Bubble-tip anemone
Entacmaea quadricolor (Leuckart in Rüppell & Leuckart, 1828)
Table of Contents
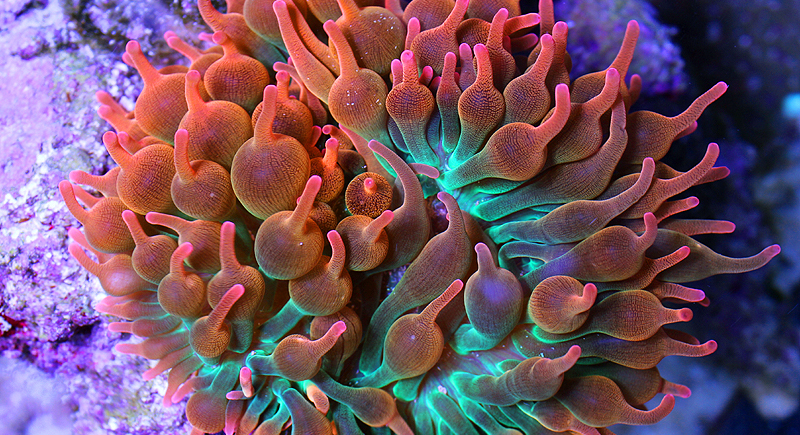
Figure 1. Rainbow bubble-tip anemone (Entacmaea quadricolor) (Source: Matthew Zahler, permission pending [37])
Overview
Entacmaea quadricolor is a sea anemone widespread throughout tropical waters, and is commonly found on Singapore's shores [21]. It is known as the bubble-tip anemone because of its distinctive bulbous tips [1]. It harbours toxins on its tentacles to catch prey [11], and is symbiotic with many species of anemonefishes, such as the common clownfish (Amphiprion ocellaris) [6]. This anemone also exhibits biofluorescence [10] and reproduces by releasing gametes directly into the water medium for external fertilisation [17]. This species is unfortunately threatened by the aquarium trade, owing to its wide variety of attractive colour morphs [23].Description
Entacmaea quadricolor have adherent bases which conform to the shape of the substratum they attach to [1]. They have smooth flared body columns which are usually rich brown but may be greenish or reddish [1][2]. The columns of small individuals range from 50mm - 100mm in length, while that of larger individuals can reach up to 500mm [1]. At the top of the column is a flattened oral disc with an oval mouth in the middle [1]. Cylindrical tentacles fringe the oral disc and inner tentacles are 2 - 3 times longer than marginal ones (Fig. 2) [2].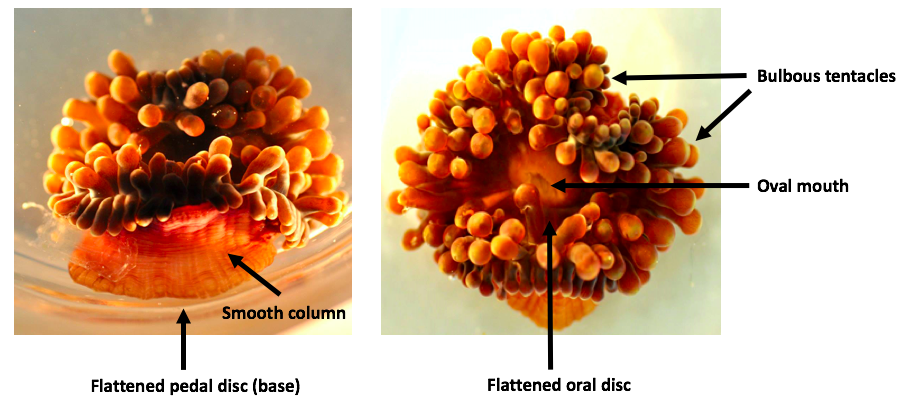
The tentacles may be bulbous at the end, with a terminal bud that usually ends in a sharp red point, or a subterminal bud which lacks a pigmented tip (Fig. 3) [1]. The bulbs may have a white ring (equator) around them (Fig. 1), or be flecked with white [2]. Some tentacles may lack a bulb and are digitiform (Fig. 3), commonly associated with the absence of symbiotic anemonefish [2]. The appearances of E. quadricolor are highly diverse, with sixteen colour morphs discovered in a study at North Solitary Island, Solitary Islands Marine Park, Australia [3]. The state of tentacle expansion can also differ, as tentacles often collapse when disturbed [1].

Figure 4, an image taken in Singapore's waters (Terumbu Semakau), clearly shows some of the diagnostic features of E. quadricolor. The greenish smooth column and bulbous tentacles with the white equator are evident in the figure.

Habitats
Individuals of E. quadricolor occur on reef environments, from portions that are nearly exposed at low tide to as deep as 40m (although rare below 20m) [1]. They exist in two morphologies - small and colonial or large and solitary. Small clustering forms occur in shallow water, typically anchored within cracks and crevices on reefs, and are sometimes attached to branches of corals [1]. Large solitary individuals are found in deeper waters on reef slopes, anchored in holes or crevices such that only the tentacles are visible. Both forms can be found at depths in between [1].Distribution
Local
The localities of E. quadricolor found on Singapore's shores are Chek Jawa (Pulau Ubin), Kusu Island, Sisters Island, Pulau Biola, Pulau Semakau and Pulau Hantu [9], among others. All known localities are shown in the map below.Figure 5 shows an individual of E. quadricolor at Sisters Island, with an interesting colour morph of brownish tentacles with a green sheen.

Global
Entacmaea quadricolor inhabit the waters of the Red Sea, eastern Africa, Indian Ocean, Micronesia, Melanesia, Japan and Australia (Fig. 6) [2].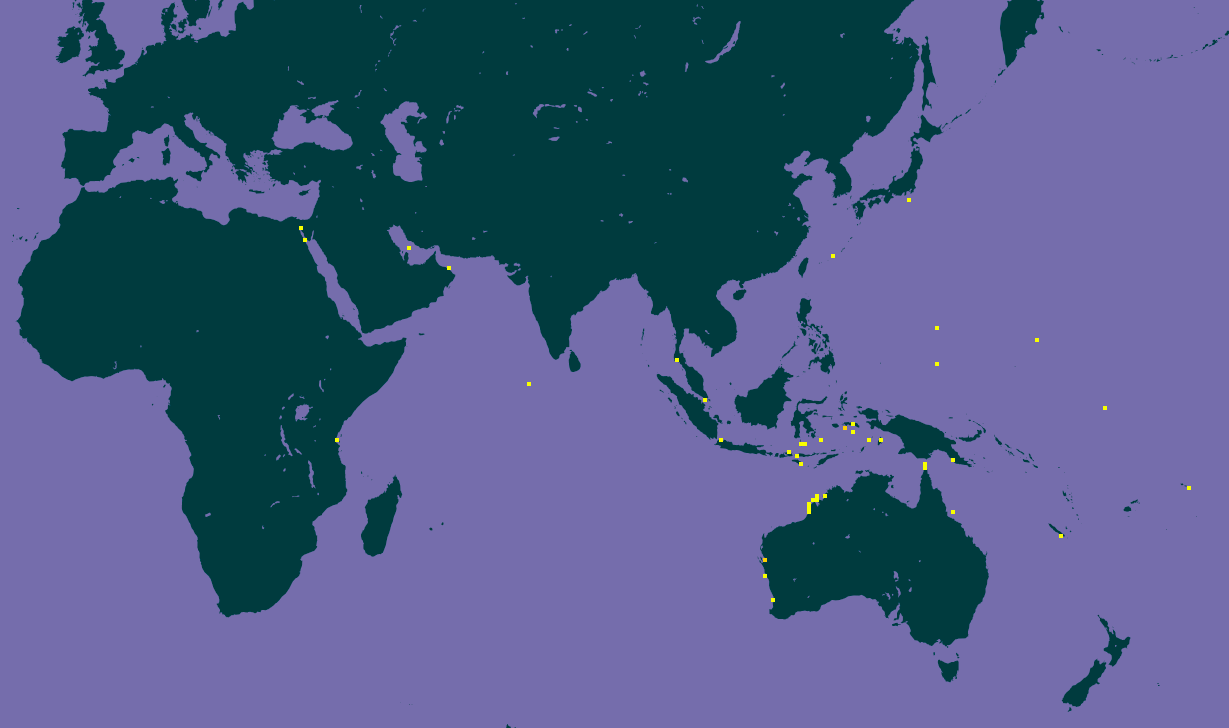
Biology
Symbiosis
Symbiosis is a close and often long-term interaction between two different biological species, known as symbionts [5]. Entacmaea quadricolor is symbiotic with some species of zooxanthellae, anemonefishes and shrimps [6][7].Endosymbionts
Entacmaea quadricolor host photosynthetic dinoflagellate algae, zooxanthellae, which supplies a rich carbon source and some essential amino acids to the host anemone [4]. The majority of the zooxanthellae (61.1%) are located in the tentacles and column walls, and belong to the genus Symbiodinium [4].Ectosymbionts
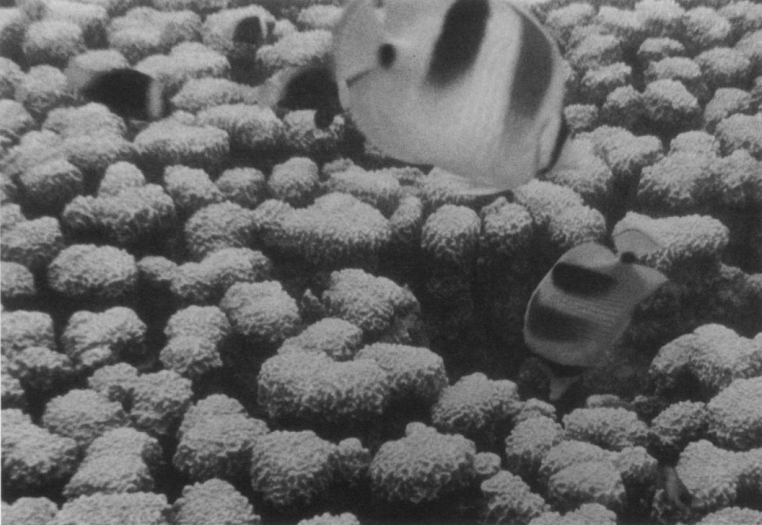
Entacmaea quadricolor are also host to several species of anemonefishes including the red sea clownfish (Amphiprion bicinctus) [5], tomato clownfish (Amphiprion frenatus), cinnamon clownfish (Amphiprion melanopus), common clownfish (Amphiprion ocellaris) and red saddleblack clownfish (Amphiprion ephippium), (Fig. 7) [6]. They also host shrimps such as the five-spot anemone shrimp (Periclimenes brevicarpalis) [7]. Anemonefishes provide aggressive protection from their main predator, the chaetodontid butterflyfish, which feeds on E. quadricolor (Fig. 8) [8]. Chaetodontids were found to prey on E. quadricolor after experimental removal of symbiotic anemonefishes, causing the elimination of some anemones and retraction of others into rock crevices [8]. Besides gaining defence against predation, ammonia excreted by anemonefishes also benefit E. quadricolor when absorbed to increase growth rates and enrich the endosymbiotic zooxanthellae [5].
Behaviour
Entacmaea quadricolor respond to predator attacks by contracting into reef holes to protect their tissues from consumption by predators, as chaetodontid butterflyfish are unable to reach into reef crevices [15]. However, remaining contracted for long periods of time may lead to to tissue degeneration as the anemone cannot feed or absorb nutrients from symbionts [15]. In some areas, Entacmaea quadricolor were observed to expand in light conditions only in the presence of symbiotic anemonefish, and in dark conditions when their major predators are inactive [15]. In Singapore, this behaviour is not observed as E. quadricolor expand in light conditions even without the presence of symbiotic anemonefish [21], possibly due to the lack of predators in local waters.While mature E. quadricolor predominantly stay attached to their substratum, they are capable of movement and detach quickly from the substratum when disturbed [10]. Entacmaea quadricolor individuals have also been observed to escape smothering by rapid sedimentation, this was done through inflating their pedal disc and column alternatively to emerge from the sediments (shown in clip below) [10].
Entacmaea quadricolor escapes from being smothered by sedimentation(Adapted from: Nicola Wood, obtained and accredited under fair use [47])
Biofluorescence
Fluorescent proteins in E. quadricolor include Green Fluorescent Protein (GFP) and Red Fluorescent Protein (RFP) [10]. A study on a specimen from Heron Island, Queensland, Australia, revealed that fluorescence was inconsistent through the anemone, with varying distributions of GFP and RFP [10]. The tentacle tips were the most fluorescent for GFP, with some fluorescence present on the tentacles and oral disc [10] (Fig. 9). Fluorescence for RFP was concentrated at the tentacle ends apart from the extreme tips, with close to no fluorescence on the oral disc [10] (Fig. 9).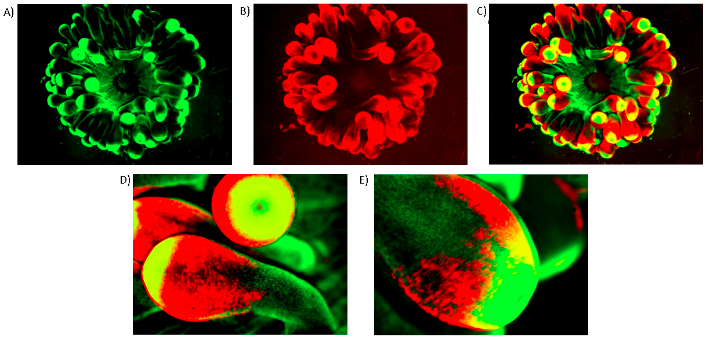
The importance of biofluorescence and to E. quadricolor has not been well studied, although it has been hypothesised that fluorescent tentacle tips may attract prey for sedentary anemones to feed on [10]. Further work is required to explore the implications of GFP and RFP distribution on the ecology of E. quadricolor.
Feeding
Entacmaea quadricolor are opportunistic carnivores that capture prey using cnidocytes on their tentacles which contain many "stinging organs" known as nematocysts (or cnidae). Larger individuals generally have larger nematocysts [1]. This occurs when prey drifts or swims into the tentacles of the anemone, get immobilised and are transported to the mouth via tentacle contraction [10]. Small prey such as plankton may get trapped in the tentacles' mucus lining, and are directed to the tentacle tips followed by the mouth via ciliary action [10]. In the video below, a white E. quadricolor is feeding on a fish and the tentacle contractions can be seen clearly. Prey can be captured because E. quadricolor utilise toxins which are injected through nematocysts or toxins associated with the mucus lining of the tentacles [11].Entacmaea quadricolor feeding on fish (Source: SquidMilk, obtained and accredited under fair use [49])
Toxins
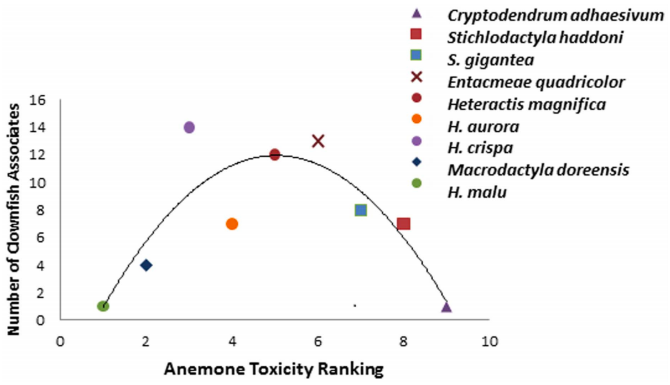
Toxins ejected from nematocysts include neurotoxins which paralyse prey, and one such toxin is acetylcholinesterase inhibitor [11]. Other toxins associated with the mucus lining of tentacles are predominantly used for defence. These toxins are lethal to some fish, causing cell lysis and gill damage [12]. However, anemonefishes are not affected by the toxins as they do not elicit nematocyst discharge from E. quadricolor when in contact with their tentacles [13]. The anemonefishes' mucus plays an important role in protection via a blocking mechanism [14]. Anemonefishes are either innately immune to E. quadricolor or they acquire protection from stinging nematocysts through acclimation [13].An interesting study published in 2014 revealed that anemones with intermediate toxicity, E. quadricolor included, had the most number of anemonefish symbionts (Fig. 10) [14]. This renders E. quadricolor a desirable host that possesses optimum toxicity for anemonefish survival, supported by the large diversity of symbiotic and highly specialised anemonefishes that inhabit E. quadricolor in nature [14].
Reproduction
Asexual
Entacmaea quadricolor can propagate asexually via longitudinal fission [1]. The anemone divides by stretching in opposite directions, starting from the base [16]. A tear is created and the tissue thins gradually, resulting in two genetically identical individuals [16] (Fig. 11). This process gives rise to individuals with irregular symmetry [1] as the individuals undergo transformations such as a change in body diameter and number of tentacles [16].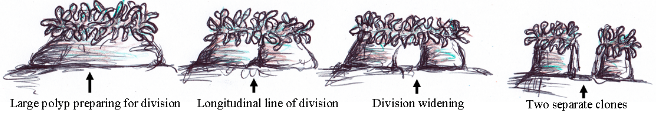
Sexual
Both sexes of E. quadricolor broadcast spawn their gametes into the water for external fertilisation and development, and the spawning was found to always occur after sunset between a full and waxing crescent moon [17]. Seasonal fluctuation in sea temperatures is an important environmental factor that influences reproduction in marine invertebrates [18]. Entacmaea quadricolor spawning events were found to occur during the warmer months of the year where sea temperatures were peaking. This can be attributed to the increased rates of embryo and larval development [19], and enhanced settlement rates associated with higher sea temperatures [20]. In Singapore, studies on E. quadricolor spawning events have yet to be published.Individuals become inflated prior to and during spawning events as the process requires forceful release of gametes, facilitated by columnar muscle contractions [17]. Males commonly release sperm in milky white streams while females release eggs singly or in large assemblages [17] (Fig. 12). Males release gametes prior to females, and this synchrony of gamete release suggests that sperm or an associated product may stimulate egg release [17]. The videos at the end of this section show the spawning of a male and female E. quadricolor.

Male E. quadricolor spawning (Source: SRKreef, obtained and accredited under fair use [53])
Female E. quadricolor spawning (Source: J Madden, obtained and accredited under fair use [54])
Conservation and threats
Status
Entacmaea quadricolor has not been evaluated in The IUCN Red List of Threatened Species, neither has it been listed among the threatened fauna of Singapore [21]. However, some of the obligate symbiotic anemonefishes whose essential habitats are provided by E. quadricolor are listed on the Red List. According to the Singapore Red Data Book, all anemonefishes, including the tomato clownfish (Amphiprion frenatus) and common clownfish (Amphiprion ocellaris) are listed as 'vulnerable' and "habitat protection and strict policing against illegal connection are required" to conserve the species [22]. Anemonefish are rarely found in Singapore anemones and thus far, only the tomato clownfish (Amphiprion frenatus) has been seen among E. quadricolor in the interdal areas of Singapore's shores [9][21] (Fig. 13, Fig. 14). Elsewhere in nature, it is unusual to see anemones without their symbiotic anemonefishes and it is likely that they have been removed by collectors in Singapore [21], threatening the local populations of both anemonefishes and their host sea anemones.

Figure 14. Tomato clownfish (Amphiprion frenatus) among E. quadricolor in Singapore's shores (Pulau Semakau)(Source: Ria Tan, under a CC BY-NC-ND 2.0 license [56])
Aquarium trade
The expanding marine aquarium trade for marine ornamentals are a threat to both anemonefishes and their host sea anemones [23]. An analysis of catch from a four-month period in Cebu, Philippines, revealed that anemonefishes and anemones comprised approximately 60% of the total catch [24]. Sea anemones that host symbiotic anemonefishes are in high demand among aquarists [23] as they represent high-value species that are preferentially harvested from the wild [24]. Harvesting of marine ornamentals has led to local extinctions of some species, and poses a threat to sea anemones globally [24]. Figure 15 shows an aquarist's tank containing E. quadricolor and symbiotic anemonefishes. The marine aquarium trade is a global problem and large numbers of individuals of E. quadricolor are sold on websites online (Fig. 16). In Singapore, there is also a demand for E. quadricolor as local stores have been found to be selling the anemone (Fig. 17). As these individuals sold online are most likely harvested from the wild, it poses a threat to both local and global populations of E. quadricolor.
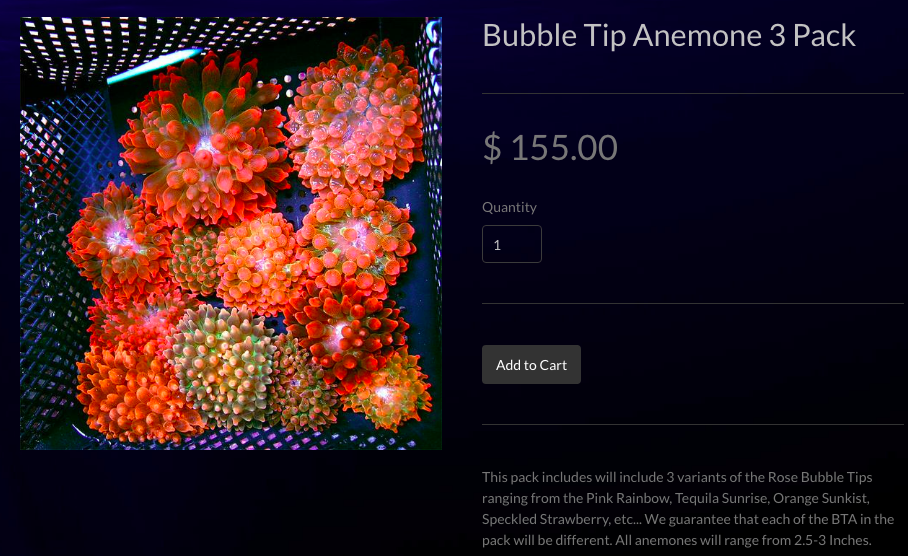
Figure 16. Individuals of E. quadricolor sold online (Adapted from: LA Reefs, permission pending [58])
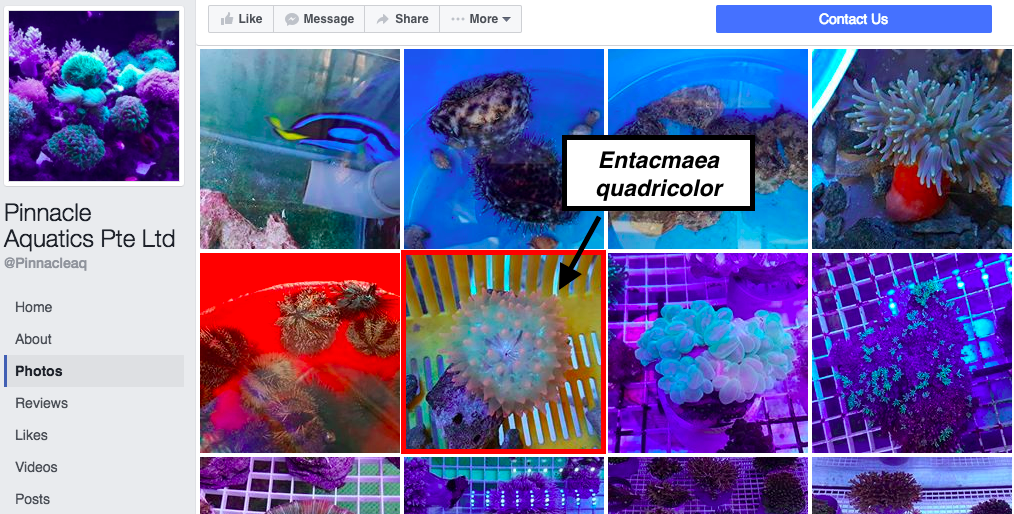
Figure 17. Entacmaea quadricolor sold by a local supplier on Facebook (Adapted from: Pinnacle Aquatics Pte Ltd, permission pending [59])
Bleaching
Rising sea temperatures can affect sea anemones through thermally-induced bleaching from the loss of symbiotic zooxanthellae, which may lead to mortality [25]. Bleaching events pose a serious threat to the viability of E. quadricolor populations and their symbiotic anemonefishes [25]. Following a bleaching event at the Keppel Islands, Australia, E. quadricolor experienced a decrease in size and abundance, and an increase in mortality rate which led to the loss of symbiotic anemonefish [25]. Bleaching is generally greatest at shallow waters where sea temperatures are highest [25], as observed in Singapore's waters where the anemones lost their endosymbionts and turned bright white [21] (Fig. 18, Fig. 19). The video below shows a pair of tomato clownfish (Amphiprion frenatus) among bleached E. quadricolor at Terumbu Raya, Singapore, in August, 2016.
Figure 18. Bleached E. quadricolor in Singapore's shores (Sisters Island)(Source: Ria Tan, under a CC BY-NC-ND 2.0 license [60])

Figure 19. Bleached E. quadricolor in Singapore's shores (Sultan Shoal)(Source: Joy Wong, used with permission [61])
Bleached E. quadricolor at Terumbu Raya, Singapore (Source: Ria Tan, under a CC BY-NC-ND 2.0 license [62])
Taxonomy
Rank and taxa names
| Kingdom |
Animalia |
| Phylum |
Cnidaria |
| Class |
Anthozoa |
| Subclass |
Hexacorallia |
| Order |
Actiniaria |
| Suborder |
Enthemonae |
| Superfamily |
Actinioidea |
| Family |
Actiniidae |
| Genus |
Entacmaea |
| Species |
Entacmaea quadricolor |
Original description
The basionym of E. quadricolor is Actinia quadricolor, originally described by Friedrich Sigismund Leuckart, a german naturalist [1][9]. It was in a publication titled "Atlas zu der Reise im nördlichen Afrika" by Rüppell & Leuckart in 1828, with its description on page 4 of the publication [1] (Fig. 20). An illustration labelled Fig. 3 was also provided [1][27] (Fig. 21).Figure 20. Original description of E. quadricolor (basionym: Actinia quadricolor)(Adapted from: Biodiversity Heritage Library. Digitized by Field Museum of Natural History Library | www.biodiversitylibrary.org)
Figure 21. Illustration of E. quadricolor (basionym: Actinia quadricolor)NB: Mislabelled as Fig. 2 in description, correctly labelled as Fig. 3 in caption [1][27](Adapted from: Biodiversity Heritage Library. Digitized by Field Museum of Natural History Library | www.biodiversitylibrary.org)
Type information
The holotype of E. quadricolor, which the original description was based on, is shown in Figure 22. It was recorded on Jan 1, 1827 [29], and the type locality is near Suez, the Red Sea, Egypt [1][2]. The specimen is now found in Senckenberg Museum, with catalog number 34 (SMF-34) [28]. It was labelled "Actinia quadricolor" and found to be a male [1].Notes on holotype: Whole animal. Oral, pedal disc diameters 40mm, length 35mm; tentacles poorly preserved. Wedges removed from margins, mid-column for microscope slides by D. G. Fautin. Labeled from Rotes Meer, attributed to Rüppell 1827 [29].
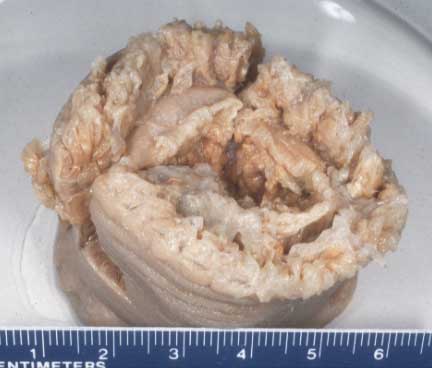
Although there is a holotype for E. quadricolor, multiple syntypes and lectotypes exist due to nomenclatural reorganisations of previously different species that are now synonymous with E. quadricolor. The list of other type specimens can be found here.
Nomenclature
The majority of sea anemone species described in the eighteenth century or early nineteenth century were originally placed in genus Actinia created by Linnaeus in 1767 [1][30]. This included E. quadricolor, which was originally named Actinia quadricolor [1]. There was much uncertainty in the assignment of species and definition of the genus Actinia [1]. The definition was later narrowed, and the subgenus Entacmaea was created by Ehrenberg in 1834 to encompass fifteen species of predominantly Red Sea and Mediterranean anemones, in which the inner tentacles are longer than marginal ones [1]. Entacmaea later became a genus of its own and currently includes two species, Entacmaea medusivora and Entacmaea quadricolor [31].Synonyms
Many synonyms exist for this species and the full inventory can be found here. The large number of synonyms could be due to the variable appearance and wide geographical range of E. quadricolor [9], together with nomenclatural reorganisations over the years. For example, the 13 species that were previously placed in Gyrostoma Kwietniewski, 1897 were moved to Entacmaea, rendering Gyrostoma a junior synonym [30].Phylogeny
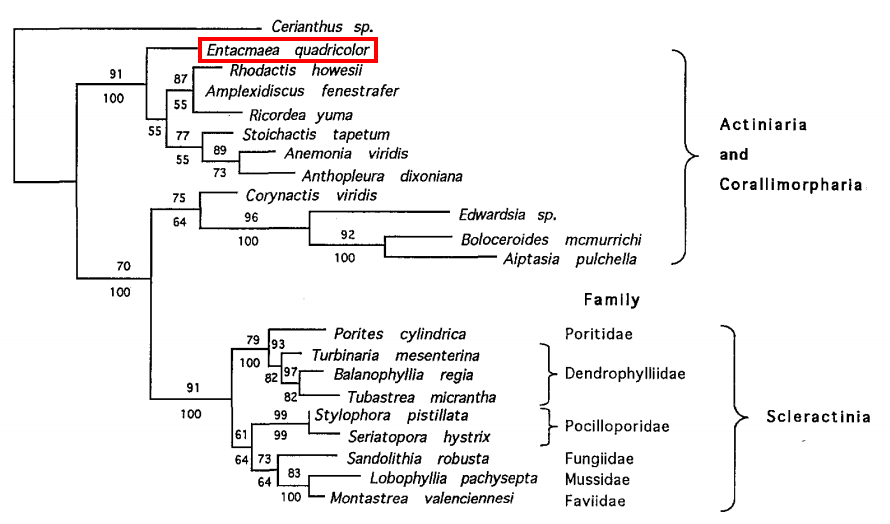
Entacmaea species have yet to be included in phylogenetic studies done within the family Actiniidae, possibly due to the small number of species present. However, the nucleotide sequence of E. quadricolor has been used in a study to investigate the systematic relationships among the orders Scleractinia (hard corals), Actiniaria (sea anemones) and Corallimorpharia (corallimorphs) within the subclass Hexacorallia, using nucleotide sequences from the 5'-end of the 28S rDNA [32]. In this study, the DNA sequences were aligned using CLUSTAL V, followed by manual editing. Both parsimony and distance methods (neighbour-joining) were used in the phylogenetic analysis [32]. Scleractinia was found to form a monophyletic group while Actiniaria and Corallimorpharia did not (Fig. 23) [32], suggesting that Actiniaria and Corallimorpharia are more closely related to one another than either one is to Scleractinia.The composition and evolutionary relationships of the subclass Hexacorallia remains unresolved because analyses have yet to provide a consistent framework for understanding the phylogeny of higher-level groups within Hexacorallia [33]. The three aforementioned orders are particularly problematic because they are morphologically very similar [32][33][34], and the relationships among the three orders are still controversial [33].
Zooming into the order Actiniaria, they are amongst the most diverse members of the subclass Hexacorallia. Relationships within Actiniaria are also unresolved due to insufficient taxon sampling conducted in previous studies to derive consistent relationships [35]. A phylogenetic reconstruction of the order Actiniaria was published in 2014 using three mitochondrial (partial 12S rDNA, 16S rDNA and cox3) and two nuclear (18S rDNA and partial 28S rDNA) markers [36]. The DNA sequences for each marker were separately aligned using MAFFT. Multiple tree optimality criteria were used, namely maximum parsimony, Maximum Likelihood (ML) and Bayesian [36]. In the maximum parsimony analysis, trees of minimum length were found at least five times, data was subjected to 1000 rounds of bootstrap sampling to assess clade support, and gaps were treated as missing data [36] - one advantage of this gap treatment is that trees can be identified solely from the pattern of gaps in an alignment, so treating gaps as missing data may decrease the input of erroneous characters while still producing a reliable phylogenetic tree. However, missing data also has the potential to result in trees that are biologically meaningless, where gaps were treated as missing data when insertion events were more likely to have occured.
In the ML analysis, clade support was conducted using bootstrapping [36]. At the internal nodes of the phylogenetic tree derived from the ML analysis, maximum parsimony ancestral state reconstructions were performed using Mesquite for seven morphological characters - acontia, basilar muscles, deciduous tentacles, endosymbionts, longitudinal ectodermal muscles in the column, marginal sphincter muscle, and nematocysts with apical flaps [36].
Although some nodes had low bootstrap support which allows for ambiguity, the resultant phylogenetic tree derived from the ML analysis of molecular data was found to be consistent with the aforementioned morphological features (Fig. 24) [36]. It was mentioned that the analysis of members in the superfamily Actinioidea, within which E. quadricolor is placed, was not extensive relative to the clade diversity [36]. Hence, more phylogenetic work needs to be done to resolve the ambiguity and increase the understanding of higher-level groups, to reveal more information about the phylogeny of Entacmaea species within the family Actiniidae.

(Adapted from: Rodríguez et al. in [36], under a CC BY 4.0 license [65]). A higher resolution image can be found here.
DNA barcode
The National Center for Biotechnology Information contains nucleotide and protein sequences for E. quadricolor, which can be found here.Glossary
Digitiform - Shaped like a finger.Endosymbionts - Smaller symbiotic partners living inside a host organism, establishing endosymbiosis.
Ectosymbionts - Partners in a symbiotic relationship that remain on the surface of its host of occupy a body cavity.
Chaetodontid - Tropical marine fish in the family Chaetodontidae (butterflyfish, bannerfish, coralfish).
Cnidocyte - Also known as a cnidoblast or nematocyte, is an explosive cell containing a secretory organelle or cnida/nematocyst.
Nematocyst - Produced by cnidocyte for capturing or paralysing prey or for defense. Each nematocyst contains a coiled, hollow thread that can have barbs or spines and often contains poison.
Acetylcholinesterase inhibitor - A chemical that inhibits acetylcholinesterase enzyme from breaking down neurotransmitter acetylcholine.
Broadcast spawn - Animals release their eggs and sperm into the water for external fertilisation.
Waxing crescent moon - A crescent moon expanding in illumination.
Basionym - The original name on which a new name is based.
Nomenclature - A system or procedure of assigning names to groups of organisms as part of a taxonomic classification.
Phylogeny - The phylogeny of an organism is the evolutionary history of the organism.
References
1. Dunn, D. F. 1981. The clownfish sea anemones: Stichodactylidae (Coelenterata: Actiniaria) and other sea anemones symbiotic with pomacentrid fishes. Transactions of the American Philosophical Society,71(1), 3-115.2. Fautin, D. G., Crowther, A. L., & Wallace, C. C. 2008. Sea anemones (Cnidaria: Anthozoa: Actiniaria) of Moreton Bay.
3. Scott, A., & Harrison, P. L. 2009. Gametogenic and reproductive cycles of the sea anemone, Entacmaea quadricolor. Marine Biology, 156(8), 1659-1671.
4. Schwarck, N. T. 1999. Carbon Budgets of the Bulb-Tentacle Sea Anemone (Entacmaea quadricolor) Symbiotic with Zooxanthellae and Anemonefish(Doctoral dissertation, Walla Walla College).
5. Porat, D., & Chadwick-Furman, N. E. 2004. Effects of anemonefish on giant sea anemones: expansion behavior, growth, and survival. Hydrobiologia, 530(1-3), 513-520.
6. Tullock, J. H. 1998. Clownfish and Sea Anemones. Baron's.
7. Fautin, D. G., Chau-Chih, G., & Jiang-Shiou, H. 1996. Costs and benefits of the symbiosis between the anemoneshrimp Periclimenes brevicarpalis and its host Entacmaea quadricolor. Oceanographic Literature Review, 7(43), 701.
8. Godwin, J., & Fautin, D. G. 1992. Defense of host actinians by anemonefishes. Copeia, 1992(3), 902-908.
9. Fautin, D. G., Tan, S. H., & Tan, R. 2009. Sea anemones (Cnidaria: Actiniaria) of Singapore: abundant and well-known shallow-water species. Raffles Bulletin of Zoology, 121-143.
10. Wood, N. 2013. Entacmaea quadricolor. Retrieved Oct 23, 2016, from http://www.gbri.org.au/SpeciesList/Entacmaeaquadricolor%7CNicolaWood.aspx
11. Mebs, D. 2009. Chemical biology of the mutualistic relationships of sea anemones with fish and crustaceans. Toxicon, 54(8), 1071-1074.
12. Samejima, Y., Yanagisawa, M., Aoki-Tomomatsu, Y., Iwasaki, E., Ando, J., & Mebs, D. 2000. Amino acid sequence studies on cytolytic toxins from sea anemone Heteractis magnifica, Entacmaea quadricolor and Stichodactyla mertensii (Anthozoa). Toxicon, 38(2), 259-264.
13. Elliott, J. K., & Mariscal, R. N. 1997. Acclimation or Innate Protection of Anemonefishes from Sea Anemones?. Copeia, 284-289.
14. Nedosyko, A. M., Young, J. E., Edwards, J. W., & da Silva, K. B. 2014. Searching for a toxic key to unlock the mystery of anemonefish and anemone symbiosis. PloS one, 9(5), e98449.
15. Fautin, D. G., Westfall, J. A., Cartwright, P., Daly, M., & Wyttenbach, C. R. (Eds.). 2005. Coelenterate Biology 2003: Trends in Research on Cnidaria and Ctenophora (Vol. 178). Springer Science & Business Media.
16. Mire, P. 1998. Evidence for stretch‐regulation of fission in a sea anemone.Journal of Experimental Zoology, 282(3), 344-359.
17. Scott, A., & Harrison, P. 2007. Broadcast spawning of two species of sea anemone, Entacmaea quadricolor and Heteractis crispa, that host anemonefish. Invertebrate Reproduction & Development, 50(3), 163-171.
18. Harrison, P. L., & Wallace, C. C. 1990. Reproduction, dispersal and recruitment of scleractinian corals. Ecosystems of the world, 25, 133-207.
19. Bassim, K., Sammarco, P., & Snell, T. 2002. Effects of temperature on success of (self and non-self) fertilization and embryogenesis in Diploria strigosa (Cnidaria, Scleractinia). Marine Biology, 140(3), 479-488.
20. Wilson, J. R., & Harrison, P. L. 1997. Sexual reproduction in high latitude coral communities at the Solitary Islands, eastern Australia. In Proc 8th int coral Reef Symp (Vol. 1, pp. 533-538).
21. Tan, R. 2008. Wild Singapore Factsheets. Bubble-tip sea anemone. Retrieved 24 Oct, 2016, from http://www.wildsingapore.com/wildfacts/cnidaria/actiniaria/entacmaea.htm
22. Davison, G. W., Ng, P. K., & Ho, H. C. 2008. The Singapore red data book: Threatened plants & animals of Singapore. Nature Society.
23. Wabnitz, C. 2003. From ocean to aquarium: the global trade in marine ornamental species (No. 17). UNEP/Earthprint.
24. Shuman, C. S., Hodgson, G., & Ambrose, R. F. 2005. Population impacts of collecting sea anemones and anemonefish for the marine aquarium trade in the Philippines. Coral Reefs, 24(4), 564-573.
25. Hobbs, J. P. A., Frisch, A. J., Ford, B. M., Thums, M., Saenz-Agudelo, P., Furby, K. A., & Berumen, M. L. 2013. Taxonomic, spatial and temporal patterns of bleaching in anemones inhabited by anemonefishes. PloS one,8(8), e70966.
26. Fautin, D. 2015. Entacmaea quadricolor. In: Fautin, Daphne G. 2013. Hexacorallians of the World. Retrieved Oct 24, 2016, from http://www.marinespecies.org/aphia.php?p=taxdetails&id=289898
27. Fautin, D. G. 2013. Entacmaea quadricolor. Hexacorallians of the World. Retrieved Oct 24, 2016, from http://hercules.kgs.ku.edu/hexacoral/anemone2/imagedetail.cfm?imageid=3818&genus=Entacmaea&species=quadricolor&subgenus=&subspecies=
28. Global Biodiversity Information Facility. 2016. Senckenberg: Collection Cnidaria SMF. Retrieved Oct 25, 2016, from http://www.gbif.org/occurrence/207633496
29. Fautin, D. G. 2013. Hexacorallians of the World. Retrieved Oct 25, 2016, from http://tinyurl.com/holotype-information
30. Fautin, D. G. 2016. Catalog to families, genera, and species of orders Actiniaria and Corallimorpharia (Cnidaria: Anthozoa). Zootaxa, 4145(1), 1-449.
31. Global Biodiversity Information Facility. 2016. Australian Ocean Biogeographic Information System (OBIS Australua): Interim Register of Marine and Nonmarine Genera. doi:10.15468/6tkudz. Retrieved Oct 25, 2016, from http://www.gbif.org/species/107950962
32. Chen, C. A., Odorico, D. M., Tenlohuis, M., Veron, J. E. N., & Miller, D. J. 1995. Systematic relationships within the Anthozoa (Cnidaria: Anthozoa) using the 5′-end of the 28S rDNA. Molecular phylogenetics and evolution, 4(2), 175-183.
33. Daly, M., Fautin, D. G., & Cappola, V. A. 2003. Systematics of the hexacorallia (Cnidaria: Anthozoa). Zoological Journal of the Linnean Society,139(3), 419-437.
34. Veron, J. E. N., Odorico, D. M., Chen, C. A., & Miller, D. J. 1996. Reassessing evolutionary relationships of scleractinian corals. Coral Reefs,15(1), 1-9.
35. Daly, M., Chaudhuri, A., Gusmão, L., & Rodriguez, E. 2008. Phylogenetic relationships among sea anemones (Cnidaria: Anthozoa: Actiniaria). Molecular phylogenetics and evolution, 48(1), 292-301.
36. Rodríguez, E., Barbeitos, M. S., Brugler, M. R., Crowley, L. M., Grajales, A., Gusmão, L., ... & Daly, M. 2014. Hidden among sea anemones: the first comprehensive phylogenetic reconstruction of the order Actiniaria (Cnidaria, Anthozoa, Hexacorallia) reveals a novel group of hexacorals. PLoS One, 9(5), e96998.
37. "Figure 1. Rainbow bubble-tip anemone (Entacmaea quadricolor)" by Matthew Zahler. Permission pending. URL: https://thereeftankblog.com/my-livestock/rainbow-bubble-tip-anemone/
38. "Figure 2. Features of E. quadricolor". Adapted from Nicola Wood. Permission pending. Modifications: combined two images and added annotations.
URL: http://www.gbri.org.au/SpeciesList/Entacmaeaquadricolor%7CNicolaWood.aspx?aid=107&PageContentID=4475
39. "Figure 3. Bubble-tip anemone tentacle forms. Tentacle with terminal bud (left), Tentacle with subterminal bud (middle), Digitiform tentacle (right)". Adapted from Nhobgood Nick Hobgood (Own work) [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons. Modifications: combined image with [40] and [41].
URL: https://upload.wikimedia.org/wikipedia/commons/4/4d/Entacmaea_quadricolor_%28Bubble_tip_anemone%29.jpg
40. "Figure 3. Bubble-tip anemone tentacle forms. Tentacle with terminal bud (left), Tentacle with subterminal bud (middle), Digitiform tentacle (right)". Adapted from Chaloklum Diving (http://www.eol.org/data_objects/31317496) [CC BY 3.0 (http://creativecommons.org/licenses/by/3.0)], via Wikimedia Commons. Modifications: combined image with [39] and [41].
URL: https://upload.wikimedia.org/wikipedia/commons/3/37/Entacmaea_quadricolor_amarilla.jpg
41. "Figure 3. Bubble-tip anemone tentacle forms. Tentacle with terminal bud (left), Tentacle with subterminal bud (middle), Digitiform tentacle (right)". Adapted from Josuevg (Own work) [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons. Modifications: combined image with [39] and [40].
URL: https://upload.wikimedia.org/wikipedia/commons/5/58/Entacmaea_quadricolor_en_acuario_de_arrecife.jpg
42. "Figure 4. Entacmaea quadricolor in Singapore's waters" by Ria Tan [CC BY-NC-ND 2.0 (https://creativecommons.org/licenses/by-nc-nd/2.0/)].
URL: https://www.flickr.com/photos/wildsingapore/14783959036/
43. "Figure 5. Entacmaea quadricolor at Sisters Island" by Ria Tan [CC BY-NC-SA 2.0 license (https://creativecommons.org/licenses/by-nc-sa/2.0/)].
URL: https://www.flickr.com/photos/wildsingapore/3792260580/
44. "Figure 6. Global distribution of E. quadricolor". Adapted from Global Biodiversity Information Facility. [CC-BY 4.0 https://creativecommons.org/licenses/by/4.0/)]. Modifications: Cropped to fit. URL: http://www.gbif.org/species/4339208
45. "Figure 7. Anemonefishes symbiotic with E. quadricolor". Adapted from: Tullock in [6]. Permission pending. Modifications: Combined multiple images into one.
46. "Figure 8. Predator Pacific double-saddle butterflyfish (Chaetodon ulietensis) feeding on tentacles of E. quadricolor" by John Godwin in [8]. Permission pending.
47. "Entacmaea quadricolor escapes from being smothered by sedimentation". Adapted from: Nicola Wood, obtained and accredited under fair use. URL: https://www.youtube.com/watch?v=IO3oOMH43dk
48. "Figure 9. Live E. quadricolor specimen under GFP and RFP fluorescent channels...". Adapted from: Nicola Wood. Permission pending. Modifications: Cropped relevant section.
URL: http://www.gbri.org.au/SpeciesList/Entacmaeaquadricolor%7CNicolaWood.aspx?aid=107&PageContentID=4474
49. "Entacmaea quadricolor feeding on fish". Source: SquidMilk, obtained and accredited under fair use. URL: https://www.youtube.com/watch?v=i0UmN01DuMw&t=42s
50. "Figure 10. Relationship between number of anemonefish symbionts and anemone toxicity ranking" by Nedosyko et al. in [14]. [CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/)]. URL: http://dx.doi.org/10.1371/journal.pone.0098449.g004
51. "Figure 11. Longitudinal fission resulting in two genetically identical individuals". Adapted from: Nicola Wood. Permission pending. Modifications: Cropped relevant section.
URL: http://www.gbri.org.au/Portals/0/Images/Classes/2013/s4196721/asexual%20reproduction.png
52. "Figure 12. Broadcast spawning of male (a) and female (b) E. quadricolor". Adapted from: Cristiana Damiano in [17]. Permission pending. Modifications: Cropped relevant section.
53. "Male E. quadricolor spawning". Source: SRKreef, obtained and accredited under fair use. URL: https://www.youtube.com/watch?v=Vrq7LEVeDdQ
54. "Female E. quadricolor spawning". Source: J Madden, obtained and accredited under fair use. URL: https://www.youtube.com/watch?v=i5JfNYAi_ZM
55. "Figure 13. Tomato clownfish (Amphiprion frenatus) among E. quadricolor in Singapore's shores (Terumbu Raya)" by Ria Tan. [CC BY-NC-ND 2.0 (https://creativecommons.org/licenses/by-nc-nd/2.0/)]. URL: https://www.flickr.com/photos/wildsingapore/20704225310/
56. "Figure 14. Tomato clownfish (Amphiprion frenatus) among E. quadricolor in Singapore's shores (Pulau Semakau)" by Ria Tan. [CC BY-NC-ND 2.0 (https://creativecommons.org/licenses/by-nc-nd/2.0/)]. URL: https://www.flickr.com/photos/wildsingapore/2767084671/
57. "Figure 15. Entacmaea quadricolor and symbiotic anemonefishes in an aquarist's tank". Adapted from: Melev's Reef, obtained and accredited under fair use. Modifications: Screen capture of relevant section. URL: https://www.youtube.com/watch?v=iXIh24pfsps
58. "Figure 16. Individuals of E. quadricolor sold online". Adapted from: LA Reefs. Permission pending. Modifications: Screen capture of relevant section.
URL: https://www.lareefs.com/products/bubble-tip-anemone-3-pack
59. "Figure 17. Entacmaea quadricolor sold by a local supplier on Facebook". Adapted from: Pinnacle Aquatics Pte Ltd. Permission pending. Modifications: Screen capture of relevant section. URL: https://www.facebook.com/Pinnacleaq/
60. "Figure 18. Bleached E. quadricolor in Singapore's shores (Sisters Island)" by Ria Tan. [CC BY-NC-ND 2.0 (https://creativecommons.org/licenses/by-nc-nd/2.0/)].
URL: https://www.flickr.com/photos/wildsingapore/30027134601/
61. "Figure 19. Bleached E. quadricolor in Singapore's shores (Sultan Shoal)" by Joy Wong, own photo. Obtained through personal communication.
62. "Bleached E. quadricolor at Terumbu Raya, Singapore". Source: Ria Tan. [CC BY-NC-ND 2.0 (https://creativecommons.org/licenses/by-nc-nd/2.0/)].
URL: https://www.flickr.com/photos/wildsingapore/28777368385/
63. "Figure 22: Holotype of E. quadricolor in Senckenberg Museum" by Daphne Fautin. Permission pending.
URL: http://hercules.kgs.ku.edu/hexacoral/anemone2/imagedetail.cfm?imageid=4483&genus=Entacmaea&species=quadricolor&subgenus=&subspecies=
64. "Figure 23. Relationships of orders (Scleractinia, Actiniaria and Corallimorpharia) within the anthozoan subclass Hexacorallia". Adapted from: Springer-Verlag in [34]. Permission pending. Modifications: Highlighted relevant section in red.
65. "Figure 24. Construction of morphological characters within Hexacorallia, with red arrow showing position of E. quadricolor". Adapted from: Rodríguez et al. in [36] [CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/)]. Modifications: Highlighted relevant section in red and added a red arrow.
URL: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4013120/figure/pone-0096998-g003/
Please contact Shu Yi at a0114038@u.nus.edu for any queries.