
Figure 1. Drupella rugosa. Image by Wu Meng Yue.
Introduction
Table of Contents
Drupella snails are capable of having a similar, if not greater, impact on coral reefs than another well-known corallivore, the crown-of-thorns sea star when present in large numbers and D. rugosa is no exception [3,4]. It is astonishing that a small marine snail that can reach up to 4 cm in length is capable of match the destruction done by large crown of thorns sea star that are generally 25 cm in diameter.
Environmental impact
Corallivory plays a role in maintaining ecosystem balance [5]. Similar to D. rugosa, Crown-of-thorn sea star is a corallivore with a preference for branching corals. Scientist have hypothesised that this sea star may a play a role in preventing the dominance of fast growing branching coral genera in reefs by their feeding [6]. Drupella rugosa may also play a similar role, but the impact of Drupella is not always positive.
Climate change and humans
Climate change and anthropogenic influences are believed to further exacerbate the negative impacts. Global warming and ocean acidification can act as stressors to the corals. Drupella rugosa had been found to prefers corals that are experiencing mechanical or environmental stressors as they may have impaired or non-functioning nematocysts [7, 8].The increase in temperature may also increase the feeding rate of D. cornus as well [9], whether this trend is found in D. rugosa is still not known. Nutrient run off from human activities can also promote the growth of Drupella juveniles [10]. Both may help increase Drupella population on the reef. With Singapore temperature rising at double the global average [33], the impact of Drupella on our local reefs needs to be addressed.
Population growth
In the last 20 years, D. rugosa population 'outbreak' in the Indo-Pacific region had been noted [11]. Population outbreak is defined as “any population of elevated density (> 2 individuals/sqm) that causes extensive mortality of corals and persists for months or years over large areas of reef” [7]. Recently, an argument was put forward to determine the difference between 'population outbreak' and 'seasonal feeding aggregation' [12].Even so, it is known that large population of Drupella can cause mass coral mortality. For example, population growth of D. rugosa and D. margariticola had caused more than 60% coral death in Hong Kong and Thailand [13,14]. This may cause a lost in coral diversity due to their feeding preference.
Thankfully, there is no evidence of a 'plague-like' population growth of Drupella since 1999 [12]. But as our climate continues change, the situation may not stand true.

Dying corals?
Coral reefs are one of the most diverse and threaten ecosystem on earth. Hence, a lot of money and manpower have been given to help conserved the reefs. To restore degraded reefs, restoration methods like coral nurseries and transplantations had been widely implemented and are essential to reef restoration methods.Coral transplants are usually fast growing branching corals that are fragmented into smaller pieces, making them susceptible to Drupella predation [15]. This can be seen in Kenya, Red sea and Hong Kong where Drupella was found to be causing coral mortality in the transplants [16,17,18]. Drupella rugosa is also found munching on our local reefs and nurseries.
Drupella are well camouflaged by the calcareous algae and tend to hide in between the coral branches making removal of Drupella difficult and labour intensive. Caution is also needed during removal when reaching into the branches of the corals where the snails are at to ensure minimal damage to the coral itself. With their ability to attract conspecifics, constant inspections have to be done to reduce coral mortality [17].
Together with the fact that Drupella population can reach very high levels, manual removal of the snails can be labour intensive. In Thailand, volunteers had removed almost 14,000 Drupella in 2016 alone! [19]

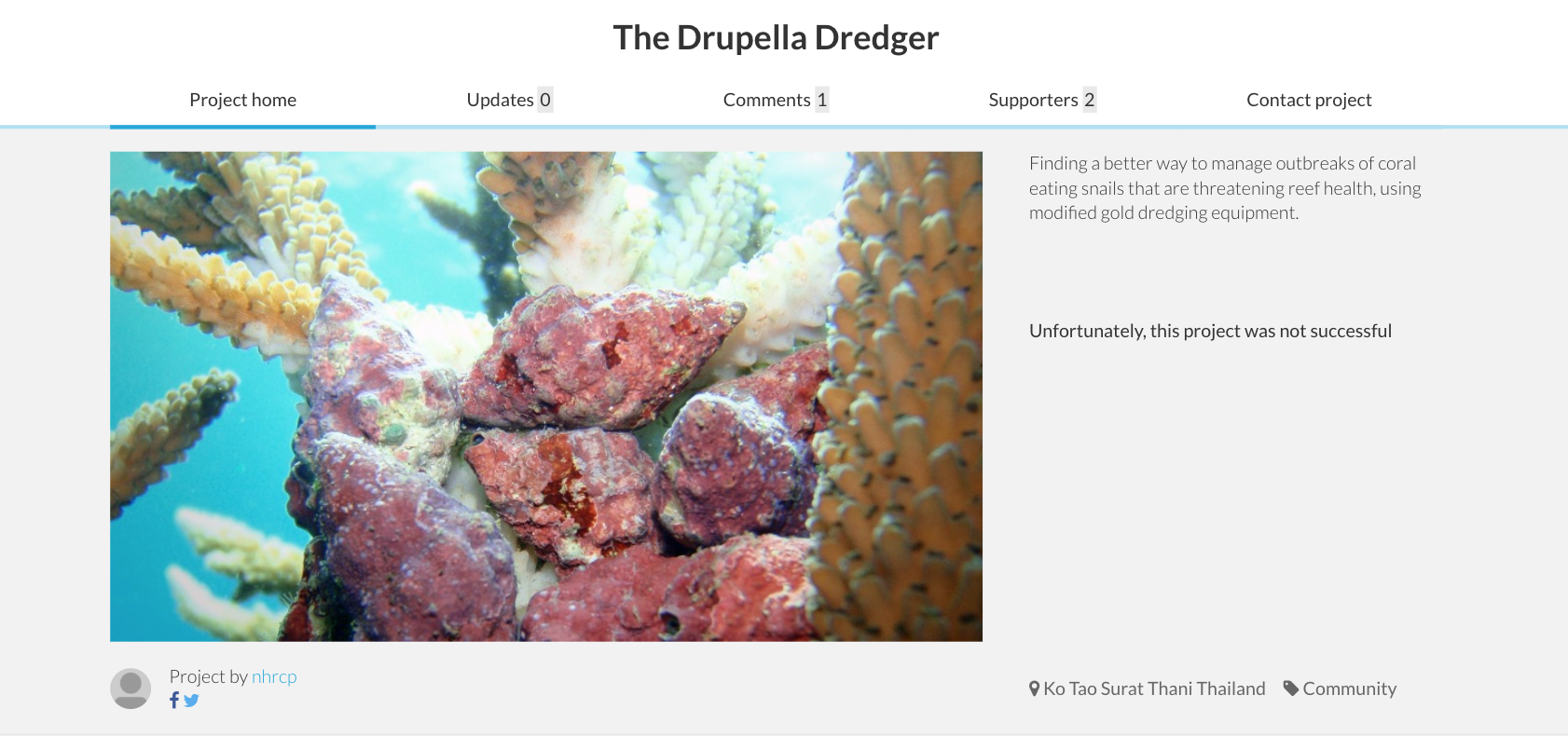
In Singapore, manual removal of Drupella is also employed since a better method had not been found. The team in Koh Tao, Thailand had tried to create a 'Drupella Dredger' to vacuum up the snails via crowdfunding, however the project was not successful. This may imply that more awareness on the impact of Drupella predation is needed to come out with more effective mitigation methods.
Biology
Adaptations to corallivory
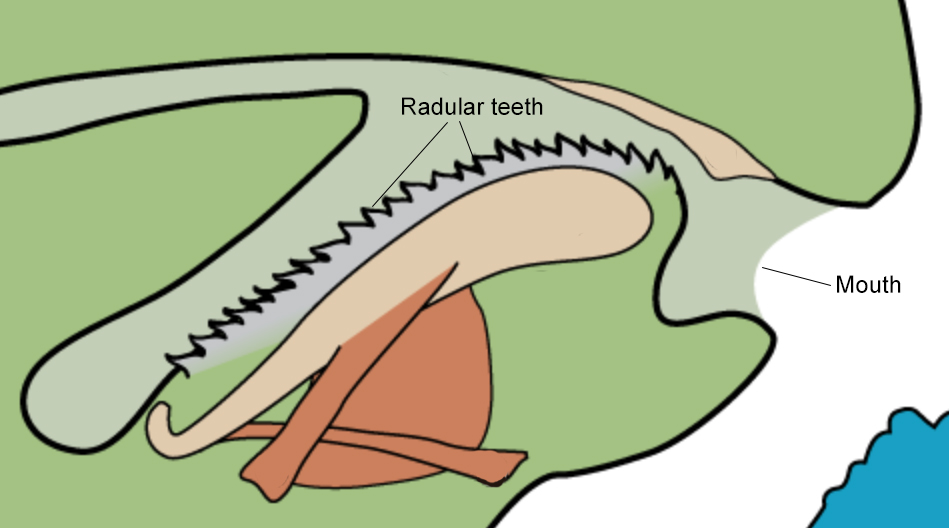
Drupella rugosa is known as an obligate corallivore, this means that live coral polyps are an essential part of its diet. Nematocysts, zooxanthellae and mucus from corals was found in its stomach content [20]. It was also found to generally only consume the coenechyme of the corals [12].As an obligate corallivore, D. rugosa possesses a specialised radula, with extremely long lateral teeth, to remove coral polyps and leave behind the white coral skeleton [3]. The radula is unlike any other muricids, including the other corallivorous subfamily coralliophilinae from the same family [21]. Interestingly, corallivorous organisms are only found in Coralliophilinae in Muricidae with Drupella (Subfamily: Ergalataxinae) being the exception, signifying multiple independent acquisition of corallivory in Muricidae [22].
Other adaptations include an external cuticularised proboscis that acts as a shield for the vulnerable tissues from the coral nematocysts [22]. An external proboscis also allows the snail to feed on adjacent live polyps while immobile [23].
Feeding behaviour
Drupella rugosa commonly feeds in groups (<20 individuals) because the feeding of an individual can attract other conspecifics [24]. This phenomena is hypothesised to be for protection and improve feeding efficiency [7]. Large feeding aggregation (~2000 individuals) is also known to occur and these aggregation tends to stay upon the prey coral colony for a longer period of time causing more damage to the reef [12]. These groups and aggregation may have more than one species of Drupella, and it will usually contain D. rugosa [7]. They are usually nocturnal feeders, but may feed during the day when present in sufficiently large numbers [4].
Drupella rugosa exhibits a preference for particular type of corals. The snails prefer branching corals such as Acropora, Montipora [4], and in Singapore reefs, Pocillipora. This does not mean that other coral species are safe from D. rugosa predation because they are capable of shifting their dietary preference to other coral species [13].
Reproduction
Drupella rugosa egg capsules can be found on recently consumed corals [25]. The coral skeleton exposed from feeding can act as a hard substrate for egg-capsule attachment [3, 25]. As they prefer branching corals, the egg capsules are found between the branches offering protection from predators and water currents [3] (Figure 7). The adults then move on to another coral colony to feed [13].
Billiform (blister-shaped) eggs capsules are attached to the coral skeleton by a wide flatten base. Each female deposited an average of 52 capsules with a mean of 67 embryos per capsule. Hence, one D. rugosa is capable of producing 3484 embryos per spawning event. The latest understanding of the life cycle of D. rugosa is summarised below.
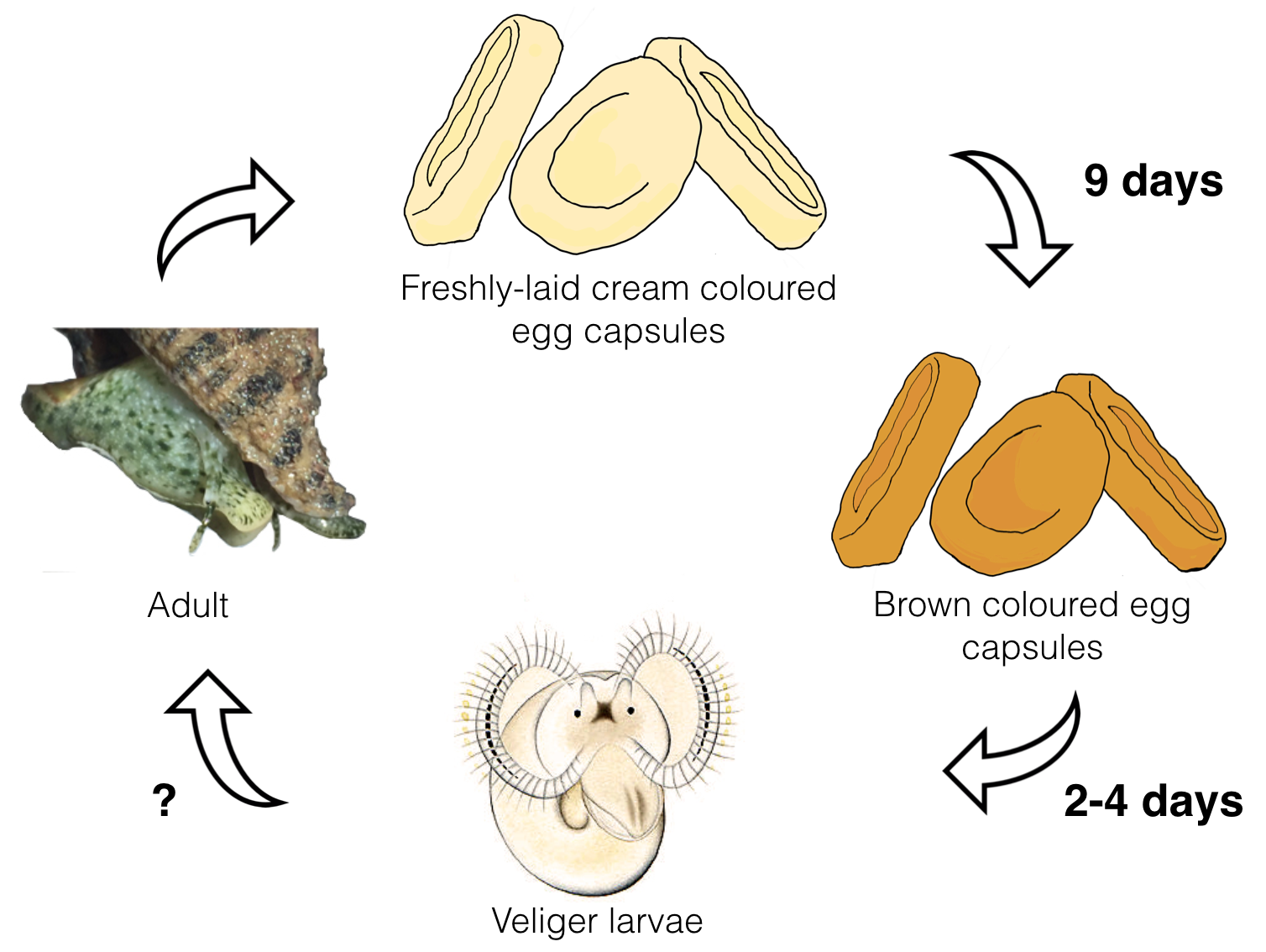
Figure 8. Life cycle of Drupella rugosa. Freshly-laid egg capsules are cream in colour, and become brown when the embryos are developed. Veliger larvae are release 15 days post-oviposition and stays near water surface or in the water column. Duration of the planktonic phase is not known to science yet. Image was created based on Sam et al. (2017) by Wu Meng Yue.
| Video 1. Pre-hatching veligers developed transparent shell and visceral mass and yolk. They move slowly within the confines of the still cream coloured egg capsules. Video extracted from Sam et al. 2017, used with permission. |
Video 2. Closed to hatching, egg capsule becomes brown and pre-hatching veligers becomes more mobile. Video extracted from Sam et al. 2017, used with permission. |
Video 3. Veliger of Drupella rugosa as it swims in the water column. Video extracted from Sam et al., 2017, used with permission. |
Habitat
Drupella rugosa is usually found in sheltered reef environments, hidden within the corals [4]. There were also records of D. rugosa found in the eulittoral zone and shallow rocky shores [26].Distribution
Drupella rugosa is found in the Indo-Pacific reefs, specifically, East Africa, Southeast and Northeast Asia.
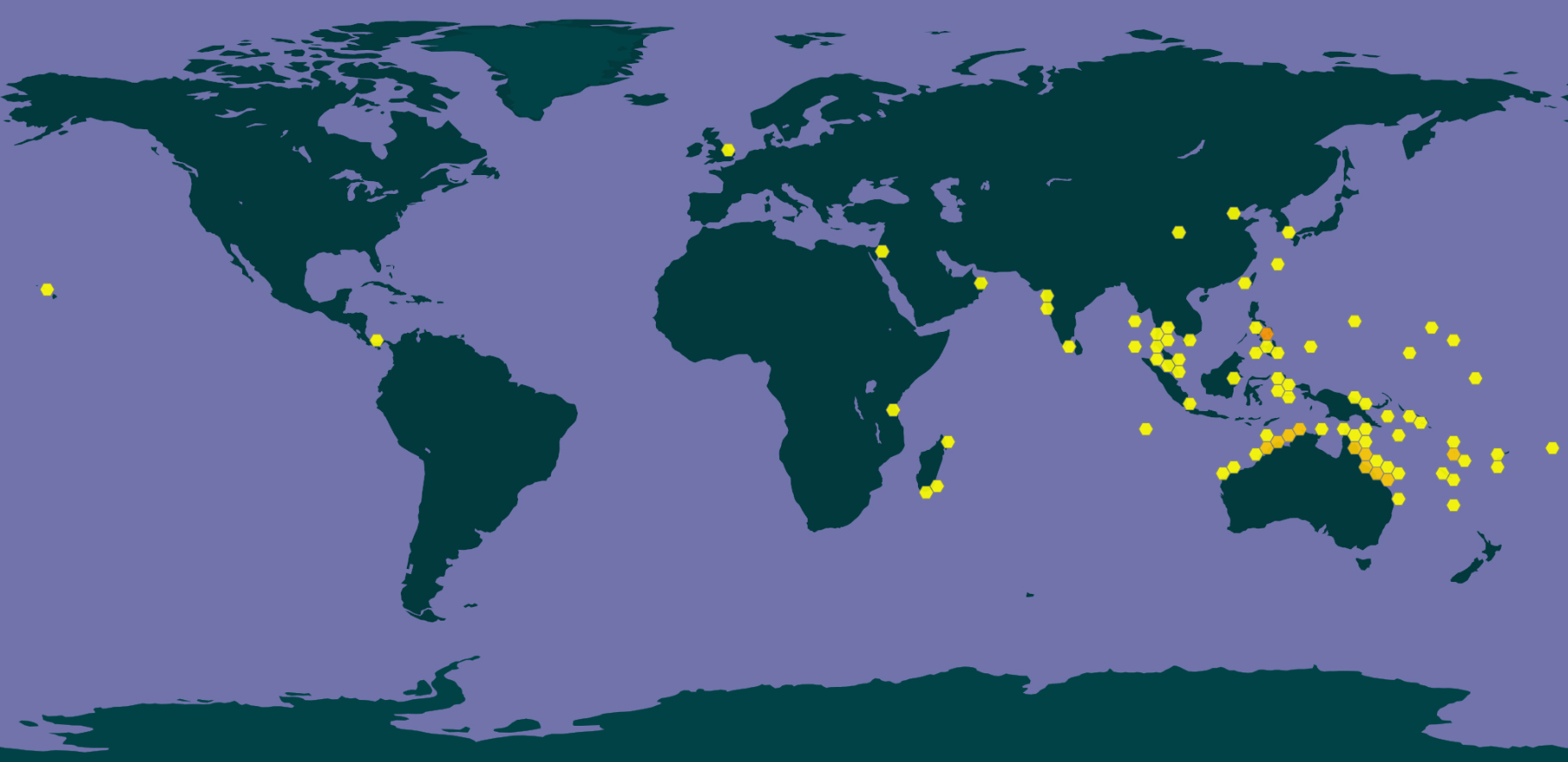
In Singapore, D. rugosa can be found in the fringing reefs of Kusu and Lazarus Island.
Description
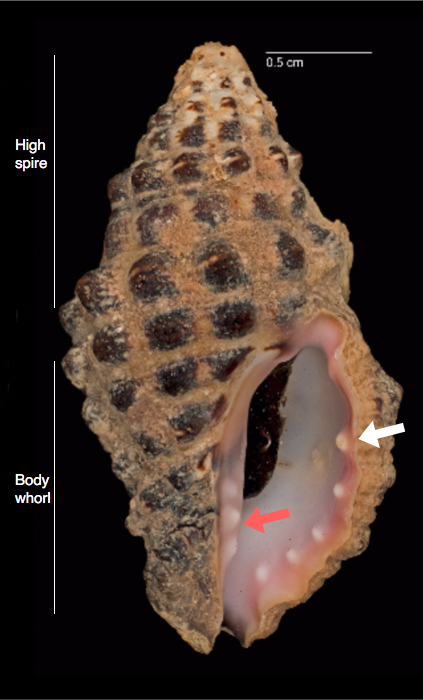 |
Description of D. rugosa given by Richmond (1997) [26] and Döring (2015) [27]:
|
 |
|
 |
|
Diagnosis
There are two known species of Drupella in Singapore – D. rugosa and D. margariticola and they tend to be found together. The two species have similar morphology (green soft body, bumpy columella, similar shell size and presence of nodules) with a couple of differences listed below.
| Distinguishing features |
Drupella rugosa Image by Astri Noorbaini Binte Samsuri, used with permission. |
Drupella margariticola Image by Astri Noorbaini Binte Samsuri, used with permission. |
| Shell morphology |
Knoddy nodules |
Parallel ridges |
| Aperture |
White elliptical aperture |
Purple elliptical aperture |

Drupella species look similar to other muricid drill snails (eg. Thais, Morula) [4,28] and tend to be misidentified without careful examination. This is a common mistake, even by taxonomists whom had erroneously placed this species into those genera due to similar morphology. An easy way to identify Drupella is the location of the snail. Drupella are usually found on or near corals because they are obligate corallivores, using corals for both food and shelter, while other genus of drill snails can be easily found on our rocky shores.
Taxonomy and Systematics
Nomenclature
Binomial name: Drupella rugosa (Born, 1778)Original combination: Murex rugosus Born, 1778 [29]
Original combination had been given erroneously as Murex rugosa in many online databases
Synonyms:
- Drupa concatenata (Lamarck, 1822)
- Drupella concatenata (Lamarck, 1822)
- Morula concatenata (Lamarck, 1822)
- Murex concatenata Lamarck, 1822 (synonym)
- Murex rugosus Born, 1778
- Purpura alveolata Reeve, 1846 (synonym)
- Purpura subturrita Blainville, 1832 (synonym)
- Ricinula concatenata (Lamarck, 1822)
- Thais alveolata (Reeve, 1846)
- Thais rugosa (Born, 1778)
Vernacular name: Rugose Drupe
Original species description
Drupella rugosa was first described by Ignaz von Born in 1778, under the name Murex rugosus in Index rerum naturalium Musei Caesarei Vindobonensis [29]. 'Rugosus', meaning 'Roughened', may have been given to this species due to the presence of numerous tubercle found on the shell.
In 1925, Thiele placed this species into a new genus Drupella because of its corallivory behaviour and apomophies of its radula [22].
With the introduction of the International Code of Zoological Nomenclature, rugosus was changed to rugosa under the 'gender agreement'. The agreement states that the species name should follow the gender of the genus, thus the masculine 'rugosus' was changed to the feminine 'rugosa'. This led to the modern name of Drupella rugosa.
Type specimen
A syntype for Purpura alveolata Reeve, 1846, now a synonym of D. rugosa, can be found at the National History Museum, London labelled as NHMUK ZOO 1993131 of unknown locality [30,31].Phylogeny
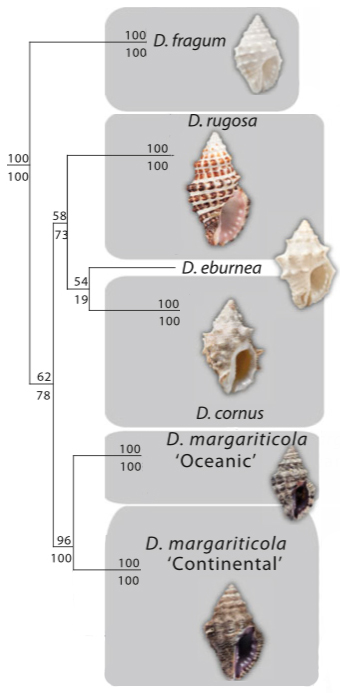
Taxonomy of the genus Drupella has be unclear, the genus had suffered excessive splits up to 12 species due to considerable morphological difference present [32]. The latest genetic study of the genus Drupella have five species and are classified under the family Ergalataxinae from the previous Rapaninae [22]. Species delimitation in that study had corresponded to the morphological species description, with the exception of D. margariticola, even though the nodes within Drupella are not well resolved (Figure 15) [22].In the study, Bayesian analysis was carried out using 28S, 12S and cytochrome oxidase I (COI). Ribosomal genes (28S, 12S) were aligned using ClustalX while mitochondria gene (COI) was aligned by eye. MrModelTest is then use to find the best nucleotide substitution models for each gene partition out of 24.
A MrBayes bayesian phylogeny was created using concatenated analysis of all three genes sequenced and BEAST/GMYC was used to delimitate species. Both MrBayes and BEAST analysis supports six Drupella species with a support value of 100 for all and Drupella being a monophyletic group (Figure 15). Yet, looking at the support values on the internal nodes of the tree, the values are quite low with one exception to D. margariticola 'Continental' (Figure 15). In conclusion, while the study support the monophyly of six Drupella species, the evolutionary relationship of this genus is still unclear.
With that conclusion, I believe the authors were using the phylogenetic species concept sensu Mishler and Theriot. Sadly, D. minuta, accepted by some authors, was not sampled and sequenced in this study. Whether this species is a valid species under this species concept is still not known.
A Species Tree Ancestral Reconstruction was also carried out in BEAST assuming a relaxed lognormal molecular clock. It had been found that Drupella diversified approximately 5.0 Ma (Figure 13) and this correspond to the previous estimate previously based on morphology [22].
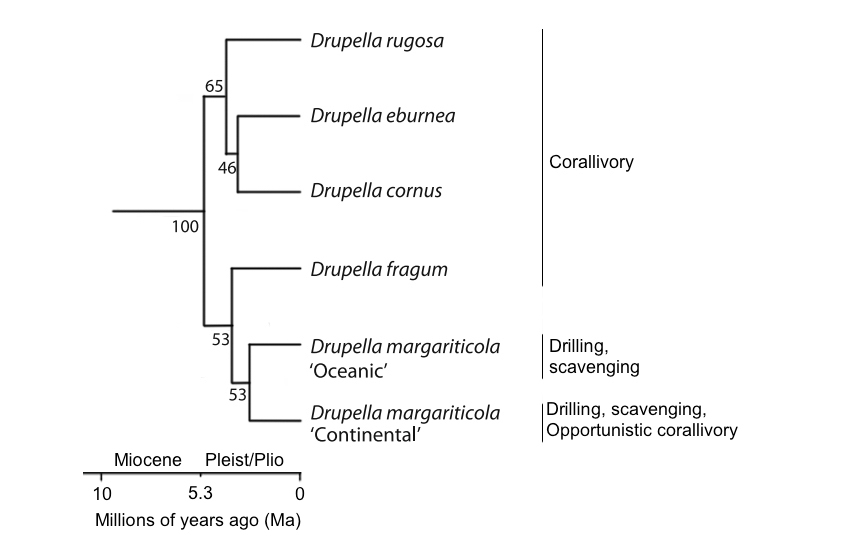
With this result, corallivory in Drupella is found to be connected to the expansion and reorganisation of coral reefs in the Miocene unlike other corallivores that obtained this trait in the Oligocene with the major appearance of major coral groups [22]. Drupella is also a monophyletic group (Figure 16).
Classification
From the latest study, the classification of D. rugosa is given below:| Kingdom |
Animalia |
| Phylum |
Mollusca |
| Class |
Gastropoda |
| Subclass |
Caenogastropoda |
| Order |
Neogastropoda |
| Superfamily |
Muricoidea |
| Family |
Muricidae |
| Subfamily |
Ergalataxinae |
| Genus |
Drupella |
| Species |
rugosa |
Glossary
Nematocysts: Specialised stinging cells of corals that act as organs for offence and defence.
Zooxanthellae: Single-celled photosynthetic organisms that are able to live in symbiosis with corals, providing corals with food.
Coenechyme: Coenechyme is the common tissue that surrounds and links the polyps in some corals.
Proboscis: An elongated appendage on the head of the snail that is used for feeding.
Apomorphy: A derived character trait that is distinct to a particular group of organisms.
Acknowledgement
I would like to thank Mr Tan Siong Kiat and Mr Daisuke Taira, without whom, this species page would not been possible.
References
[1] Boucher, L. M. (1986). Coral predation by muricid gastropods of the genus Drupella at Enewetak, Marshall Islands. Bulletin of marine science, 38(1), 9-11.
[2] Rotjan, R. D., & Lewis, S. M. (2008). Impact of coral predators on coral reefs. Marine Ecology Progress Series, 367, 73-91. doi: 10.3354/meps07531
[3] Turner, S. J. (1994). The biology and population outbreaks of the corallivorous gastropod Drupella on Indo-Pacific reefs. Oceanography and Marine Biology: An Annual Review, 32.
[4] Cumming, R. L. (2009). Case Study: Impact of Drupella spp. on reef-builging corals of the Great Barrier Reef. Great Barrier Reef Marine Park Authority.
[5] Rotjan, R. D., & Lewis, S. M. (2008). Impact of coral predators on coral reefs. Marine Ecology Progress Series, 367, 73-91. doi: 10.3354/meps07531
[6] Enochs, I. C., & Glynn, P. W. (2017). Corallivory in the eastern Pacific. In Coral Reefs of the Eastern Tropical Pacific (pp. 315-337). Springer Netherlands.
[7] Cumming, R. L. (2009). Population outbreaks and large aggregations of Drupella on the Great Barrier Reef.
[8] Cumming, R. L. (1999). Predation on reef-building corals: multiscale variation in the density of three corallivorous gastropods, Drupella spp. Coral Reefs, 18(2), 147-157.
[9] Al-Horani, F., Hamdi, M., & Al-Rousan, S. (2011). Prey selection and feeding rates of Drupellacornus (Gastropoda: Muricidae) on corals from the Jordanian Coast of the Gulf of Aqaba, Red Sea.Jordan Journal Of Biological Sciences, 4(4),191-198
[10] Brodie, J., Fabricius, K., De’ath, G., & Okaji, K. (2005). Are increased nutrient inputs responsible for more outbreaks of crown-of-thorns starfish? An appraisal of the evidence. Marine pollution bulletin, 51(1), 266-278.
[11] Tsang, R. H. L., & Ang, P. (2015). Cold temperature stress and predation effects on corals: their possible roles in structuring a nonreefal coral community. Coral Reefs, 34(1), 97-108.
[12] Morton, B., & Blackmore, G. (2009). Seasonal variations in the density of and corallivory by Drupella rugosa and Cronia margariticola (Caenogastropoda: Muricidae) from the coastal waters of Hong Kong:‘plagues’ or ‘aggregations’?. Journal of the Marine Biological Association of the United Kingdom, 89(1), 147-159.
[13] Hoeksema, B. W., Scott, C., & True, J. D. (2013). Dietary shift in corallivorous Drupella snails following a major bleaching event at Koh Tao, Gulf of Thailand. Coral Reefs, 32(2), 423-428.
[14] Baird, A. (1999). A large aggregation of Drupella rugosa following the mass bleaching of corals on the Great Barrier Reef. Reef Research, 9, 6-7.
[15] Forde, M. J. (1992). Populations, behaviour and effects of Drupella cornus on the Ningaloo Reef, Western Australia. Drupella cornus: A Synopsis. Department of Conservation and Land Management, Western Australia, 4550.
[16] Cros, A., & McClanahan, T. (2003). Coral transplant damage under various management conditions in the Mombasa Marine National Park, Kenya. Western Indian Ocean Journal of Marine Science, 2(2), 127-136.
[17] Shafir, S., & Rinkevich, B. (2010). Integrated long-term mid-water coral nurseries: a management instrument evolving into a floating ecosystem. University of Mauritius Research Journal, 16, 365-386.
[18] Lam, K., Shin, P. K., & Hodgson, P. (2007). Severe bioerosion caused by an outbreak of corallivorous Drupella and Diadema at Hoi Ha Wan marine park, Hong Kong. 2Coral Reefs, 26(4), 893-893.
[19] The Gap Year Guidebook (2017). Podvolunteers tells us of their 2016 successes. Retrieved from http://www.gap-year.com/news/pod-volunteer-tells-us-of-their-2016-successes
[20] Taylor, J. D. (1978). Habitats and diet of predatory gastropods at Addu Atoll, Maldives. Journal of experimental marine Biology and Ecology, 31(1), 83-103.
[21] Vermeij, G. J., & Carlson, S. J. (2000). The muricid gastropod subfamily Rapaninae: phylogeny and ecological history. Paleobiology, 26(1), 19-46.
[22] Claremont, M., Reid, D. G., & Williams, S. T. (2011). Evolution of corallivory in the gastropod genus Drupella. Coral Reefs, 30(4), 977-990.
[23] Turner, S. J. (1992). The egg capsules and early life history of the corallivorous gastropod Drupella cornus (Röding, 1798). The Veliger, 35(1), 16-25.
[24] Morton, B., Blackmore, G., & Kwok, C. T. (2002). Corallivory and prey choice by Drupella rugosa (Gastropoda: Muricidae) in Hong Kong. Journal of Molluscan Studies, 68(3), 217-223.
[25] Sam, S. Q., Toh, T. C., Kikuzawa, Y. P., Ng, C. S. L., Taira, D., Afiq-Rosli, L., ... & Chou, L. M. (2017). Egg capsules and veligers of the corallivorous muricid gastropod Drupella rugosa (Born, 1778). Invertebrate Reproduction & Development, 1-8.
[26] Richmond, M. D. (1997). A guide to the seashores of Eastern Africa and the Western Indian Ocean islands. Sida/Department for Research Cooperation, SAREC.
[27] Döring, M. (2015). Drupella rugosa. Retrieved from https://www.gbif.org/species/113490381
[28] Tan, R. (2016). Drills. Retrieved from http://www.wildsingapore.com/wildfacts/mollusca/gastropoda/muricidae/muricidae.htm
[29] Born, I. (1778) Index rerum naturalium Musei Caesarei Vindobonensis. Verzeichniss der Natürlichen Seltenheiten des K.K. Naturalien Kabinets zu Wien. Erster Theil, Schalthiere. Pars 1, Testacea. Retrieved from https://www.biodiversitylibrary.org/bibliography/11581#/summary
[30] Global Biodiversity Information Facility (n.d.). Drupella rugosa. Retrieved from https://www.gbif.org/species/4363679
[31] Global Biodiversity Information Facility (n.d.). Purpura alveolata. Retrieved from https://www.gbif.org/occurrence/1056799360
[32] Johnson, M. S., & Cumming, R. L. (1995). Genetic distinctness of three widespread and morphologically variable species of Drupella (Gastropoda, Muricidae). Coral Reefs, 14(2), 71-78.
[33] Cheng, K. (2016) S'pore temperatures rising at double global average. Retrieved from http://www.todayonline.com/singapore/singapore-growing-warmer-twice-global-average