(Hymenoptera: Formicidae: Formicinae)

Overview
Dinomyrmex gigas is one of the largest ants in the world and the largest in Southeast Asia [17][21]. It is also more commonly known as the giant forest ant. This large charismatic ant can be found in the forests of Singapore and Southeast Asia, however it is not often sighted by the public due to its predominant nocturnal activity [17][20][21]. Despite being considerably well studied compared to its smaller counterparts, most people know little about the giant forest ant beyond its large size. Most studies on this species focuses on the populations found in Malaysia, Sabah [19][20][21][22][23][27] and Brunei [17][18]. These studies have characterized many aspects of the natural history and biology of this species including its foraging behaviour [16][19][20], diet [16][20][22], territoriality [21], reproduction [18] and colony structure [18][20][21]. While much is known about the biology of this species in Sabah and Brunei, little is known about the populations in Singapore, where small and fragmented forest habitats may have an impact on the viability and behaviour of this majestic giant ant of the forest.
Table of Contents

Figure 2. Dinomyrmex gigas photographed in the Central Catchment Nature Reserve (natural habitat) at night. Image © Pierre Escoubas (Used with permission)
There is some confusion in the scientific name of this species, with most people knowing it by the latin name Camponotus gigas. Until the start of 2016, this ant was previously known as Camponotus gigas, under the subgenus Dinomyrmex, before new phylogenetic studies concluded that it was from a distinct lineage, resulting in the resurrection of the genus Dinomyrmex and the new combination Dinomyrmex gigas and Dinomyrmex gigas borneensis [25] as recognised latin names for the giant forest ant.
General Information
Latin name: Dinomyrmex gigas (Latreille, 1802)Common name: Giant forest ant / Malaysian giant ant / Giant ant
Distribution
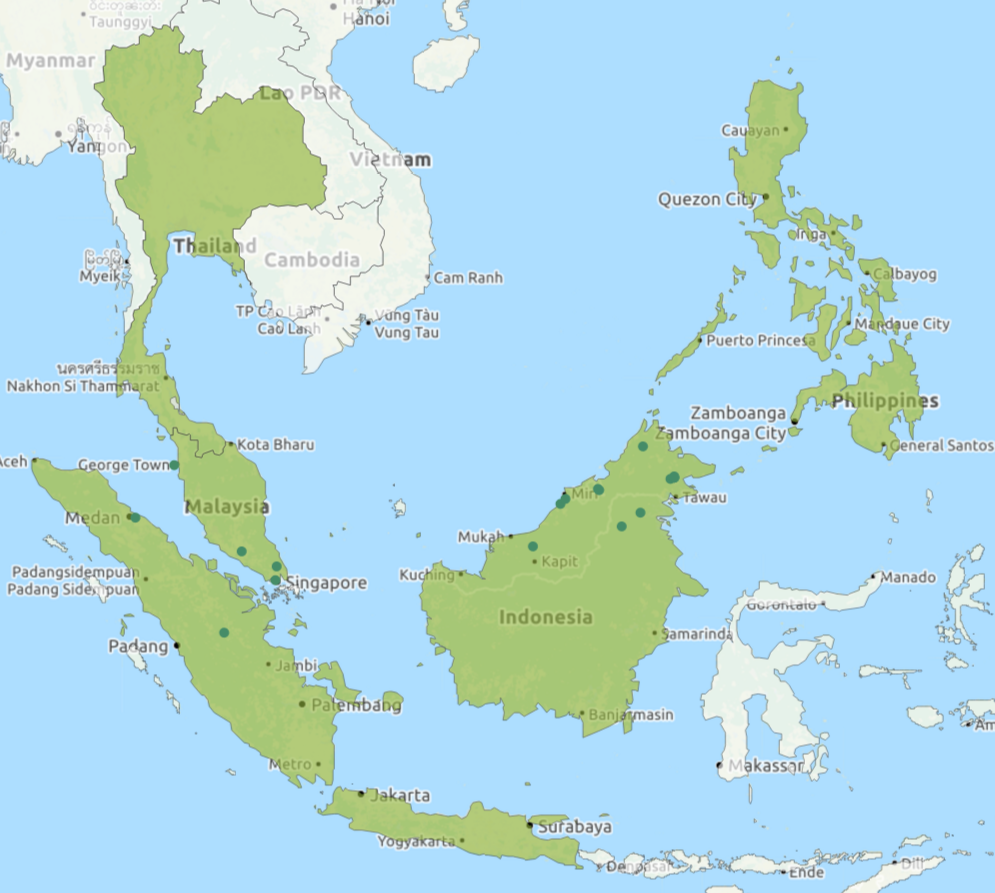
The giant forest ant is a common inhabitant of the rainforest in Southeast Asia from Thailand, Sumatra, Borneo, Malaysia to Singapore, with one recognized subspecies, Dinomyrmex gigas boreensis found in South Borneo [21]. It is found in a range of habitats from the peat swamps of a mangrove forest and up to montane forest 1500m above sea level [21].Figure 3. Map showing distribution of Dinomyrmex gigas retrieved from antmaps.org (pending permission). Note that the giant forest ant is not found in Philippines (Christian Peeters, pers comm.) The green dots on the map represent physical specimens that have been collected at that location. Click on image to see an interactive version of map with GPS coordinates.
How to identify the Giant Forest Ant in Singapore
The giant forest ant is one of the largest ants in the world comparable to Paraponera clavata (Bullet ant) and Dinoponera gigantea with head widths of more than 4mm [21] and the biggest ant you can find in Singapore and Southeast Asia! It is about the size of a one dollar coin (Figure 4) and can only normally be found in a good forest patch at night!
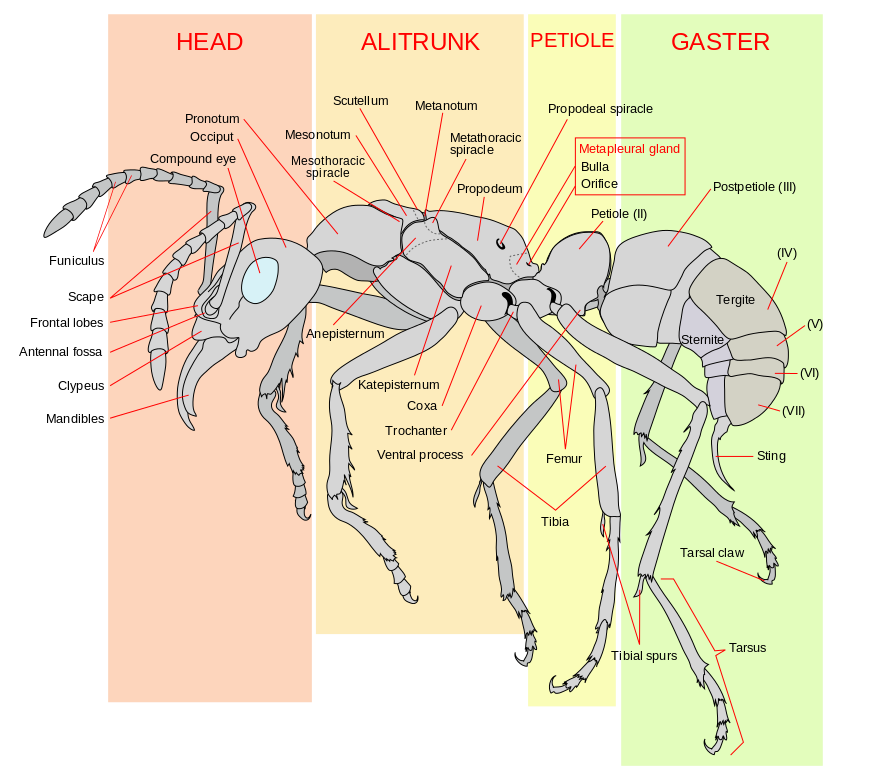
Morphology of ants
The image below gives a quick look at the morphology of an ant from the side view. The presence of a 'constricted waist' differentiates ants (Formicidae) from most other Hymenoptera. Note the following parts on the picture that will be important in the more accurate and scientific diagnosis to recognize Dinomyrmex gigas: The position of the metapleural gland for the species and the femur for the separation between species and subspecies.
Diagnosis
The following combination of features are used to identify the giant forest ant, Dinomyrmex gigas [25](i) Its large size. (Figure 4)
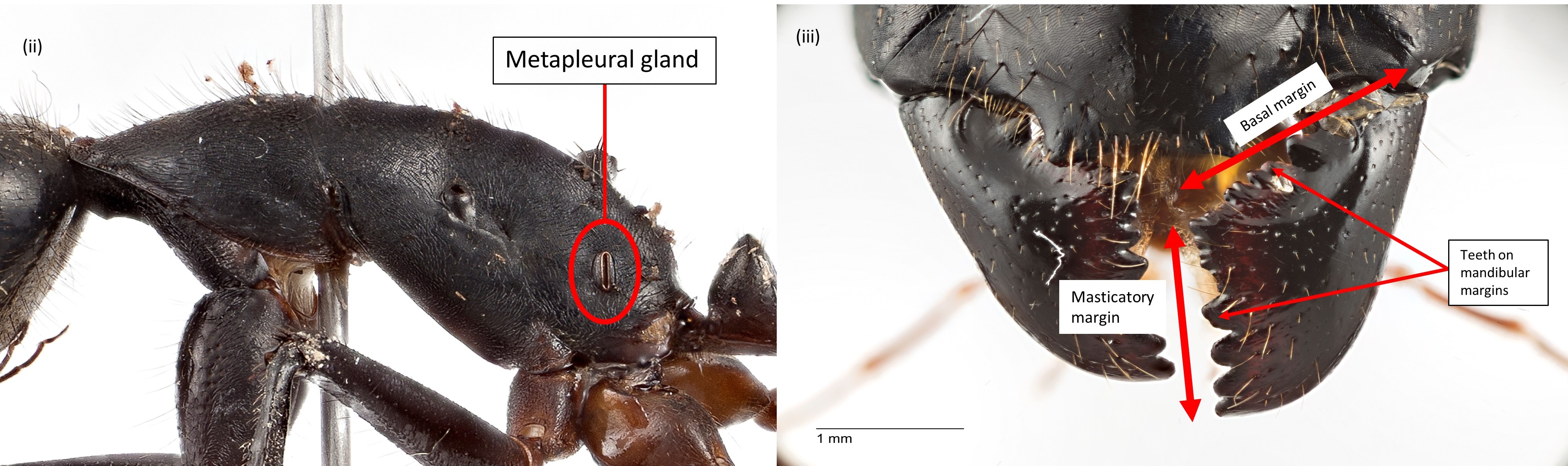
(ii) Presence of metapleural gland on the propodeum.
(iii) Presence of teeth on the masticatory and basal margin of the mandibles.
The following feature can be used to distinguish between the species Dinomyrmex gigas and its subspecies Dinomyrmex gigas borneensis
(i) The presence of yellow femur on the all legs of Dinomyrmex gigas borneensis compared to the dark brown femurs on all legs of Dinomyrmex gigas

Eusociality and Caste system
The term ‘eusocial’ was first coined by Suzanne Batra in 1966 to describe the nesting behaviour in Halictine bees. The terminology was subsequently extended to include other social insects such as ants, wasps and termites by E. O Wilson in 1971 [26]. He described the following three main features in ‘eusocial’ organisms:
1. Reproductive division of labour (with or without sterile caste)
2. Overlapping generations
3. Cooperative care of young
Most eusocial insects have a caste system of reproductive females and males and sterile workers that take care of the brood and soldiers that protect and defend the colony [13]. The castes in Dinomyrmex gigas includes the alates (winged reproductive males and females) [2], gynes (female reproductive or queen of the colony) [2] and bimorphic workers with major and minor workers in two separate subcaste. On average, major workers weighed 372 mg while minor workers weighed 135 mg [20][21]. Both minor and major workers exhibited size polymorphism while each caste differed in allometric growth of the head [21].





 Figure 8. Image of the different caste of Dinomyrmex gigas. Click on the red labels to look at head, top and side view of each subcaste. Images © Gordon Yong Wenjie
Figure 8. Image of the different caste of Dinomyrmex gigas. Click on the red labels to look at head, top and side view of each subcaste. Images © Gordon Yong WenjieFemale alate
The female winged reproductive of the species, the female performs mating flights and mates with male alates before losing its wings to become a gyne and found a new colony. Notice the swollen gaster of the female alate, it is swollen with eggs and food reserves, this is also known as a physogastric gaster. The female also has three ocelli on the top of its head and an enlarged thorarcic wing segment that house muscles that are essential for flight [24].

Figure 9. (a) Front view (b) lateral view (c) top view of Dinomyrmex gigas alate with scale bars attached. Images © Gordon Yong Wenjie
Major Worker
The major worker is a sterile female whose primary role is to defend the nest and protect foragers. Notice the large heart shaped head of the major and the large mandibles with sharp mandibular teeth! The major workers also possess a single reduced ocellus on the top of its head, its function however is still a mystery but suggests a distinct development pattern from minor worker (Christian Peeters, pers comm.). These ants are also rarely involved in foraging [17][21] but perform colony border patrol and ritualistic fights [22] and have a strong bite that would result in a small cut on human hands (Gordon Yong, pers obs.). Figure 10. (a) Front view (b) lateral view (c) top view of Dinomyrmex gigas major worker with scale bars attached. Images © Gordon Yong Wenjie
Figure 10. (a) Front view (b) lateral view (c) top view of Dinomyrmex gigas major worker with scale bars attached. Images © Gordon Yong WenjieMinor Worker
Large Minor Worker
Minor workers are highly polymorphic with a big size range of average head width 3.56mm (SD=0.53mm). They lack ocelli unlike the major workers or alates. Minor workers are also most involved in foraging and transporting of food from nest to nest [20][21]. Note the more rectangular-ovalish head shape compared to the heart shape of the major worker, these two caste are often confused for each other.

Figure 11. (a) Front view (b) lateral view (c) top view of Dinomyrmex gigas large minor worker with scale bars attached. Images © Gordon Yong Wenjie
Small Minor Worker
Note the smaller size and the more ovalish head shape for the smaller minor worker compared to the large minor worker. Small minor workers do not grow up to become large minor workers but remain the same size throughout their lifespan.

Figure 12. (a) Front view (b) lateral view (c) top view of Dinomyrmex gigas small minor worker with scale bars attached. Images © Gordon Yong Wenjie
Male alate
The male alate is the winged reproductive male of the colony and engages in mating flights to with female alates. Male alates are not always produced by the colony and are only produced for the sole purpose of mating with winged females, they have a short lifespan of about 1 to 8 days [19]. Note the small head compared to the body and the enlarged thoracic wing segment on the thorax (alitrunk) that house the muscles for mating flights. The males also have 3 well developed ocelli on the top of the their heads that are essential for flight.

Figure 13. (a) Front view (b) lateral view (c) top view of Dinomyrmex gigas male alate with scale bars attached. Images © Gordon Yong Wenjie
Natural history and biology
Colony structure and foraging range
The giant forest ant has a moderately sized colony compared to other ant species, however its large size allows it to forage over a much greater area. The best studied colony in Sabah had a population of about 7000 workers with a foraging range of 0.8 hectares. Numerous studies concluded the giant forest ants to have polydomous colonies, with workers of each colony distributed unevenly among numerous nests [20][21]. These nest were most often found in the soil between the buttress roots of emergent trees and also found in the hollows of fallen or decaying tree trunks and at the base of smaller trees [21]. Excavation of a queenright nest in Brunei also yielded 784 minor workers, 45 major workers, 2 male alates, 157 larvae and 34 eggs [18]. The giant forest ant was found to be monogynous, with only one queen per colony. Excavation of nests in Sabah also revealed some ant guests staying in the nest of the gigas, namely a myrmecophilous cricket, Camponophilus irmi Ingrisch 1995 and a cockroach, Eroblatta borneensis Shelford [21].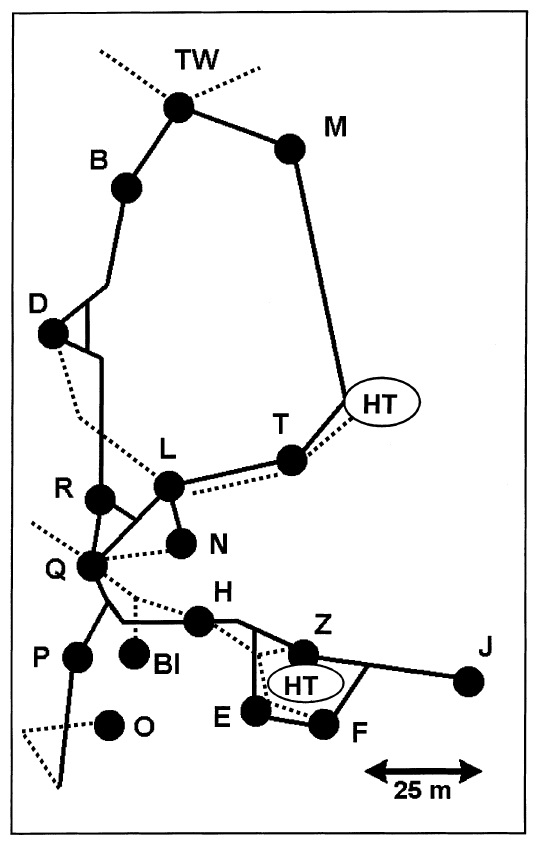
Figure 14. Image showing the distribution of nests of a single colony studied in Sabah. Each black dot represents a single nest, with Q being the nest with the queen of the colony. Black solid lines represent artificial trails made of bamboo used to link the nests and broken lines represent natural trails in the canopy that were abandoned by the giant forest ants after bamboo trails were introduced. HT represents the foraging trees with large groups of associated trophobionts. Figure from Pfeiffer & Linsenmair, 1998 [20] (Fair use).
Foraging
The giant forest ant is active and forages predominatly at night [17][20][21]. Some reasons hypothesized to explain its nocturnal activities include the high temperatures and low humidity in the canopy where they forage in the day, the need to reduce predation on Phorid flies that need light to lay their eggs in the wounds of majors and to avoid competition for foraging trails such as trunks and branches, which are used by other diurnal ant species for foraging during the day [20]. The giant forest ants also use a system of dispersed central foraging as a result of their polydomous colonies. Such a system involves the foragers bringing food back from a food source such as a trophobiont associated tree, into satellite or 'source' nest within their territory such as nest E and Z in Figure 11 on one night. Trophollaxis from these foragers to specialized transporter ants waiting in these nest then occur before these specialized transporter ants carry the food from the 'source' nests to the 'sink' nest where the queen and most of the brood are found [21].

Before the start of each foraging night, large number of ants have been observed to accumulate at the entrance of each nest (Christian Peeters, pers comm.) at dusk, before the sudden leaving of these foragers in large numbers from the nest within a short span of 50 min [20][21]. This sudden leaving of foragers have been termed as an exodus by researchers describing this phenomenon [21]. The exodus of foragers often climb into the canopy, where they use a network of natural trunk trails such as tree branches, fallen trees, bamboo and vines as highways to navigate to foraging trees with large groups of associated trophobionts in Sabah [21][23]. In Brunei, foragers have been observed to travel quickly on such highways from a fixed distance, before ending in a slow meandering search pattern at the end of such highways [17].

Diet
In Sabah, the giant forest ants collect honeydew as their main food source but also take some bird dropping and other insects such as winged termites to supplement nitrogen intake. It was suggested that rainfall may affect the items they collected for nitrogen supplements, with termites being collected after heavy rains that wash away the bird droppings and bird droppings collected when there was no or little rain [21]. These ants collect honeydew in large amounts from different trophobiotic associations with wax cicadas, Membracidae, Fugloridae and and Coreidae [21][23]. The giant forest ant also frequently collects extrafloral nectar, plant sap and fed on rubber directly from open wounds on the plant [21]. In Brunei however, fungi was found to be the main bulk of the diet of the giant forest ant, supplemented by arthropods, small fruits and seeds [17]. In Singapore, the giant forest ant has been observed to be foraging in large numbers up a tree at night and coming down with full gasters, suggesting the collection of honeydew (Gordon Yong, pers obs.). The ants were also observed to collect insect parts, termite alate without wings and bird droppings on separate ocassions (Gordon Yong, pers obs.).

For its main food source of honeydew, the giant forest ants depend on the excretions of Homoptera, hence forming a trophobiotic interaction with these insects. In particular, Dinomyrmex gigas was observed to herd but not protect Bythopsyrna circulata (wax cicadas) and provide protection to Mictis cf. longicornis (coreid bugs) to obtain honeydew from these insects. Three distinct behavioural patterns were observed in the trophobiotic association of the giant forest ant with wax cicadas. The first was collecting which involved the flicking of honeydew droplets by the wax cicada and the catching of the flicked droplet using the head or leg of the waiting ant. The wax cicada has no specific morphological adaptations to control the output of honeydew droplets, resulting in the flicking of the droplet. The second pattern is antennating from ahead which involves an ant sitting laterally or above the cicada while antennating its front part. The last pattern was secondary gathering which involved a worker ant shuttling between different collector ants and grooming them when needed. The grooming involved gathering the droplets that had hit them and that they were unable to collect by themselves. These worker ants also frequently prompted the collectors to perform trophallaxis to transfer collected honeydew from the collector to the worker before heading back to the nest once they are full [23].
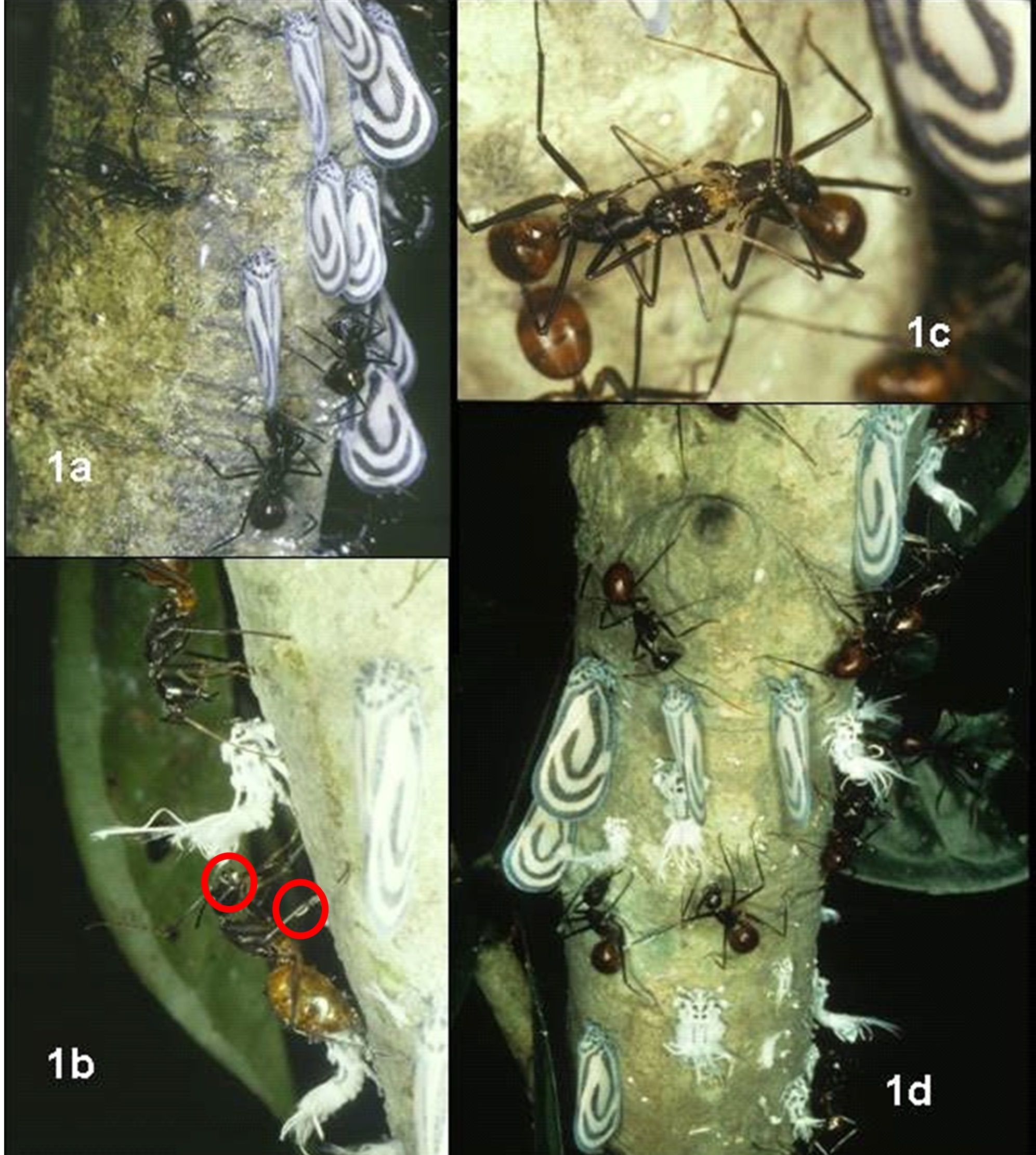
Territoriality
With a large foraging range, the majors of the giant forest ants conduct patrols along foraging trails to defend its territory from invaders, ants and other insects. These patrols normally occur throughout the night while the foraging trail are still active, with the majors only retreating in the morning. To defend key points important for food or access to the canopy, sentinels in the form of a group of 3 to 4 major workers are often deployed at these points at the start of foraging. These major workers would stand guard throughout the night only retreating in the morning when trail activity ceases [22].

Due to its large foraging range, it is inevitable that the giant forest ant has to deal with threats from intraspecific (other colonies of D. gigas) and interspecific (other ant species and insects) threats. In defending its territory from interspecific ants, the giant forest ants almost always fought violently with intruders spraying acid and using poison glands, often leading to death from both sides. However, in dealing with intraspecific ants, the major workers of each colony ants engage in ritualistic one-to-one battles with one another. These battles can rage on for as many as 30 days in what is known as an ‘ant war’ but rarely result in the death of either party. Only majors are involved in these battles. Each battle between one pair of ants always followed a steady pattern of behaviours starting with the meeting of both aggressor and the defending sentry. Occasionally, the aggressor would drum the ground with its gaster producing an audible sound before both parties open their mandibles and raised its first pair of legs. Once the aggressor touches the defender, the fighting begins. Intense antennation of up to 3-4 Hz and vibrating gasters occur throughout the fight. Both ants also raised their body with open mandibles to threaten each other in a “slit-like” posture (Figure 20). Front leg boxing then occurs with the continuous sweeping of front legs alternatively up and down to produce a “paddling” motion. Very often, the fight is decided at this stage, with the ant that held its leg up longer becoming the winner of the ‘round’ and the loser retreating immediately. However, occasionally the fight progresses into mandible grabbing with each ant trying to grab and drag the other ant on the ground in a tug-of-war. A single win however does not stop the confrontation, with the majors taking a break by retreating to their territories and grooming their antennae and legs before returning to the tournament site to fight again. The fight continues throughout the night, with tired major substituted and ritualistic fighting continuing [22].
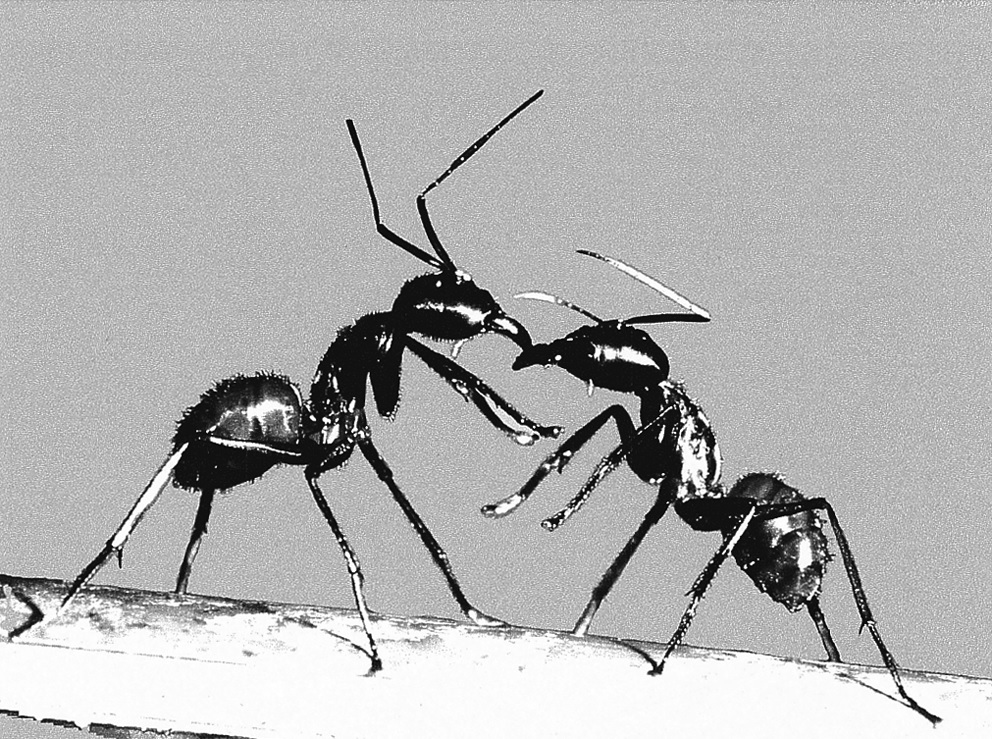
Reproduction
Ants are exogamous and invest a lot of energy into reproduction, therefore it is essential that mating flights match their neighbouring colonies to prevent energetic waste. Many different cues can be used to synchronize the mating flights of different ant colonies of the same species. These cues are called zeitgeber and include, temperature, humidity, global radiation and the development of a brood which is intricately linked to the seasonality in temperate zones. However, in the tropics which are mostly aseasonal and development of brood is not limited by temperature other cues have to be sought. The giant forest ant follows a circa-semiannual nuptial flight pattern where the time between each nuptial flight was observed to be 188 ± 5 days. This suggests a strong endogenous or ‘internal clock’ component involved in the reproductive cycle of the giant forest ant [19].The behaviour before and after the nuptial flight has also been recorded for the giant forest ant. Before the nuptial flight one hr before the exodus of foragers, hundreds of workers will begin to surround the nest and climb the trunk of the nest tree. This is followed by the appearance of winged sexuals at the entrance of the nest. Just as the sky was getting dark, some males begin to fly off in swarms while the queens and other males begin to climb the tree to start at a greater height. Bats were observed to hunt the swarm intensively as they rose. About one and a half hours later most of the winged alates have left coupled with the retreat of most of the workers back into the nest. After their mating flight, queens were seen to be climbing down the trunks of trees within the study area suggesting that the queens do not disperse very far [19].
Notes on tagging of workers
Marking of ants was often use to observe and collect data about all aspects of the biology of the ant from ant foraging behaviour to movement between nests. Such marking was done on the thorax and/or abdomen of the ant using either durable numbered plastic tags [23] or acrylic hobby paint of different colours [17].

Figure 21. Major worker marked with two colours (left) and minor worker marked with one colour (right) for observational studies. Images © Gordon Yong Wenjie
Status in Singapore
While many studies have been conducted on the populations of Dinomyrmex gigas found in Sabah and Brunei, little is known about their distribution or biology in Singapore. More studies are desperately needed on the populations of this large charismatic ant in Singapore.Distribution
While not much is known of the distribution of Dinomyrmex gigas in Singapore, it is known to inhabit only good quality forests which are diminishing in numbers. The large territory that each colony occupies coupled with reducing forest patch sizes and fragmentation could spell trouble for the viability of this species in Singapore. The map below details the current known distribution of Dinomyrmex gigas in Singapore using data obtained from collected specimens. Anyone with a sighting of the giant forest ant is invited to comment on this page with a picture of the ant, locality and date to update the map!Figure 22. Google MyMaps showing the known locations where Dinomyrmex gigas has been collected or sighted in Singapore.
Taxonomy and Phylogeny
Disclaimer: The next section maybe technically challenging to the layman reader but one is encouraged to read on to gain more information on the science of naming and delimiting species!Taxonavigation
Kingdom: AnimaliaPhylum: Arthropoda
Class: Insecta
Order: Hymenoptera
Family: Formicidae
Subfamily: Formicinae
Genus: Dinomyrmex
Species: Dinomyrmex gigas
Taxonomic history
1 subspeciesDescribed as Formica gigas Latreille, 1802 "GRANDES-INDES".
Smith, 1858a: 14 (w.q.m.). Combination in Camponotus: Mayr, 1862: 669; in Dinomyrmex: Ashmead, 1905c: 384; in Camponotus (Myrmogigas): Forel, 1912j: 91; in Camponotus (Dinomyrmex): Forel, 1914a: 268; in Dinomyrmex: Ward, Blaimer & Fisher, 2016: 355. See also: Bingham, 1903: 369.
Current subspecies: nominal plus Dinomyrmex gigas borneensis.
Type specimen:
No holotype, syntype, lectotype known for Dinomyrmex gigas.
Syntype for Dinomyrmex gigas borneensis (No holotype, lectotype unknown)
1.Type locality: Borneo, Sarawak, MALAYSIA
Collected by: G. Doria
Date collected : 31 Dec 1865
Life stage: 1 worker (Major)
Currently at: Museum d’Histoire Naturalle in Geneva, SWITZERLAND
Antweb link: https://www.antweb.org/specimen.do?name=casent090518
2. Type locality: Borneo, Sarawak, MALAYSIA
Collected by: Bedot
Date collected : Date unknown
Life stage: 1 worker
Currently at: Museum d’Histoire Naturalle in Geneva, SWITZERLAND
Antweb link: https://www.antweb.org/specimen.do?name=casent0905186
Sources: Antweb (2016) and Antcat.org (2016)
Phylogeny
Dinomyrmex gigas is part of the subfamily Formicinae, which is a large group with about 3030 described species. It is a successful group with a global distribution across a wide range of different terrestrial environments [3][8][11]. Within this subfamily lies some well-known taxa such as the Weaver ants (Oecophylla), carpenter ants (Camponotus) and about 50 other diverse taxas.The first methods used to classify this subfamily followed the Morphological species concept, looking at specifically the morphological features of the proventriculus, an internal organ of the ant. This progressed to the use of a suite of morphological characters [1][8] aligning with the Phylogenetic Species Concept sensu Wheeler and Platnick, which resulted in the recognition of nine tribes and two informal tribe groups by Bolton [8]. Subsequent methods were largely based on the Phylogenetic Species Concept sensu Mitcher and Theorit and used molecular data to build phylogenetic trees to assign ‘ranks’ based on monophyletic groupings. The first of such molecular phylogenetic analysis of this subfamily by Johnson et al [14] using two mitochondrial genes yielded the results that were mostly in agreement with Bolton [8]. However, later studies using multiple nuclear genes and more extensive taxon sampling [10][15][16] suggests that some recognized taxa such as the genus Camponotus was not monophyletic.
This led to a new analysis using a phylogenomic data set of almost 1000 genes and a broad sampling taxa, in an attempt to give a more robust and comprehensive picture of the evolution of this subfamily [6]. A total of 959 ultraconserved element (UCE) loci was sequenced in 48 out of 51 currently recognized formicine genera and eight outgroups. A more traditional data set using Sanger sequencing of 10 nuclear genes was also generated for comparison. The comparison led to the conclusion that the use of UCEs and phylogenomic analysis was far more superior in estimating formicine relationships, with better resolution of both shallow and ancient relationships, increased node support and a more resolved phylogeny [6]. Both datasets were analysed using Bayesian and Maximum likelihood (ML), with stronger node support and resolution in the phylogenomic tree which is shown in Figure 19. To supplement the molecular analysis, morphological was also conducted for the tribe of Camponotini, a complex tribe with the previously non-monophyletic genus of Camponotus which the giant forest ant used to belong to before the revision.
On a subfamily level, the new analysis revealed six strongly supported clades and five other long branched lineages with little species who have weaker support and uncertain positions on the resultant tree [25]. This prompted the authors to designate each monophyletic clade as a tribe, giving a total of 11 tribes for this subfamily. Of interest and relevance to the giant forest ant is the tribe Camponotini.
Camponotini is a tribe with 10 genera. Before the current revision, the giant forest ant belonged in the genus Camponotus, a large genus with 46 valid genera before the latest revision. However, many have commented that many of the subgenera within Camponotus was poorly defined, and most certainly represent artificial groupings [7][9][12]. This phylogenomics study has shown that Camponotus is in fact made up of three separate lineages, Camponotus sensu stricto, Camponotus gigas (Giant forest ant) and Camponotus (Colobopsis), all supported with high Maximum likelihood bootstrap values of 100 for each node (Figure 19). This result led to the resurrection of the subgenus Dinomyrmex to genus level and the new combination Dinomyrmex gigas as the recognized scientific name for the giant forest ant.
References
- Agosti, D. (1991). Revision of the oriental ant genus Cladomyrma, with an outline of the higher classification of the Formicinae (Hymenoptera: Formicidae). Systematic Entomology, 16, 293–310. http://dx.doi.org/10.1111/j.1365-3113.1991.tb00690.x
- Agosti, Donat; Majer, Jonathan D.; Alonso, Leeanne E.; Schultz, Ted R., eds. (2000). Ants: Standard Methods for Measuring and Monitoring Biodiversity. Smithsonian Institution Press. ISBN 1560988851.
- AntCat. (2016). An online catalogue of the ants of the world. Available from: http://antcat.org/
- AntWeb. (2016). Available from http://www.antweb.org. Accessed 24 November 2016
- Batra, Suzanne W. T. (Jan 1968). "Behavior of Some Social and Solitary Halictine Bees Within Their Nests: A Comparative Study (Hymenoptera: Halictidae)".Journal of the Kansas Entomological Society.41(1): 120–133.
- Blaimer, B.B., Brady, S.G., Schultz, T.R., Lloyd, M.W., Fisher, B.L. & Ward, P.S. (2015). Phylogenomic methods outperform traditional multi-locus approaches in resolving deep evolutionary history: a case study of formicine ants. BMC Evolutionary Biology, 15, 271. http://dx.doi.org/10.1186/s12862-015-0552-5
- Bolton, B. (1995). A new general catalogue of the ants of the world. Harvard University Press, Cambridge, Massachusetts, 504 pp.
- Bolton, B. (2003). Synopsis and classification of Formicidae. Memoirs of the American Entomological Institute, 71, 1–370.
- Brady, S.G., Gadau, J. & Ward, P.S. (2000) Systematics of the ant genus Camponotus (Hymenoptera: Formicidae): a preliminary analysis using data from the mitochondrial gene cytochrome oxidase I. In: Austin, A.D. & Dowton, M. (Eds.) Hymenoptera. Evolution, biodiversity and biological control. CSIRO Publishing, Collingwood, Victoria, pp. 131–139.
- Brady, S.G., Schultz, T.R., Fisher, B.L. & Ward, P.S. (2006). Evaluating alternative hypotheses for the early evolution and diversification of ants. Proceedings of the National Academy of Sciences of the United States of America, 103, 18172–18177. http://dx.doi.org/10.1073/pnas.0605858103
- Brown, W.L. Jr. (2000). Diversity of ants. In: Agosti, D., Majer, J.D., Alonso, L.E. & Schultz, T.R. (Eds.), Ants. Standard methods for measuring and monitoring biodiversity. Smithsonian Institution Press, Washington, pp. 45–79
- Clouse, R.M., Janda, M., Blanchard, B., Sharma, P., Hoffmann, B.D., Andersen, A.N., Czekanski-Moir, J.E., Krushelnycky, P., Rabeling, C., Wilson, E.O., Economo, E.P., Sarnat, E.M., General, D.M., Alpert, G.D. & Wheeler, W.C. (2015) Molecular phylogeny of Indo-Pacific carpenter ants (Hymenoptera: Formicidae, Camponotus) reveals waves of dispersal and colonization from diverse source areas. Cladistics, 31, 424–437.
- Encyclopedia Britannica. (n.d.). Eusocial species. Retrieved November 23, 2016, from https://global.britannica.com/science/eusocial-species
- Johnson, R.N., Agapow, P.-M. & Crozier, R. H. (2003) A tree island approach to inferring phylogeny in the ant subfamily Formicinae, with especial reference to the evolution of weaving. Molecular Phylogenetics and Evolution, 29, 317–330. http://dx.doi.org/10.1016/S1055-7903(03)00114-3
- Moreau, C.S. & Bell, C.D. (2013). Testing the museum versus cradle tropical biological diversity hypothesis: phylogeny, diversification, and ancestral biogeographic range evolution of the ants. Evolution, 67, 2240–2257. http://dx.doi.org/10.1111/evo.12105
- Moreau, C.S., Bell, C.D., Vila, R., Archibald, S.B. & Pierce, N.E. (2006). Phylogeny of the ants: diversification in the age of angiosperms. Science, 312, 101–104. http://dx.doi.org/10.1126/science.1124891
- Orr AG, Charles JK (1994). Foraging in the giant forest ant, Camponotus gigas (Smith)(Hymenoptera: Formicidae): evidence for temporal and spatial specialisation in foraging activity. Journal Natural History, 28:861-872
- Orr AG, Charles JK, Yahya Hj HR, Sharebini Hj N (1996). Nesting and colony structure in the giant forest ant Camponotus gigas (Latreille) (Hymenoptera: Formicidae). The Raffles Bulletin of Zoology 44:247-251
- Pfeiffer M, Linsenmair KE (1997). Reproductive synchronization in the tropics: the circa-semiannual rhythm in the nuptial flight of the giant ant Camponotus gigas Latreille (Hym./Form.). Ecotropica, 3:21-32
- Pfeiffer M, Linsenmair KE (1998). Polydomy and the organization of foraging in a colony of the Malaysian giant ant Camponotus gigas (Hym./Form.). Oecologia, 117:579-590
- Pfeiffer M, Linsenmair KE (2000). Contributions to the life history of the Malaysian giant ant Camponotus gigas (Hymenoptera / Formicidae). Insectes Sociaux, 47:123-132
- Pfeiffer M, Linsenmair KE (2001). Territoriality in the Malaysian giant ant Camponotus gigas (Hymenoptera /Formicidae). Journal of Ethology, 19:75-85
- Pfeiffer, M. A. R. T. I. N., & Linsenmair, K. E. (2007). Trophobiosis in a tropical rainforest on Borneo: Giant ants Camponotus gigas (Hymenoptera: Formicidae) herd wax cicadas Bythopsyrna circulata (Auchenorrhyncha: Flatidae). Asian Myrmecol, 1, 105-119.
- Schwarz, S., Wystrach, A., & Cheng, K. (2011). A new navigational mechanism mediated by ant ocelli. Biology letters, 7(6), 856-858.
- Ward, P. S., Blaimer, B. B., & Fisher, B. L. (2016). A revised phylogenetic classification of the ant subfamily Formicinae (Hymenoptera: Formicidae), with resurrection of the genera Colobopsis and Dinomyrmex. Zootaxa, 4072(3), 343-357.
- Wilson, Edward O. (1971). The Insect Societies. Cambridge. Massachusetts: Belknap Press of Harvard University Press.
- Yamane S, Itino T, Nona AR (1996). Ground ant fauna in a Bornean dipterocarp forest. Raffles Bulletin of Zoology, 44:253-262