| Phylym |
Arthopoda |
| Subphylum |
Crustacea |
| Superclass |
Multicrustacea |
| Class |
Malacostraca |
| Subclass |
Eumalacostraca |
| Superorder |
Eucarida |
| Order |
Decapoda Latreille, 1802 |
| Suborder |
Pleocyemata |
| Infraorder |
Anomura MacLeay, 1838 |
| Superfamily |
Paguroidea Latreille, 1802 |
| Family |
Diogenidae Ortmann, 1892 |
| Genus |
Clibanarius Dana, 1852 |
| Species |
Clibanarius infraspinatus Hilgendorf, 1869 |
Based on morphological evidences, individuals are classified under each taxon by fulfilling the criterion of different taxonomic groupings.
- Infraorder Anomura
Despite being labelled a 'crab', C. infraspinatus is not considered a true crab, which belongs to the infraorder Brachyura. Hermit crabs like C. infraspinatus instead belongs to its sister infraorder Anomura, or referred to as Anomala by some authors [24]. These two clades of Anomura and Brachyuran constitutes Meiura [25], which literally translates to having a 'reduced tail'. The clade Meiura is also classified morphologically based on synamorphies such as (a) 1st atennna bearing short asymmetrical flagella (b) Z-shape of exopods on all three maxillipeds (c) chelae present only on the 1st pereiopods (d) similar phenomenon of carcinization in obtaining a crab-like form [26].
- Superfamily Paguroidea
Within the 7 superfamilies of infraorder Anomura, all hermit crabs share the classification of superfamily Paguroidea (Latreille, 1802) with king crabs (lithodids). This classification of superfamily is based on the dextral shell inhabitation in the hermit crabs, or in other words, having an asymmetrical body plan to fit into the spirals of the gastropod shell .
- Family Diogenidae
Being a hermit crab with fingers of near-equal sizes opening in front of them horizontally, C. infraspinatus is placed in the family of Diogenidae (Ortmann, 1892). It is interesting to note that while Anomura has been a well-established monophyletic taxon in the recent two to three decades, the construction of interrelationships in the superfamilies and families remains rather contentious.
- Genus Clibanarius
Characteristic features such as (a) chelipeds equal or subequal, with fingers opening horizontally and ending in spoon-shaped corneous tips (b) presence of 13 pairs of biserial gills (c) elongate, subrectangular shield (d) short rostrum (e) both sexes with four left biramous pleopods on each of pleonal somites 2-5 [27, 28], and more.
- Clibanarius infraspinatus
Defining an individual as C. infrapsinatus is based largely on the unique combination of character states that an individual it has, instead of other evidences such as its monophylyl, evolutionary fate, possibility of interbreeding or even reproductive isolaion. The species concept applied here is thus most probably following the phylogenetic species concept sensu Wheeler and Platnick (2000).
5.4 Phylogeny
Since the past, the exact construct of the hermit crab phylogenetic tree have always been under constant shifts and revisions. Even at family level, the family Diogenidae that C. infraspinatus belongs to, are being placed and re-positioned into different groupings, resulting in drastically different evolutionary relationships among the Anomurans. It is of no surprise that C. infraspinatus has yet to found its placing in any of the phylogenies as more needs to be done before the evolutionary history of C. infraspinatus can be determined.
With an increase in data availability, accessibility and technological advancements, there has been increasing cladistic analyses performed based on a combination of morphological data with molecular data. Doing so, it would provide us with a better and perhaps more accurate visualisation of where C. infraspinatus stands, upon the revelation of the Paguroidea and Diogenidae family. Paguroidea would serve to inform us about the origin of the asymmetrical body plan of C. infraspinatus hermit crab while Diogenidae would inform us more about the origin of the equal sizing of the C. infraspinatus pincers.
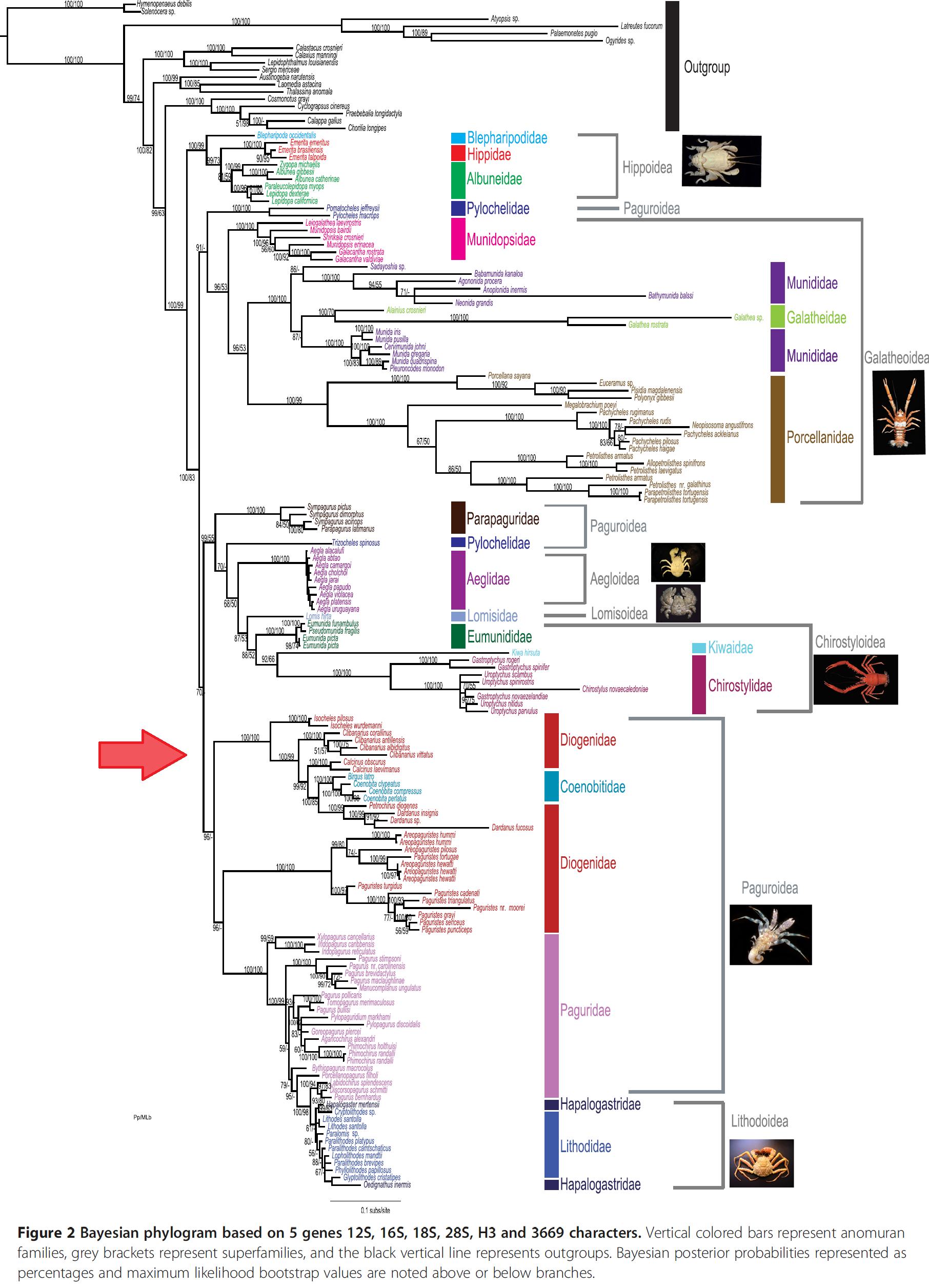
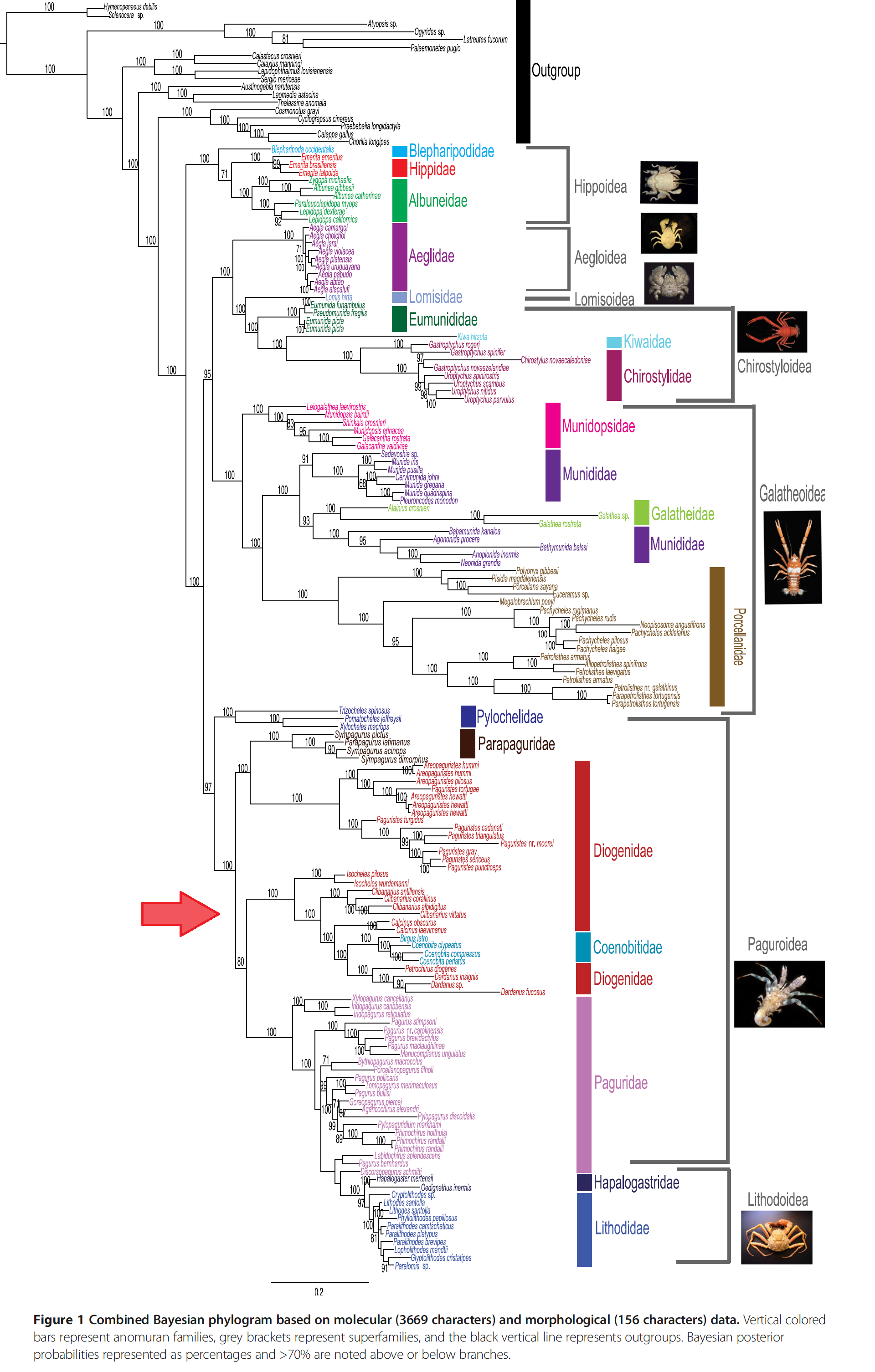
| Over the years, the anomuran interrelationships have been under constant revision as more data are available and advanced cladistic analysis are made possible. This figure shows some of the proposed relationships among the superfamilies and families in Anomura. The Diogenidae family of C. infraspinatus are highlighted in red box. (A) based on Martin & Abele (1986); (B) based on Morrison et al. (2002); (C) based on Perez-Losada et al. (2002); (D) based on Ahyong & O'Meally (2004); (E) based on Porter et al. (2005); (F) based on Macpherson et al. (2005); (G) based on McLaughlin et al. (2007). Picture: Adapted from Figure 2 in Ah yong et al. (2009) |
While there have been multiple studies done on the anomuran evolutionary history, one of the more trustworthy phylogeny construct would be the most recent attempt in 2013 by Bracken-Grissom et al. [29] reconstructed the anomuran evolutionary history through a combination of 3669 molecular characters (from 2 mitochondrial and 3 nuclear markers) and 156 morphological characters.
In their study:
- 19 families of anomurans were studied, of which included 26 species from family Diogenidae and 4 from the Clibanarius genus, C. albidigitus Nobili 1901, C. antillensis Stimpson, 1859, C. corallinus (H. Milne-Edwards, 1848) and C.vittatus Bosc, 1802.
- A Bayesian Inference (BI) of 50% majority-rule consensus was performed on both molecular data alone (Figure 2 below) and on a combination of both molecular and morphological data (Figure 1 below).
- Both non-protein coding and protein coding genes are utilised, ensuring that there is a wider representation of the valuable and informative genes.
- In both of the phylogenetic trees, the 4 closely-related species of C. infrapsinatus are placed in the superfamily Paguroidea and family Diogenidae, of which both are non-monophyletic.These are supported by high Bayesian values.
- Apart from so, the paper has also suggested at:
- Infraorder Anomura origination was possibly in the late Permian (~259 MYA) from a symmetrical crab-like ancestor
- Superfamily Paguroidea origination was possibly after the Late Trissaic period radiation (~205 MYA) from a squat-lobster-like ancestor.
- The consistent paraphylyl and/or polyphyly of Diogeneidae, with other past studies, suggests that in looking at the origin of hermit crabs, there has been independent derivations of the asymmetry in the hermit crabs or dextral shell inhabitation. This could possibly be done through 3 separate clades of Paguridae, Coenobitidae + Diogenidae, and Parapaguridae [30]. One of such origination might have dervied once around 200-187 MYA or 173-167 MYA.
- The paper is however, not without any doubts as follow:
- In alignment of the gene sequences, MAFFT was employed. Considering that there might be possible long leading gaps present due to user input differences that comes from the experiment itself and not because of gene differences, the exact alignment across such a huge quantity of gene sequences might not have been achieved.
- Although non-protein coding genes are utilised, certain homologies were trimmed and excluded and this might have resulted in a loss of valuable piece of information in determining the evolutionary relationships in the anomuran and to C. infraspinatus.
- The usage of Bayesian Inference (BI) analysis in reconstructing the phylogenetic tree, instead of proceeding with their Maximum Likelihood analysis which has already been programmed to run, is dubious. This is especially when the posterior probabilities are grossly overestimated.
5.5 DNA barcoding
C. infraspinatus mitogenome has been sequenced to be of 16,504 base pairs circular DNA, with 67.95% A and T content and contains 13 protein-coding genes, 2 ribosomal subunit genes, 22 transfer RNAS and a putative 1500 bp non-coding AT-rich region [31]. The complete mitochondrial genome can be found on NCBI Nucleotide Database with accession numbers NC_025776.1 and LN626968.1| Gene organization in the C. infraspinatus mitogenome with genes to scale and transcribed in the direction indicated by arrows and abbreviated with the exception of tRNA genes which are assigned their corresponding amino acid letter code. Picture: Gan et al. (2016) |
Glossary
- Antennae: One of a pair of long, slender, segmented appendages on the heads of some animals.
- Antennules: One of a pair of small mobile appendages on the heads of crustaceans in front of the antennae, usually having a sensory function.
- Bayesian Inference (BI): A method of statistical inference in which Bayes' theorem is used to update the probability for a hypothesis as more evidence or information becomes available.
- Benthos: The flora and fauna found on the bottom, or in the bottom sediments, of a sea or lake.
- Biramous: A limb dividing to form two branches and each branch consists of a series of segments attached end-to-end.
- Carapace: Dorsal (upper) section of the exoskeleton or shell.
- Carcinization: A hypothesised process whereby a crustacean evolves into a crab-like form from a non-crab-like form
- Carrions: Decaying flesh of dead animals.
- Cephalothorax: Fused head and thorax region.
- Cheliped: A leg bearing a chela or a pincer.
- Clade: A group of organisms believed to comprise all the evolutionary descendants of a common ancestor.
- Cladistic: An approach to biological classification in which organisms are categorized based on shared derived characteristics that can be traced to a group's most recent common ancestor and are not present in more distant ancestors.
- Desiccation: State of extreme dryness.
- Detritivore: An animal which feeds on dead organic material, especially plant detritus.
- Dextral shell: Coiling clockwise or of right-handedness.
- Ecosystem: Includes all of the living things (plants, animals and organisms) in a given area, interacting with each other, and also with their non-living environments (weather, earth, sun, soil, climate, atmosphere).
- Endolithic: Living inside the surface of plants or living animals.
- Epibiotic: Living on the surface of plants or living animals.
- Estuarine: Pertaining to a partially enclosed coastal body of brackish water with one or more rivers or streams flowing into it, and with a free connection to the open sea.
- Exopods: The outer ramus of a biramous limb of a crustacean.
- Exoskeleton: External skeleton that supports and protects an animal's body.
- Eyestalks: A movable stalk that bears an eye near its tip, especially in crabs, shrimps, and related crustaceans, and in some molluscs.
- Filter-feeding: A form of food procurement in which food particles or small organisms are randomly strained from water.
- Gastropod: More commonly known as snails and slugs, are a large taxonomic class within the phylum Mollusca.
- Genus: a principal taxonomic category that ranks above species and below family, and is denoted by a capitalized Latin name, e.g. Clibanarius.
- Glaucathoe: Final post-larval stage.
- Holotype: A single type specimen upon which the description and name of a new species is based.
- Homology: The state of having the same or similar relation, relative position, or structure.
- Interbreeding: To breed with individuals of another species, subspecies, or variety.
- Inter-tidal: Area that is above water at low tide and under water at high tide.
- IUCN Red List: World's most comprehensive inventory of the global conservation status of biological species.
- Locomotion: Movement or the ability to move from one place to another.
- MAFFT: Acronym for Multiple Alignment using Fast Fourier Transform, which is a high speed multiple sequence alignment program.
- Maxillipeds: Appendages modified to function as mouthparts.
- Maximum Likelihood (ML): Procedure of finding the value of one or more parameters for a given statistic which makes the known likelihood distribution a maximum.
- Megalopa: The final larval stage found in decapod crustaceans.
- Meiura: A clade consisting of two groups of Anomura and Brachyura.
- Metamorphosis: The process of transformation from an immature form to an adult form in two or more distinct stages.
- Mid-littoral: Belonging to or designating the zone of a seashore which is both covered and uncovered by neap tides.
- Mitochondrial: Pertaining to the organelle in an eukaryote cell that acts as the power plants of the cell in making energy.
- Mitogenome: Mitochondrial genome.
- Monophyletic: A group of organisms that forms a clade, which consists of all the descendants of a common ancestor.
- Morphology: The study of form and structure of organisms and their specific structural features.
- Moulting: The process of shedding and replacing a rigid exoskeleton with a new, larger one that allows an animal to grow.
- Native (species): A species that normally lives and thrives in a particular ecosystem.
- Nauplius: The first crustacean larva to emerge from the egg.
- Nutrient cycling: Describes how nutrients move from the physical environment into living organisms, and subsequently are recycled back to the physical environment.
- Omnivorous: Consumption of materials originating from plant and animal origin.
- Osmoregulatory: Active regulation of the osmotic pressure of an organism’s body fluids to maintain the fluid balance and concentration of electrolytes in an organism (homeostasis).
- Ovigerous: Bearing or modified for the purpose of bearing eggs.
- Paraphyletic: A group of organisms that includes an ancestor but not all of its descendants.
- Pereiopod: any of the thoracic appendages of a decapod that are used for walking (and for gathering food).
- Phylogeny: A diagrammatic hypothesis about the history of the evolutionary relationships of a group of organisms.
- Planktonic: Small organisms that float or drift in great numbers in bodies of salt or fresh water.
- Polyphyletic: A group of organisms that are classified into the same group but came from different ancestors.
- Primary producers: Organisms in an ecosystem that produce biomass from inorganic compounds.
- Protozoea: A larval stage preceding the zoea in some decapod Crustacea.
- Ribosomal: Pertaining to ribosomes, which are large and complex molecular machine that catalyzes the synthesis of proteins, referred to as translation.
- Rostrum: A beak-like process or extension of some part.
- Salinity: A measure of all the salts dissolved in water.
- Sexual dimorphism: The differences in appearance between males and females of the same species, such as in colour, shape, size, and structure, that are caused by the inheritance of one or the other sexual pattern in the genetic material.
- Singapore Red List: a objective and scientific system for determining threat status of species in Singapore.
- Species: the lowest taxonomic rank, and the most basic unit or category of biological classification.
- Species concept: A set of species limitation set to define a taxa.
- Spermatophore: A protein capsule containing a mass of spermatozoa, transferred during mating.
- Synamorphies: A characteristic present in an ancestral species and shared exclusively (in more or less modified form) by its evolutionary descendants.
- Syntypes: A collection of specimens that together serve as the definition of the species where a holotype was never selected.
- Synonyms: A scientific name that applies to a taxon that now goes by a different scientific name, although the term is used somewhat differently in the zoological code of nomenclature.
- Taxon: A taxonomic group of any rank, such as a species, family, or class.
- Telson: Posterior-most division of the body of an arthropod.
- Uropod: Flattened lateral appendages of the last abdominal segment of a crustacean.
- Zoea: A free-swimming planktonic larval form of many decapod crustaceans and especially crabs that has a relatively large cephalothorax, conspicuous eyes, and fringed antennae and mouthparts.
Links to other useful sources
- BOLD systems
- Encyclopedia of Life 2982606
- GenBanks NCBI Nucleotide Database:
- GBIF ID 4311775
- The National Checklist of Taiwan (TaiBNet) ID 310937
- OBIS Taxon ID 423946
- WildSingapore
- WoRMS Aphia ID 208960
- uBio Portal ID 6176234
References
- Henderson, J. R., 1915. Hermit-crabs from the Chilka Lake. Records of the Indian Museum, 11: 25-29.
- Rahayu, D. L., 1996. Notes on littoral hermit crabs (excluding Coenobitidae)(Crustacea: Decapoda: Anomura) mainly from Singapore and peninsular Malaysia. The Raffles Bulletin of Zoology, 44(2): 335-356.
- Teoh, H.W., 2014. Ecology of hermit crabs (family diogenidae) in Matang mangrove estuary and adjacent coastal waters / Teoh Hong Wooi. PhD thesis, University of Malaya, Malaysia.
- Morton, B. & J. Morton, 1983. The sea shore ecology of Hong Kong (Vol. 1). Hong Kong University Press, Hong Kong. 156 pp.
- Hazlett, B. A., D., Rittschof & C. E., Bach, 2005. The effects of shell size and coil orientation on reproduction in female hermit crabs, Clibanarius vittatus. Journal of Experimental Marine Biology and Ecology, 323(2): 93-99.
- Ajmal Khan, S. & R. Natarajan, 1984. Hermit crabs of Proto Novo coast. Records of the Zoological Survey of India, Occasional Paper, 67: 25
- Nirmal, T., 2015. A taxonomic study of hermit crabs along selected districts of Maharashtra Coast. M. F. Sc. Dissertation, Central Institute of Fisheries Education, Versova, Mumbai.
- McLaughlin, P. A., D. L. Rahayu, T. Komai & T. -Y. Chan, 2007. A Catalog of the Hermit Crabs (Paguroidea) of Taiwan. National Taiwan University, Keelung, Taiwan. 376 pp.
- Teoh, W.H. & V.C. Chong, 2015. Allometric relationships and sexual dimorphism in three ubiquitous hermit crab species (Anomura, Diogenidae) from a tropical mangrove estuary. Crustaceana, 88(10-11): 1127-1138.
- Hess, G. S. & R. T. Bauer, 2002. Spermatophore transfer in the hermit crab Clibanarius vittatus (Crustacea, Anomura, Diogenidae). Journal of Morphology, 253(2): 166-175.
- Turra, A., 2005. Reproductive behavior of intertidal hermit crabs (Decapoda, Anomura) in southeastern Brazil. Revista brasileira de Zoologia, 22(2): 313-319.
- Brossi-Garcia, A. L., 1987. Morphology of the larval stages of Clibanarius sclopetarius (Herbst, 1796)(Decapoda, Diogenidae) reared in the laboratory. Crustaceana, 52(3): 251-275.
- Harms, J., 1992. Larval development and delayed metamorphosis in the hermit crab Clibanarius erythropus (Latreille)(Crustacea, Diogenidae). Journal of Experimental Marine Biology and Ecology, 156(2): 151-160.
- Turra, A. & F. P. Leite, 2000. Population biology and growth of three sympatric species of intertidal hermit crabs in south-eastern Brazil. Journal of the Marine Biological Association of the United Kingdom, 80(6): 1061-1069.
- Hazlett, B., 1981. The Behavioral Ecology of Hermit Crabs. Annual Review of Ecology and Systematics, 12: 1-22.
- Gherardi, F. & E. Tricarico, 2010. Chemical ecology and social behavior of Anomura. In: Breithaupt T., Thiel M. (eds), Chemical Communication in Crustaceans. Springer New York, New York, NY. 297-312 pp.
- McDermott, J. J., J. D. Williams, & C. B. Boyko, 2010. The unwanted guests of hermits: a global review of the diversity and natural history of hermit crab parasites. Journal of Experimental Marine Biology and Ecology, 394(1): 2-44.
- Williams, J. D. & L. M. Schuerlein, 2005. Two new species of branchial parasitic isopods (Crustacea: Isopoda: Bopyridae: Pseudioninae) from hermit crabs collected in Singapore. Proceedings of the Biological Society of Washington, 118(1): 96-10.
- Kunsook, C. & P. Dumrongrojwatthana, 2017. Species Diversity and Abundance of Marine Crabs (Portunidae: Decapoda) from a Collapsible Crab Trap Fishery at Kung Krabaen Bay, Chanthaburi Province, Thailand. Tropical life sciences research, 28(1): 45.Briffa, M. & S.L. Mowles, 2008. Hermit crabs. Current Biology, 18(4): R144-R146.
- Hilgendorf, F., 1869. Crustaceen. In K. Otto (eds), Baron Carl Claus von der Decken's Reisen in Ost-Afrika in den Jahren 1959 bis 1865, volume III. C. F. Winter'sche Verlagshandlung, Leipzig & Heidelberg, Germany. Pp. 69–116.
- Dana, J. D., 1852. Crustacea. Part I. United States Exploring Expedition. During the Years 1838, 1839, 1840, 1841, 1842. Under the Command of Charles Wilkes, U. S. N. (Vol. 13). C. Sherman, Philadelphia (reprinted Antiquariaat Junk, Lochem, 1972). 1-685 pp.
- Man, J.G., 1888. Report on the Podophthalmous Crustacea of the Mergui Archipelago, collected for the Trustees of the Indian Museum, Calcutta, by Dr. John Anderson, FRS, Superintendent of the Museum.—Part V. Zoological Journal of the Linnean Society, 22(140): 241-305.
- Das, I., & A. A. Tuen, 2016. Naturalists, Explorers and Field Scientists in South-East Asia and Australasia (Vol. 15). Springer.
- Davie, P. J. F., 1983. Zoological Catalogue of Australia, volume 19.3B (Crustacea: Malaccostraca: Eucariadia (Part 2). Decapoda – Anomura, Brachyura). Csiro Publishing, Australia. 45 pp.
- McLaughlin, P. A., & Holthuis L. B., 1985. Anomura versus Anomala. Crustaceana, 49(2): 204-209.
- Scholtz, G. & S. Richter, 1995. Phylogenetic systematics of the reptantian Decapoda (Crustacea, Malacostraca). Zoological Journal of the Linnean Society, 113(3): 289-328.
- Borradaile, L.A., 1916. Crustacea. Part II. Porcellanopagurus: An instance of carcinization. In: British Antarctic ("Terra Nova") Expedition, 1910. Natural History Report. Zoology. 3(3): 111-126.
- Rahayu, D. L., & J. Forest, 1992. Le genre Clibanarius (Crustacea, Decapoda, Diogenidae) en Indonésie, avec la description de six espèces nouvelles. Bulletin du Muséum National d’Histoire Naturelle, Section A, Zoologie, Biologie et Ecologie Animales, Paris 14(3-4):745-779
- Forest, J. & M. de Saint Laurent, 1968. Résultats scientifiques des campagnes de la “Calypso”, Part VII. Campagne de la Calypso au large des côtes Atlantiques de l’Amérique du Sud (1961–1962). 6. Crustacés Décapodes: Pagurides. In: Annales de l’Institut Océanographique de Monaco. Monaco, Paris, Masson. 45-172 pp.
- Bracken-Grissom, H. D., M. E. Cannon, P. Cabezas, R. M. Feldmann, C. E. Schweitzer, S. T. Ahyong, D. L. Felder, R. Lemaitre & K. A. Crandall, 2013. A comprehensive and integrative reconstruction of evolutionary history for Anomura (Crustacea: Decapoda). BMC Evolutionary Biology, 13(1): 128.
- Ahyong, S. T., K. E. Schnabel & E. W. Maas, 2009. Anomuran phylogeny: new insights from molecular data. Decapod crustacean phylogenetics, 18: 399-414.
- Gan, H. Y., H. M. Gan, M. H. Tan, Y. P. Lee & C. M. Austin, 2016. The complete mitogenome of the hermit crab Clibanarius infraspinatus (Hilgendorf, 1869), (Crustacea; Decapoda; Diogenidae) – a new gene order for the Decapoda. Mitochondrial DNA Part A, 27(6): 4099-4100.