Grapsus albolineatus (Latreille in Milbert, 1812) - the Sally Lightfoot Crab
Table of Contents
Frequently asked question:
Q: "Can eat one or not?"
A: No. They're toxic💀[1]. Please don't.
Name
Binomial: Grapsus albolineatus (Latreille in Milbert, 1812)
Vernacular: Sally-Light-Foot Crab, Mottled Crab (Kardousha et al., 2016)[2], Mottled Sally-Light-Foot Crab (Gumert & Malaivijitnond, 2012)[3], Mottled Lightfoot Crab (Kudlai et al., 2016)[4]
Fun fact:
This crab is cited as 'Latreille in Milbert (1812)' as Milbert had used Latreille's notes to describe Grapsus albolineatus while on his voyage around the Indian Ocean [5]. It is sometimes also cited as Lamarck, 1818, but the correct citation should be 'Latreille in Milbert, 1812' [5].
Etymology
The Genus 'Grapsus' refers to a group of sally lightfoot crabs that have a four-sided carapace (shell), a capacious posterior and short eyestalks [6]. The species name 'albolineatus' is derived from the Latin words 'alba' and 'lineatus', meaning 'white' and 'lined' respectively, and indeed, white lines can be observed on the crab's body (Figure 2). Grapsus albolineatus is one of the nine quasi-terrestrial crabs in the genus Grapsus [7]. Grapsus albolineatus belong to the Family 'Grapsidae', sometimes referred to as 'shore' or 'talon' crabs [8].
Figure 2: side-view of G. albolineatus found on Big Sisters' Island (photo taken by Gan Su Xuan)
Distribution in Singapore
In Singapore, they can be found on the shores of Sentosa, East Coast Park, Tanah Merah, Pulau Sekudu, Sisters' Islands, Kusu Island and St. John's Islands (Figure 3). In the figure below, the areas circled in red are the areas where G. albolineatus has been spotted.
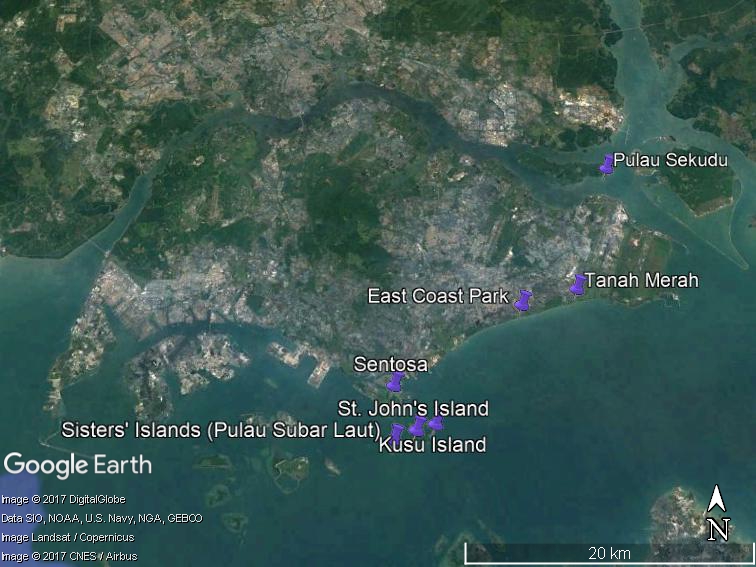
Figure 3: distribution of Grapsus albolineatus in Singapore (information obtained from Ria Tan's blog, Wild Singapore)[9]
Habitat
In general, this colourful crab is often found in intertidal areas, mangrove mudflats, rocky shores and open mudflats [10]. It usually forages at low tide levels, and as it moves very quickly and is rather shy, it is rarely spotted - one would have to be very patient when attempting to spot them!
Figure 4: the habitats that G. albolineatus can usually be found in. (photos A-B taken by Gan Su Xuan, photos C-D taken by Ria Tan on the Wild Singapore blog)[11, 12]: A and B show two of the intertidal shores of Singapore, where A is Pulau Semakau and B is Changi beach. C is the rocky shore of Labrador Park, and D is Kranji's mangrove mudflat.
Distribution in the World
Globally, Grapsus albolineatus can be found in certain parts of the Indo-West Pacific, around the Red Sea, western Indian Ocean, Japan and Polynesia [13].Biology
Behaviour
In general, the crab has been observed to stay above the water level at all times, even in intertidal areas [14]. Crab feeding periods tend to be limited to periods of low tide, and at high tide levels, G. albolineatus would stay put in one position and not move [14]. Based purely on observation, Kennish (1998) has found that the main predators of the crab are likely to be birds and other crabs when in the intertidal zones, and they sometimes also act as bait for groupers [14].Feeding Habits
Grapsus albolineatus was previously known to graze on algae and believed to be herbivorous - however, in a study by Kennish (1996), they are now also known to consume animal matter when available [15, 16].The crab's diet changes throughout the year, as algae are seasonal. According to a study by Kennish et al. (1996) in Hong Kong, G. albolineatus tends to feed preferentially on green filamentous algae, such as Enteromorpha spp. and Cladorphora spp., despite how foliose algae such as Ulva fasciata are more abundant in winter. In summer, as both foliose and filamentous algae are not present, the crabs would feed exclusively on encrusting algae [15]. It is interesting to note that despite foliose algae containing a higher nutrient content compared to filamentous algae, G. albolineatus would still preferentially feed on filamentous algae when given the choice [17]. According to Kennish & Williams (1997), the 'growth of G. albolineatus is enhanced and mortality reduced when given a diet of filamentous algae as opposed to foliose algae', and they eventually concluded that the morphology of the algae encouraged this preference. The crabs also generally forage at low tides, meaning that their window of time for feeding is very short [14].

GIF of G. albolineatus feeding on algae encrusted on rocks! GIF was created with the aid of Lightworks and Giphy. Video was taken by Paladej Srisuk. Original video here
A guide to algae morphs can be found here.
Fun facts:
The reproductive state, season and cycle of G. albolineatus have also been found to be affected by algae seasonality [18].
Though filamentous algae are neither the most nutritious nor easily-digested algae, the crab consumes so much of it that it is able to overcome this problem [18]!
Reproduction
According to Christy (1987), female brachyuran crabs often tend to moult before mating. However, it is uncertain if G. albolineatus, specifically, also moults before mating[19].
Figure 5: an example of a planktonic crab zoea (unidentified) (photo taken by Gan Su Xuan)
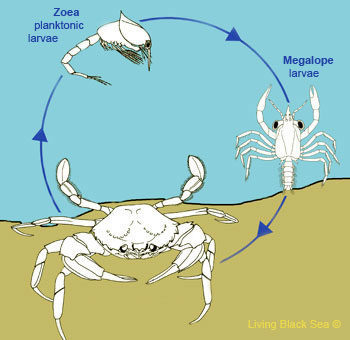
Figure 6: an example of the life cycle of the crab. Crab featured: Geocarcoidea lalandii (Image obtained from here)
The life cycle of crabs generally includes an egg-laying stage and larval stages, the larval zoea and megalopa stages. Larvae zoea and megalopa are planktonic in nature, and in these stages, they moult frequently as they grow, before finally obtaining distinctive adult crab features [20].
As the zoea moults, limbs and appendages would develop after each moult, before reaching the megalopa stage [20]. Zoea and megalopa larvae have different swimming patterns, where zoea tend to swim in a backwards manner, while megalopa swim in a forward direction [20]. In terms of feeding patterns, crab zoea can capture prey by lashing its tail, and also feeds on plant matter. Crab megalopa capture prey with their claws (chelae).
Parasites
A well-known parasite of the crab is known as the Rhizocephalan barnacle, and it is a rather gruesome and disturbing parasite. Its name is derived from the Greek words 'rhiza' and 'cephale', meaning 'root' and 'head' respectively, which likely alludes to how the barnacle takes over the crab.Rhizocephalans are a suborder of barnacles (infraclass: Cirripedia) that specialize in parasitizing Crustacea. Its life cycle is quite different from other barnacles, where its body does not have segments, a digestive system and appendages in certain life stages [21].
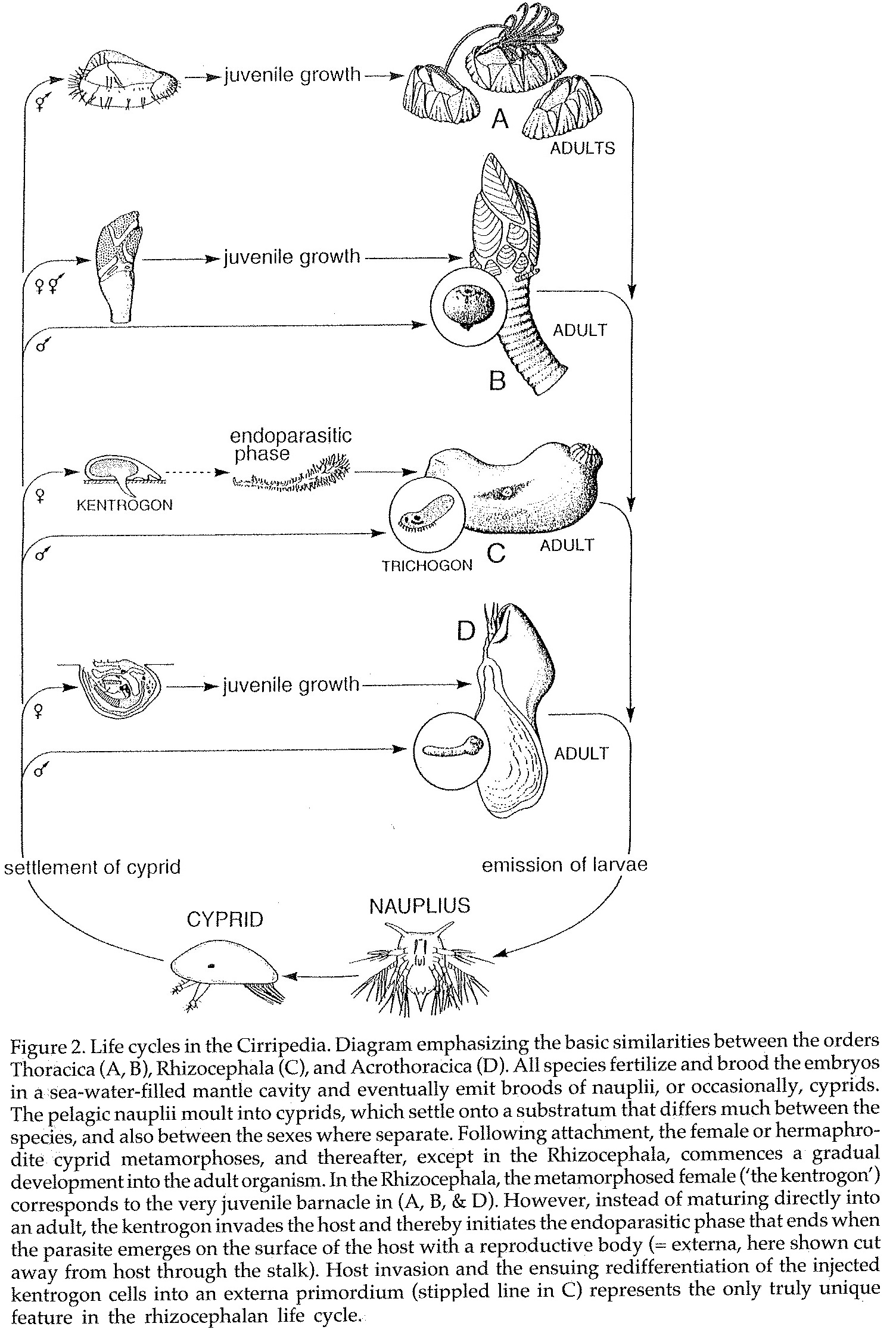
Figure 7: Reproductive cycles of different barnacles in the Cirripedia infraclass (image obtained from Hoeg, 1995)[22]
Figure 7 details the various different life cycles of barnacles, where C represents the life cycle of Rhizocephalan barnacles, which differs greatly from the two other life cycles.
Different species of Rhizocephalan barnacles can infect host crabs in different ways. The cyprid larva has two main modes of infection, where one involves the cyprid actively searching for a suitable site on the external surface of the crab to anchor itself on, and the other involves the cyprid attaching itself to the crab's gills [21]. A few days after attachment, the parasite then spreads a network of appendages throughout the crab, ultimately taking over the crab completely, where a cross-section would likely yield a crab whose innards have a disturbing spread of veins throughout the crab, including its legs, which is sometimes described as being 'root-like' (hence its name) [21]. The parasite would then survive by leeching its nutrients from the crab's tissues [23]. The cyprid that is attached to the crab is usually female, and at the site of attachment, a tumorous growth can be observed if the crab is dissected. This tumorous growth later emerges as something known as the 'externa', which can be observed as an outgrowth on the crab on the ventral site of its abdomen, and at this point, the crab stops moulting altogether [22, 24]. The parasite then begins to produce chemical substances to attract male Rhizocephalan cyprids to mate with it [22]. The male would attach to the female, which acts as a male organ, effectively turning the female parasite embedded in the crab into a hermaphrodite (cryptogonochorism) [22]. These male organs would then be able to fertilize the eggs of the female parasite, and cyprid larvae may develop and later be expelled from the host, or expelled directly as eggs [21].

Figure 8: the Rhizocephalan larvae taking over the entire body of the crab (image obtained from Wikipedia)
Alternatively, stephanieseashore does a very good explanation on the parasitism of Rhizocephala on crabs!
Fun fact:
The invasion of this parasite destroys the host's sexual organs, which can oftentimes also cause the abdomen of the crab to broaden, causing male crabs to sometimes be mistaken for female crabs, while female crabs experience little change in their external morphology [24, 25].
As a Potential Bioindicator
Studies have shown that Grapsus albolineatus can potentially play a role as a bioindicator. In a study done by Li (2004), G. albolineatus crabs have been found and observed to exhibit changes in its body symmetry (fluctuating asymmetry) depending on the stress levels experienced by the crab [26]. G. albolineatus has also been found to experience a very high rate of mortality when infected by the White Spot Syndrome Virus, which is a serious viral disease that can wipe out entire populations of shrimps in the aquaculture industry [27].Diagnosis
In intertidal areas, due to its swift speed and mosaic colouration, this species may sometimes be confused with the scaly rock crab, Plagusia squamosa. Additionally, both crabs are found in the same habitat, and in similar parts of Singapore (Sentosa, East Coast Park and Kusu Island), giving more opportunities for misidentification. Upon closer inspection, however, differences can be observed in the shells (carapace) and colouration of the crabs. Grapsus albolineatus has a more circular shell compared to P. squamosa, which has a squarish body (Figure 5).
Figure 9: Grapsus albolineatus on the left in Big Sisters' Island (photo taken by Marc Chang) and Plagusia squamosa on the right in East Coast Park (photo taken by Loh Kok Sheng)

Figure 10: Plagusia squamosa at Raffles Lighthouse (photo taken by Loh Kok Sheng) here, you can see its squarish, block-like carapace that differs from G.albolineatus' rounded carapace
Description
Naderloo (2017) gives a very thorough description of Grapsus albolineatus, and also provides a taxonomic key to allow one to distinguish the crab from other species (see "Original description")[8]. Glen Westbroek gives an example of a taxonomic key and how to use one here.The figures below feature the G. albolineatus, and its colouration (or rather, decolourisation) is due to its preservation in 70% ethanol. The specimen was collected by Aden Ip from Little Sisters' Island.
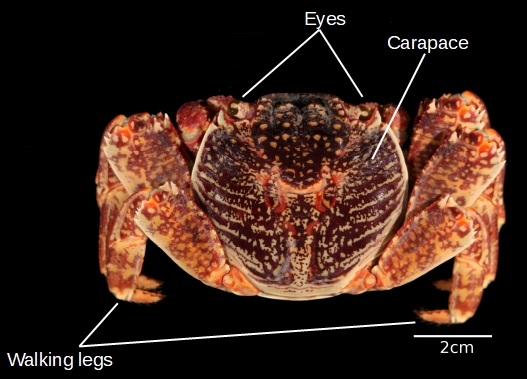
Figure 11: dorsal (top) view of G. albolineatus (photo taken by Gan Su Xuan)
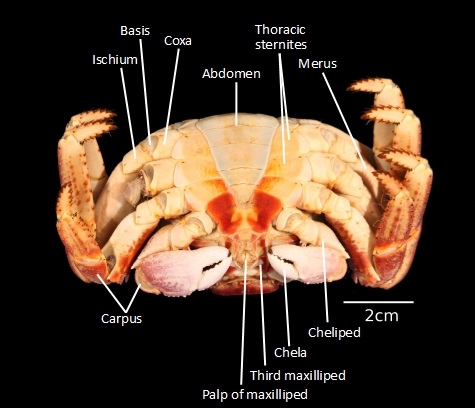
Figure 12: ventral (bottom) view of G. albolineatus (photo taken by Gan Su Xuan
Notably, G. albolineatus has four pairs of walking legs, and no paddle legs. The walking legs of the crab generally determine the type of habitat the crab is more commonly found in - the walking legs of G. albolineatus likely implies that it spends most of its time on land, moving at swift speeds, while the sharp claws on its legs may aid the crab in clinging onto surfaces when tides get rough [9]. In contrast, the legs of the spotted moon crab (Ashtoret lunaris) are all paddle-like instead of sharp and stilt-like like G. albolineatus.
A more detailed description of crab anatomy can be found here.
Original description
Description by Naderloo (2017)[8]:"Carapace quadrate to subcircular, dorsal surface moderately flat, usually with transversal ridges; lateral margins entire or with one dentation behind exorbital angle, converging posteriorly, junction of anterolateral and posterolateral margins not distinct. front broad, strongly deflexed, broadly bilobed. Orbits well developed, shorter than front, eyestalks moderately long. Antennules folded transversely. Antennae with basal segment bent, flagellum short. Third maxillipeds with broad rhombidal gap between, mandibles visible when third maxillipeds closed, no longitudinal setose ridge on outer surface of ischium and merus. Chelipeds medium-sized, merus usually with developed spines on anterodistal margin, fingers apically spoon-shaped. Walking legs robust, distinctly flattened, usually armed with spines and bristles. Abdomen of males and females with seven freely-moving somites. G1 robust, nearly straight, usually with dense setae on apical part; G2 much smaller than G1. Genital openings of males and females sternal.
Lateral margins of carapace arched, with postorbital tooth; frontal region with granules.
Lateral margins of carapace markedly arches; outer infraorbital angle with acute tooth; third maxillipeds with long merus, slightly shorter than ischium; male abdomen with somite 5 about 2.3 times as broad as long; G1 slightly curved; large sized species."
Size
According to Hartnoll (2009), the width of its carapace has been recorded to reach up to 71mm in length [28]. However, in a description by Naderloo (2011), the longest carapace length recorded for G. albolineatus is 50.27mm (male) and 59.14mm (female), while the longest breadths are 54.77mm and 63.80mm for males and female crabs respectively [29].Coloration
Grpasus albolineatus has been described by Vannini & Valmori (2013) to have a reddish body and reddish legs, white claws with dark tips and a body that is mottled and marbled with brown [30].It has also been described to sometimes have a bluish carapace, light red/orange walking legs, a light-coloured underside with a reddish-orange mosaic pattern (Figure 13) [29, 31].

Figure 13: picture of the alternative colouration of G. albolineatus, with a bluish carapace and orange walking legs (picture obtained from Wikipedia)
Sexing
To distinguish the male and female crabs of the species, one would have to observe the ventral side (underside) of the crab. Crabs with wider abdominal widths are usually female, while those with thinner abdominal widths would be males. It is likely that the crab featured in figures 11 and 12 is female.Phylogeny
In a study by Kitaura et al. (2002), they sequenced the 16S mitochondrial ribosomal RNA gene, 12S rRNA and 16s rRNA genes of 25 crab species in the Grapsidae, Ocypodidae and Camptrandiidae crab families [32]. In addition to this, they also used the sequencing data of 9 other crab species in an older dataset to construct the tree below (Figure 14) [32, 33].In this study, the authors applied maximum parsimony (MP) analysis, maximum likelihood (ML) and neighbour-joining (NJ) methods to analyze the phylogenetic relationships between these crab species. The figure below represents the MP analysis, where ML and NJ methods are not featured as the trees are quite similar in terms of twhere G. albolineatus is on the tree. The authors took into account the chances of DNA base transitions (TS) and transversions (TV) in their analysis, where the numbers indicate the weight (amount of emphasis put on) the particular analysis.
DNA base transversions refer to the swapping of purine bases with purine bases (A/G) or pyrimidine bases with pyrimidine bases (C/T). DNA base transversions refer to switches between pyrimidine and purine bases. By assigning weighted values of 1, 2 or 3 to the changes, it affects the building of the tree and the position of the organism on the tree. The values correspond to the number of base pair changes that can occur before the data is turned down - in a sense, room for error. More about weights here
In MP analysis, the tree obtained is the simplest tree which best explains the relationships of the organisms with each other [34]. It is somewhat similar to the principle of Occam's Razor, which states that the simplest route to explaining one's data is the most preferred.
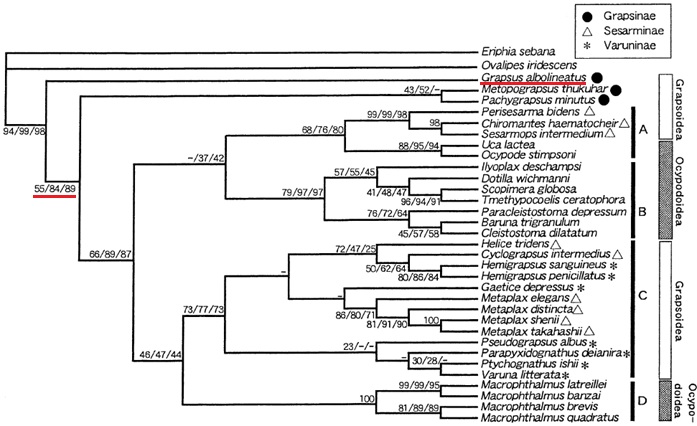
Figure 14: the phylogenetic relationships of grapsid and ocypodoid crabs through maximum parsimony analysis (tree obtained from Kitaura et al. (2002))[32]. Underlined in red is the location of G. albolineatus on the tree, and its TS1TV1/TS1TV2/TS1TV3 values.
According to Kitaura et al. (2002), this tree (Figure 14) is "The single most-parsimonious tree of 19 grapsid and 13 ocypodoid species and two outgroup taxa. A–D denotes four clusters commonly inferred from all of the tree reconstruction methods employed. Numbers at each branch indicate bootstrap values obtained after 1,000 replicates for each weighting scheme (TS1TV1/TS1TV2/TS1TV3). A single number indicates that the values were the same for all weightings. A dash indicates that a node was not recovered in bootstrap analysis." [32]
Interestingly, the tree shows that the family of Grapsidae crabs is polyphyletic, while the subfamilies are monophyletic [32].
Various trees have been constructed for Brachyuran crabs, but most have low confidence levels in terms of which group G. albolineatus falls under. According to the study by Tsang et al. (2014), the age of the Brachyuran infraorder may be as old as 180Ma, which is about as far back as the Jurassic age [35].
Taxonavigation
Table 1: table showing the classification of G. albolineatus| Kingdom |
Animalia |
| Phylum |
Arthropoda |
| Subphylum |
Crustacea |
| Superclass |
Multicrustacea |
| Class |
Malacostraca |
| Subclass |
Eumalacostraca |
| Superorder |
Eucarida |
| Order |
Decapoda |
| Suborder |
Pleocyemata |
| Infraorder |
Brachyura |
| Section |
Eubrachyura |
| Subsection |
Thoracotremata |
| Superfamily |
Grapsoidea |
| Family |
Grapsidae |
| Subfamily |
Grapsinae |
| Genus |
Grapsus |
| Species |
Grapsus albolineatus |
Synonyms
Cancer strigosus Herbst, 1799.Grapsus strigosus (Herbst, 1799).
Grapsus (Goniopsis) strigosus de Haan, 1835.
Grapsus (Goniopsis) flavipes MacLeay, 1838.
Grapsus albolineatus Lamarck, 1818.
Grapsus longipes Stimpson, 1858.
Grapsus peroni Milne Edwards H., 1853.
Grapsus granulosus Milne Edwards H., 1853
Grapsus strigosus Dana 1852.
Grapsus albolineatus Holthuis, 1958.
Type Information
The type information of G. albolineatus specifically refers to where a specimen of the crab which has been described is currently residing. According to Castro (2011), the type locality of the crab is Île de France, aka, Mauritius, but it is unknown where a specimen of the crab originally described may be found [38].
Conservation Status
The Mottled Lightfoot crab is not listed in the International Union for the Conservation of Nature (IUCN) Red List.
Literature and References
[1] Llewellyn L. E. & Endean R., 1988. Toxic coral reef crabs from Australian waters. Toxicon, 26: 1085–1088.[2] Kardousha M. M., Al-Muftah A. & Al-Khayat J. A., 2016. Exploring Sheroah Island at South-Eastern Qatar: first distributional records of some inland and offshore biota with annotated checklist. Journal of Marine Science: Reseeach & Development, 6: 191.
[3] Gumert M. D. & Malaivijitnond S., 2012. Marine prey processed with stone tools by Burmese long-tailed macaques (Macaca fascicularis aurea) in intertidal habitats. American Journal of Physical Anthropology, 149(3): 447–457.
[4] Kudlai O., Cribb T. H. & Cutmore S. C., 2016. A new species of microphallid (Trematoda: Digenea) infecting a novel host family, the Muraenidae, on the northern Great Barrier Reef, Australia. Systematic Parasitology, 93(9): 863–876.
[5] Ng P. K. L., Guinot D. & Davie P. J. F., 2008. Systema Brachyurorum: Part I. An Annotated Checklist of Extant Brachyuran Crabs of the World. Raffles Bulletin of Zoology, 17: 1–286.
[6] Luque J., Christy J. H., Hendy A. J. W., Rosenberg M. S., Portell R. W., Kerr K. A. & Palmer A. R., 2017. Quartenary intertidal and supratidal crabs (Decapoda, Brachyura) from tropical America and the systematic affinities of fossil fiddler crabs. Journal of Systematic Paleontology, 1–19.
[7] Araujo M., 2014. The leaping behavior of the sally lightfoot crab Grapsus grapsus (Crustacea: Decapoda: Brachyura) at an oceanic archipelago. Journal of Research in Biology, 4(4): 1357–1364.
[8] Naderloo R., 2017. Chapter 30: Family Grapsidae MacLeay, 1838 (Shore Crabs, Talon Crabs) In Atlas Crabs of the Persian Gulf (pp. 337–345). Cham, Switzerland: Springer International Publishing.
[9] Tan R., 2016. Sally-light-foot crab. Accessed from: WildSingapore, URL:
http://www.wildsingapore.com/wildfacts/crustacea/crab/grapsidae/albolineatus.htm. Date accessed: 2 Dec 2017.
[10] Jigneshkumar T., Gadhavi M. K. & Vachhrajani K. D., 2012. Diversity and habitat preference of brachyuran crabs in Gulf of Kutch, Gujarat, India. Arthropods, 1(1): 13–23.
[11] Tan R., 2017. Rocky shore ecosystem. Accessed from: WildSingapore. URL: http://www.wildsingapore.com/wildfacts/concepts/rocky.htm. Date accessed: 3 Dec 2017.
[12] Tan R., 2011. Rare seagrass at Kranji Nature Trail. Accessed from: Wild Singapore. URL: http://wildshores.blogspot.sg/2011/02/rare-seagrass-at-kranji-nature-trail.html#.WiO5ikqWbIU. Date accessed: 3 Dec 2017.
[13] Holthuis L. B., 1977. The Grapsidae, Gecarcinidae and Palicidae (Crustacea: Decapoda: Brachyura) of the Red Sea. Israel Journal of Zoology, 26: 141–192.
[14] Kennish R., 1998. Foraging behaviour of the tropical herbivorous crab, Grapsus albolineatus: time minimiser or nutrient maximiser? In: Morton L. B. (ed) The Marine Biology of the South China Sea III: Proceedings of the Third International Conference on the Marine Biology of the South China Sea: Hong Kong, 28 October-1 November 1996. Hong Kong University Press, Hong Kong, pp. 227–238.
[15] Kennish R., Williams G. A. & Lee S. Y., 1996. Algal seasonality on an exposed rocky shore in Hong Kong and the dietary implications for the herbivorous crab Grapsus albolineatus. Marine Biology, 125: 55–64.
[16] Kennish R., 1996. Diet composition influences the fitness of the herbivorous crab Grapsus albolineatus. Oecologia, 105: 22–29.
[17] Kennish R. & Williams G. A., 1997. Feeding preferences of the herbivorous crab Grapsus albolineatus: the differential influence of algal nutrient content and morphology. Marine Ecology Progress Series, 147: 87–95.
[18] Kennish R., 1997. Seasonal patterns of food availability: influences on the reproductive output and body condition of the herbivorous crab Grapsus albolineatus. Oecologia, 109(2): 209–218.
Ahyong S. T., Lai J. C. Y., Sharkey D., Colgan D. J. & Ng P. K. L., 2007. Phylogenetics of the brachyuran crabs (Crustacea: Decapoda): the status of Podotremata based on small subunit nuclear ribosomal RNA. Molecular Phylogenetics and Evolution, 45: 576–586.
[19] Christy J. H., 1987. Competitive mating, mate choice and mating associations of Brachyuran crabs. Bulletin of Marine Science, 41(2): 177–191.
[20] Warner G. F., 1977. Chapter 8: Life Histories. In: The Biology of Crabs. Elek Science London, University of Virginia, pp.119–140.
[21] Hoeg J. T. & Lutzen J., 1995. Life cycle and reproduction in the Cirripedia Rhizocephala. Oceanography and Marine Biology: an Annual Review, 33: 427–485.
[22] Hoeg J. T., 1995. The biology and life cycle of the Rhizocephala (Cirripedia). Journal of the Marine Biological Association of the UK, 75: 517–550.
[23] Takahashi T. & Matsuura S., 1994. Laboratory studies on moulting and growth of the shore crab, Hemigrapsus sanguineus de Haan, parasitized by a Rhizocephalan barnacle. Biological Bulletin, 186(3): 300–308.
[24] Li M-H., 2002. Fluctuating asymmetry and intersexuality in the shore crab Grapsus albolineatus near a coastal landfill site in northern Taiwan. Bulletin of Marine Science, 70(1): 75–88.
[25] Reinhard E. G., 1956. Parasitic castration of crustacea. Parasitology, 5: 79–107.
[26] Li M-H., 2004. Morphological biomarkers of shore crabs (Grapsus albolineatus) living in the vicinity of a coastal landfill site. Fresenius Environmental Bulletin, 13(6): 485–490.
[27] Hameed A. S. S., Balasubramanian G., Musthaw S. S. & Yoganandhan K., 2003. Experimental infection of twenty species of Indian marine crabs with white spot syndrome virus (WSSV). Diseases of Aquatic Organisms, 57: 157–161.
[28] Hartnoll R. G., 2009. Sexual maturity and reproductive strategy of the rock crab Grapsus adscensionis (Osbeck, 1765) (Brachyura, Grapsidae) on Ascension Island. Crustaceana, 82(3): 275–291.
[29] Naderloo R., 2011. Grapsoid crabs (Decapoda: Brachyura: Thoracotremata) of the Persian Gulf and the Gulf of Oman. Zootaxa, 3048: 1–43.
[30] Vannini M. & Valmori P., 2013. Researches on the coast of Somalia. The shore and the dune of Sar Uanle. Monitore zoologico italiano, 6: 57–101.
[31] Banerjee, S.K., 1960. Biological results of Snellius Expedition 18. The genera Grapsus, Geograpsus and Metopograpsus (Crustacea: Brachyura). Temminckia, 10: 132–199.
[32] Kitaura J., Wada K. & Nishida M., 2002. Molecular Phylogeny of Grapsoid and Ocypodoid crabs with special reference to the genera Metaplax and Macrophthalamus. Journal of Crustacean Biology, 22(3): 682–693.
[33] Kitaura, J., K. Wada, and M. Nishida. 1998. Molecular phylogeny and evolution of unique mud-using territorial behavior in ocypodid crabs (Crustacea: Brachyura: Ocypodidae). Molecular Biology and Evolution, 15: 626–637.
[34] Kannan L. & Wheeler W. C., 2012. Maximum parsimony on phylogenetic networks. Algorithms for Molecular Biology, 7(1): 9.
[35] Tsang L. M., Schubart C. D., Ahyong S. T., Lai J. C. Y., Au E. Y. C., Chan T-Y., Ng P. K. L. & Chu K. H., 2014. Evolutionary history of true crabs (Crustacea: Decapoda: Brachyura) and the origin of freshwater crabs. Molecular Biology and Evolution, 31(5): 1173–1187.
[36] Davie P., 2010. Grapsus albolineatus Latreille in Milbert, 1812. Accessed through: World Register of Marine Species. URL: http://www.marinespecies.org/aphia.php?p=taxdetails&id=207523. Date accessed: 3 Dec 2017.
[37] Marine Species Identification Portal, 2017. Grapsus albolineatus. Accessed from: Marine Species Identification Portal. URL:
http://species-identification.org/species.php?species_group=crabs_of_japan&menuentry=soorten&id=1653&tab=classificatie. Date accessed: 3 Dec 2017.
[38] Castro P., 2011. Catalog of the anomuran and brachyuran crabs (Crustaces: Decapoda: Anomura, Brachyura) of the Hawaiian Islands. In: Zootaxa 2947. Magnolia Press, Auckland, New Zealand. 154pp.
