 |
| Beccari's seagrass (Halophila beccarii). Notice the stripes on the leaf? That is why some local Singaporeans calls it the 'Tiger seagrass'. Image courtesy of Janet Chia. |
What are seagrasses? | General Biology | Distribution | Conservation status of Beccari's seagrass | How neglected are seagrasses globally and locally? | Developmental Biology | Taxonomy | Seagrass barcoding system
What are seagrasses?
Seagrass are true flowering plants living in marine ecosystems. They should not be confused with seaweeds, and can be easily differentiated by their true roots system as well as veins on their leaves.
Seagrass are the only group of plants that successfully invaded the marine world, evolving from terrestrial plants. They possess many structure to adapt to their marine existence, such as aerenchyma and use of inorganic carbon in water for photosynthesis[5]. Still, seagrasses are restricted by light requirements, and have to be in the photic zone. They are one of the most valuable ecosystems worldwide[6], providing the services of blue carbon[7], high primary productivity, habitat and various other ecological roles.
Seagrass is a misnomer, as it is more closely related to lilies than true grasses. In particularly, not all seagrasses are found in the sea, as Beccari’s seagrass is predominantly found in mangroves and estuaries. As the smallest seagrass in Singapore, Beccari’s seagrass is likely to be missed or dismissed as algae on mangrove mud, yet its thick mat is important as a habitat and substrate stabiliser.
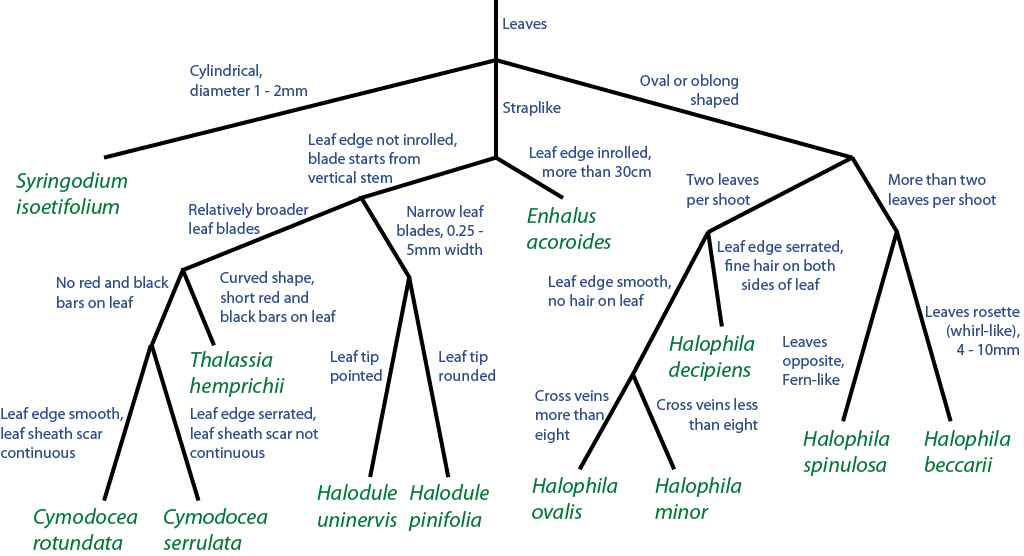 |
| Flowchart showing the use of leaf morphology for quick identification of our local seagrass species. Beccari’s seagrass (Halophila beccarii) is on the right end of the chart. Adapted from McKenzie et al. (2009). While Halophila decipiens and Beccari’s seagrass are one of the smallest local species, they can be easily distinguished by their leaf arrangement (the former is in pairs, while the latter is in a rosette). For more information on our local seagrass species, do visit here. |
General Biology
Habitat
Beccari's seagrass is found on muddy to sandy substrates[8-11]. The seagrass is euhaline (salinity range: 0 ppt to 33 ppt[11]), but has a small depth range of the upper intertidal areas[1,8]. As such, these peculiar plants are grow dense meadows[9] in estuarine areas, such as the mudflats, sandy shores, as well as with mangroves and salt marshes[8,12].
 |
| Beccari's seagrass meadow found growing on the muddy areas among the mangrove trees at Mandai mangrove, Singapore.Image courtesy of Lee Chengfa. |
Life Strategy
Beccari’s seagrass can be either annual[13] or perennial[12,14]. This would affect the plant’s phenology to either reproducing be throughout the year, or episodically[13]. Besides local environment resulting in difference in life strategy, Beccari’s seagrass can also be limited by resources availability, resulting in seasonality of growth[13,15]. A large portion of seagrass reproduction is asexual, as it provides the quick expansion of seagrass required for a species with colonisation as a strategy[16].
Ecological Importance
Beccari's seagrass are pioneer species in the estuary. The rhizomes bind to the substrate, thereby both stabilising the substrate, and helping in sediment accretion. The stabilised substrate is then conducive for recruitment of successive species such as mangrove, while accretion helps in seaward expansion of mangroves[15]. All seagrasses help in waves attenuation, which reduces water carrying capacity and promote sedimentation[7]. This includes Beccari’s seagrass, although its size has diminished this function.
As a habitat, Beccari’s seagrass meadows are home to crabs, molluscs[16,17], and the medically important horseshoe crabs[18]. The meadows are also nursery to other animals[15]. The uneven topography of the meadow traps algae, thereby increasing habitat complexity and primary production of the meadows[9].
| Beccari's seagrass meadow recolonising an abandoned land in Taiwan.Image courtesy of Ko Chih-Jen. |
Distribution
Global
Beccari's seagrass has been recorded to be at the coastlines of East Asia, South East Asia, Central Asia, Kenya and Tanzania. This lies in the Indo-Pacific region of global seagrass distribution, which is the richest in seagrass diversity in the world[8].
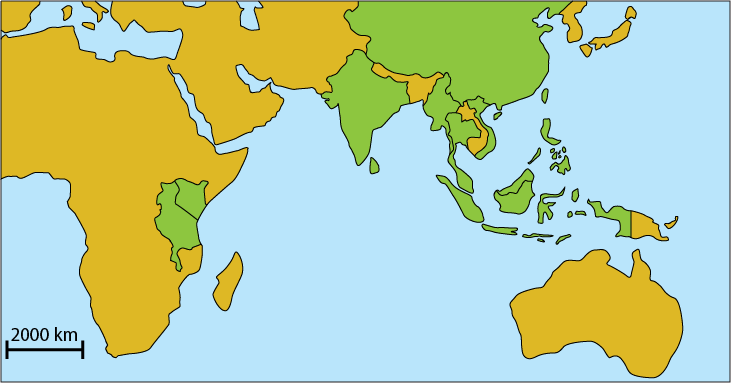 |
| Global distribution of Beccari's seagrass by country, based on literature. Regions with records of Beccari’s seagrass are Madagascar, India, Sri Lanka, Chaina, Myanmar, Thailand, Malaysia, Singapore, Indonesia, Vietnam, Hong Kong, Taiwan and Philippines. Map obtained from Google Maps @2012. Image courtesy of Lee Chengfa. <image pending amendment> |
Local status
Much of Singapore has undergone coastal development, resulting in an altered coastline and loss of many coastal ecosystems, such as the mangroves that Beccari's seagrass are found in, and the seagrass meadows. Beccari’s seagrass have been previously collected in Lim Chu Kang, Kranji, Pulau Buloh (small island in Sungei Buloh Wetland Reserve), Woodlands, and Tanah Merah Beach (which has since been reclaimed for the construction of Changi International Airport). Today, Beccari’s seagrass is known to be at the mangroves of Mandai, Sungei Buloh Wetland Reserve, Kranji Nature Trail and Chek Jawa (Pulau Ubin)[2].
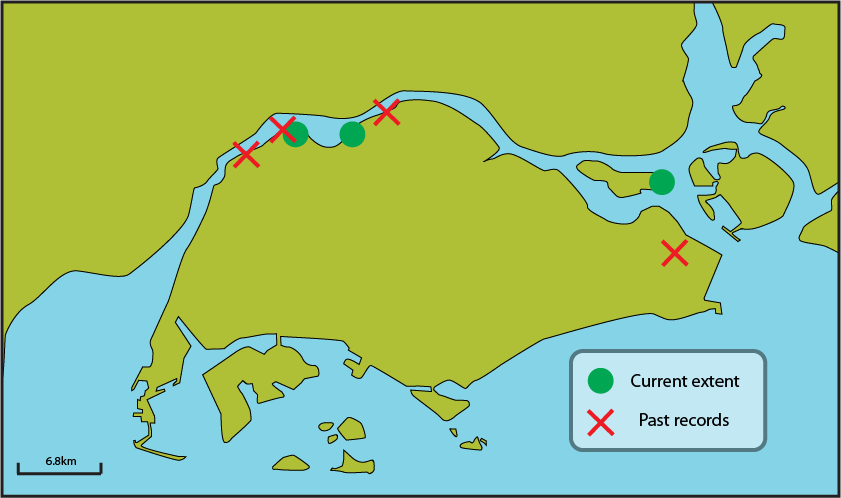 |
| Distribution of Beccari's seagrass in Singapore. Red crosses mark locations of old botanical records, while green circle marks current known distribution.Image courtesy of Lee Chengfa. |
Conservation status of Beccari's seagrass
One-third of global seagrass distribution has been lost and reducing, despite their highly valued ecosystem services. Furthermore, the Indo-Pacific region of seagrass has the largest data gap, despite being richest in seagrass diversity[19]. This has implications on Beccari’s seagrass, which lies in the Indo-Pacific region, and is also reducing in cover worldwide.
| IUCN Status |
Vulnerable[1] |
| Singapore Red List |
Critically endangered[20] |
Anthropogenic threats
Globally, humans have threatened seagrass habitats through coastal development and reclamation, reducing water quality as well as alien introduction. These results in situations ranging from total loss of the ecosystem to impacted environment that restricts seagrass growth and survival[19].
Similarly, Beccari’s seagrass is susceptible to the same threats, especially owing to its restricted range and habitat requirement. It is thus vulnerable to coastal development and reclamation[19,21], where its preferred habitats might be lost and there are no other suitable habitats. Declining water quality from human-induced eutrophication[17,22], pollution[9,22] and sedimentation[8,19] also impact seagrass survivability by either reducing light reaching seagrass, or smouldering and burying the seagrass. As a small seagrass species, Beccari’s seagrass is unable to within long periods of burial or insufficient light for photosynthesis, owing to low storage supplies from colonisation strategy and would die off.
Indirectly, seagrass meadows are impacted by our harvesting of marine resources. Besides impacts from destructive fishing practices such as explosive fishing, seagrass meadows are trampled and uprooted during gleaning of meiofauna or net trawling too[17,22].
Restoration
Seagrass restoration is difficult and minimally successful, especially as transplantation is underwater[7]. Additionally, seagrass meadows effect a positive feedback on themselves, as wave attenuation also allows for seagrass recruitment and development. Therefore, there will be a minimum threshold to be crossed to ensure sustainability of restoration.
How neglected are seagrasses globally and locally?
The proportion of academic research might be almost similar to that of mangroves and salt marshes internationally, but there is very low publicity for seagrass among the general public, such that the media reports per scientific paper is the least. Thus, impacts on seagrass are not widely known to the public, and lack public support for conservation of seagrass[19,23].
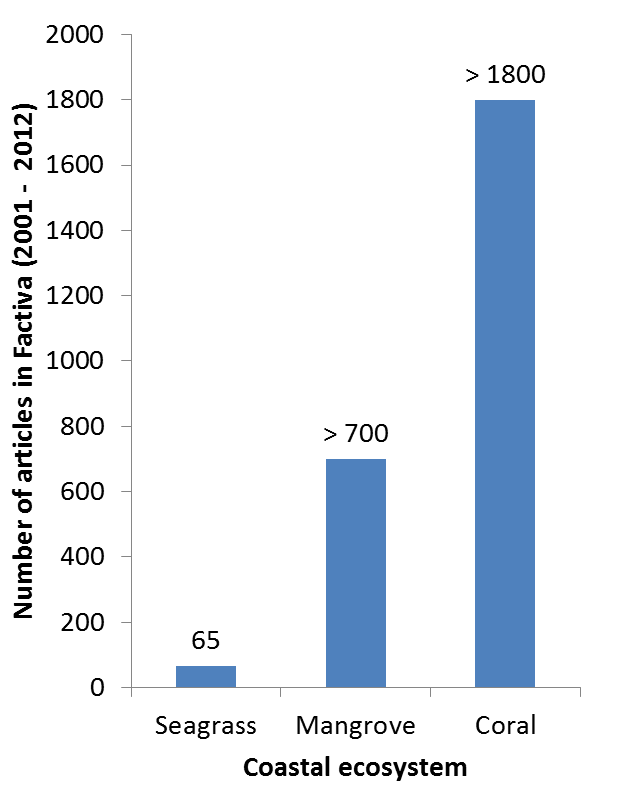
In Singapore, both research and media efforts are still establishing. In fact, of the 65 mentions of ‘seagrass’ in local media, many are in tandem with other coastal ecosystems such as mangroves and corals and do not provide much information on seagrass to the public. More can still be done to raise the awareness of seagrasses in Singapore, and the importance of protecting these threatened habitat.
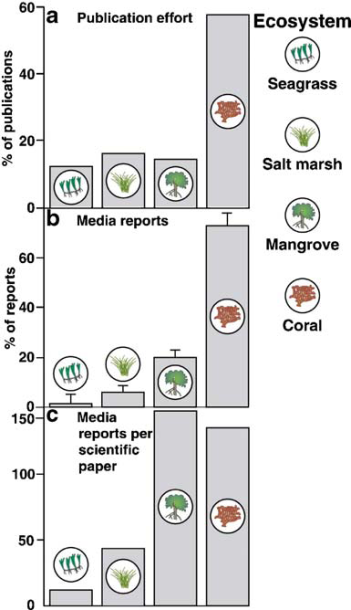 |
 |
| Graph showing total percentage of seagrass research, media reports and media reports per scientific paper, when compared to other coastal ecosystems. Image courtesy of Duarte C. M. |
Graph showing amount of media reports in Singapore. Separate searches were done on Factiva using the keywords 'seagrass', 'mangrove' and 'coral', from 2001 to March 2012, and limited to print and online news from the region of Singapore. Image courtesy of Lee Chengfa. |
Developmental Biology
Reproduction
Beccari’s seagrass can reproduce both sexually and asexually[15,24].
Asexual reproduction
The asexual reproductive method used by Beccari's seagrass is vegetative propagation. The basic unit or ramet of a seagrass comprises of a seagrass shoot and an internode, which the seagrass will continuously repeat by rhizome elongation[16]. Besides elongation, the rhizome also branches periodically, which increases the complexity of rhizome networks[16], forming dense meadows[9].
As a small seagrass species like other Halophila species, Beccari’s seagrass have a quick rhizome elongation rate[25], which helps the Beccari’s seagrass rapidly colonise as well as stabilise sediments to allow it to expand further[15]. Smaller seagrass species tend to also have shorter lifespans, so quick clonal growth will offset their lifespans, causing high turnover and positive population growth[25]. In addition, resources could also be shared, by lateral transfer through rhizomes, allowing the Beccari’s seagrass as a whole to adapt to habitat heterogeneity[16]. Nevertheless, cloning results in low genetic variation, which reduces resilience of the seagrass population[26].
Sexual reproduction
Beccari’s seagrass is a monoecious plant that pollinates through water, a mechanism termed as hydrophilous pollination. Flowers develop on a shoot in a pair, usually a male and female flower[9,27]. To reduce chances of self-fertilisation, the female flowers of Beccari’s mature first when the male flowers are still immature, in a mechanism known as protogyny[9]. When the male flowers are mature, pollen grains will be dispersed at night[9], either singly or in long chains (filiform)[9,28]. The latter pollen structure is an adaptation to the three dimensional nature of water and water currents, thereby increasing chances of pollination in water28.
Similarly, fruits dispersal of Beccari’s seagrass is through water, hydrophilous dispersal. The fruits tend to be carried to upper intertidal areas by the tides, whereby the fruits release their negatively buoyant seeds onto the substrate for recruitment[14]. Beccari’s seagrass has a large production of seeds. Although slower, sexual reproduction is still necessary to increase population resilience, especially in highly disturbed areas[25].
| The flowers of Beccari's seagrass comes in a pair.The label 'sf' is pointing to the male flower, and 'pf' to the female flower.Image courtesy of Ko Chih-Jen. |
Development of seedlings
Beccari’s seagrass seeds remain dormant until re-exposed from shallow burial. A seedling develops a pioneer shoot and three pioneer leaves from the seed, and continues producing leafs. By the eighth pioneer leaf, the cotyledons would have been shed while rhizome develops for propagation. Rhizome elongation and branching then initiates, and more shoots develop. Following, the seedling matures into a juvenile plant, as both the hypocotyl and the pioneer shoot leaves are lost in the original shoot[13].
| The first cotyledons 'C' developing from the hypocotyl 'HC' of Beccari's seagrass.The radicle 'R' is also seen. Image taken from Muta Harah (2002), pending permission. |
The seedling has already produced a unilateral rhizome system, comprising of two shoots.The pioneer shoot 'PS', lateral rhizome 'A', and lateral root 'LR'.Image taken from Muta Harah (2002), pending permission. |
Taxonomy
Beccari's seagrass was first described by Paul Friedrich August Ascherson in 1871. The seagrass was named after its holotype collector, Oroardo Beccari, an Italian naturalist who pioneered collection of plants in Borneo and made many contributions to Sarawak botany during his time[29]. A holotype is an individual that is used as the reference for the species description. Subsequent identification of other conspecifics will be based on this description.
 |
| Holotype of Beccari's seagrass, collected by Odoardo Beccari from Sarawak, Borneo.The holotype is currently kept in the Natural History Museum (specimen BM000958598).Image courtesy of Natural History Museum, London. |
Taxonavigation
| Eukaryote |
||||||||||||
| Plantae |
||||||||||||
| Viridiplantae |
||||||||||||
| Streptophyta |
||||||||||||
| Tracheophyta |
||||||||||||
| Euphyllophytina |
||||||||||||
| Spermatophytae |
||||||||||||
| Angiospermae |
||||||||||||
| Monocots |
||||||||||||
| Alismatales |
||||||||||||
| Hydrocharitaceae |
||||||||||||
| Halophila Thouars |
||||||||||||
| Microhalophila Ascherson |
||||||||||||
Description
The species name in taxonomy is an important label, as it associates any research information to a label. As such, it is important to be clear about the descriptions, so that we will not misidentify or mislabel an individual. If any of the three errors in taxonomy occurs (i.e. wrong description, wrong identification, wrong naming), then the research related to the erroneous taxonomy might be at best wrongly attributed to another species, or at worst a waste of time and effort producing useless scientific data.
Beccari's seagrass is a monocotyledon plant, under the group Angiospermae (flowering plant). Some of the morphological terms used for description are shown in the image below.
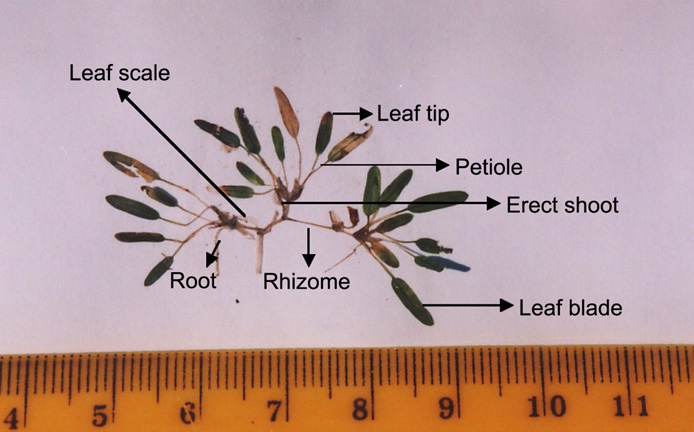 |
| Beccari's seagrass, labelled. Image courtesy of Abu Hena M K. |
Leaves
The leaves are arranged in pseudowhorls of 4 to 10 leaves, giving rise to a rosette arrangement[8,30]. The petiole and leaf blade are well differentiated[31]. Petioles are sheathed[30]. The leaf blades have been recorded to be either linear[8,10], elliptical[10] or lanceolate[32], with a width of less than 4 mm[10] and length of 6 to 11m[32].
Rhizome
The rhizome is a stem is horizontal and grows underground. There is a growing apex, where rhizome lengthening and cloning occurs. At regular intervals, branching would occur, resulting in a new branch of clones growing along side the original plant.
 |
| Rhizomes of Beccari's Seagrass. Image courtesy of 空空. |
Flowers
A pair of flowers will develop together, from the centre of the rosette[13]. The pair of flowers are usually of mixed gender (i.e. one male and one female flower), although single gender pairs have been observed before.
 |
| The leaves of Beccari's seagrass grows out in a pseudowhorl. Flowers will develop among the pseudowhorl (or rosette), as shown by the red arrow. Image courtesy of Lee Chengfa. |
Male flowers
Male flowers are reduced, have a curved bud, slender pedicels of maximum length 8mm, and three tepals of maximum width 2.5mm and length 1.5mm. Each flower has three anthers. The flower is erect at maturity. Pollen produced is ellipsoidal in shape, and is dispersed either singular or in a chain[9].
| Unopened male flower of Beccari's seagrass. Image courtesy of Ko Chih-Jen. |
Female flowers
The female flowers are reduced, comprising of an ovary of maximum length 3mm that contains 2 to 4 ovules, a hypanthium of maximum length 2mm that is protected by a sheath and two styles of maximum length 1.5cm. One style is usually longer than the other, and may split at midway to form three filament-like stylar branches[9].
| Female flower of Beccari's seagrass.Notice that the two styles 'st' are of different lengths.Image courtesy of Ko Chih-Jen. |
Dissected femal flower of Beccari's seagrass, showing the ovules 'fn'.Image courtesy of Ko Chih-Jen. |
Fruit
The fruit is oval shaped with a curved apex, membranous reddish brown pericarp (seed coat) that is reticulate, and grows to a maximum of length 4.5mm and width 1.2mm. There are 1 to 4 seeds in each fruit. The seeds are hard and globular, to a maximum of length 1.2mm and width 1mm[9].
Seagrass barcoding system
To better aid conservation of seagrass, seagrass barcoding systems are proposed to manage seagrass identification worldwide. This is made more urgent, as molecular data of seagrasses of the species rich Indo-Pacific region are not well documented despite much loss in seagrass meadows. Parataxonomist can use DNA barcoding to more accurately identify a species, especially when identifying highly plastic species in a meadow such as Halophila[33].
In order to have a comparison between species, a gene is chosen as a reference for barcoding. This gene has to evolve sufficiently fast, so as to be able to distinguish between sister species, but yet not too fast such that even genetic variation within species is considered as a new species[34]. Also, the chosen gene should be easily amplified, to reduce the minimum sample amount required for cross referencing the barcoding system[35]. However, it should still be noted that the chosen gene might evolve at different rates in different clades, or be inadequate in resolving between species that are too closely related[34]. As such, COI sequence is adequate for DNA barcoding of animal but not for plant35. To substitute in plants and seagrass barcoding system, the use of a multilocus approach[34] – matK (best distinguishing capability), rbcL (most conservative) and trnH-psbA[33,35] – is implemented.
Lucas et al. (2012) proposed a seagrass barcode system, based on their final consensus tree using four optimality criterion – maximum parsimony, distance, maximum likelihood and Bayesian inference. Most of the seagrass sequences used are also stored in a database for future reference[33]. Three major clades of seagrasses are observed, which is reflective of the polyphyletic nature of seagrasses[28]. However, Halophila genus is still mostly unresolved. Fortunately, this excludes Beccari’s seagrass, which has character attributes so distinct that it is in a different clade, and unknown species A can be identified as Beccari’s seagrass. Despite the success in creating a seagrass barcoding system, some sceptism should still be reserved, as manual alignment was used at multiple instances in this system, which is not easily replicable to the same results by another scientist or parataxonomist. Nevertheless, the high support values might still suggest useful taxonomic identification[33].
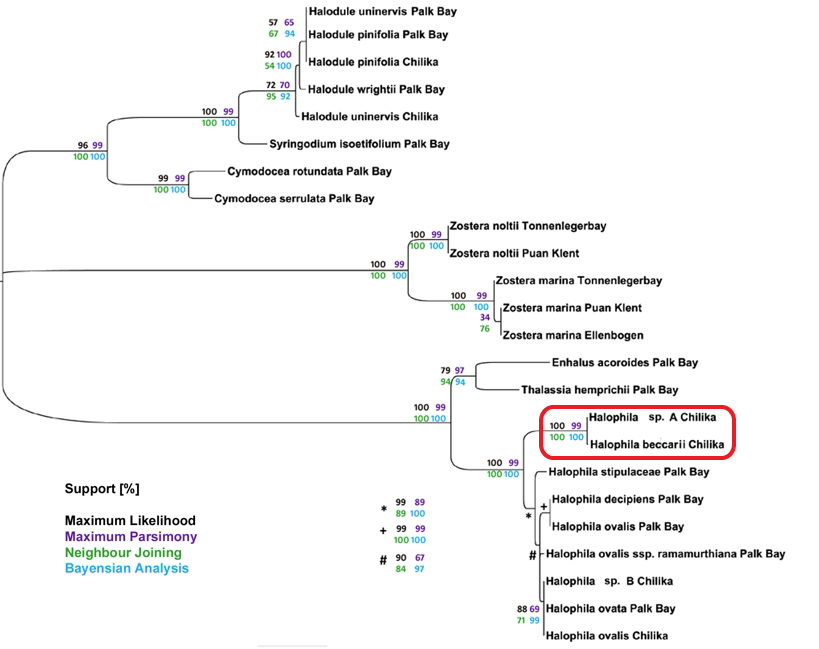 |
| Phylogeny tree of seagrass, with Beccari's seagrass circled in red. Image courtesy of Dr. Jutta Papenbrock, from Lucas et al. (2012). |
External websites
IUCN page on //Halophila beccarii//
EOL page on //Halophila beccarii//
Hudong online encyclopedia on //Halophila beccarii// [Chinese]
A webpage by a nature enthusiast on the marine biodiversity of Singapore
Wildsingapore page on //Halophila beccarii//
A webpage seeking collaborators to set up a seagrass barcoding system.
Global Initiative to barcode seagrasses
A local branch of the international seagrass monitoring exercise
TeamSeagrass homepage
HTML Comment Box is loading comments...
Reference
- Short, F. T., Coles, R., Waycott, M., Bujang, J. S., Fortes, M., Prathep, A., Kamal, A. H. M., Jagtap, T. G., Bandeira, S., Freeman, A., Erftemeijer, P., La Nafie, Y. A., Vergara, S., Calumpong, H. P. & Makm, I. (2010). Halophila beccarii. IUCN Red List of Threatened Species Version 2012.2. Retrieved 24 October, 2012, from www.iucnredlist.org
- Tan, R., Yaakub, S. M., & Dinesh, A. (2011). Beccarii: Tiny but mighty. Seagrass-Watch, 12–15.
- Short, F., Carruthers, T., Dennison, W., & Waycott, M. (2007). Global seagrass distribution and diversity: A bioregional model. Journal of Experimental Marine Biology and Ecology, 350(1-2), 3–20.
- McKenzie, L. J., Yaakub, S. M., & Yoshida, R. L. (2009). Seagrass-Watch: Proceedings of a Workshop for Monitoring Seagrass Habitats in Singapore, 1st–3rd May 2009.
- Touchette, B. W., & Burkholder, J. M. (2000). Overview of the physiological ecology of carbon metabolism in seagrasses. Journal of Experimental Marine Biology and Ecology, 250, 169–205.
- Costanza, R., d’Arge, R., de Groot, R., Farberk, S., Grasso, M., Hannon, B., Limburg, K., Naeem, S., O'Neill, R. V., Paruelo, J., Raskin, R. G., Suttonkk, P. & van den Belt, M. (1997). The value of the world’s ecosystem services and natural capital. Nature, 387, 253-260.
- Nellemann, C., Corcoran, E., Duarte, C. M., Valdés, L., De Young, C., Fonseca, L. & Grimsditch, G. (2009). Blue Carbon: A Rapid Response Assessment. Norway: Birkeland Trykkeri AS.
- Green, E. P., & Short, F. T. (2003). World Atlas of Seagrass. Berkeley, USA: UNEP–WCMC by the University of California Press.
- Parthasarathy, N., Ravikumar, K., & Ramamurthy, K. (1988). Floral Biology and Ecology of Halophila beccarii Aschers. (Hydrocharitaceae). Aquatic Botany, 31, 141–151.
- Ko, C.-J. (2003). A Taxonomic and Distributional Study of Seagrass in Taiwan. National Sun Yat-sen University.
- Jagtap, T. G., & Untawale, A. G. (1981). Ecology of seagrass bed Halophila beccarii (Aschers.) in Mandovi estuary, Goa. Indian Journal of Marine Science(16), 215–217.
- Abu Hena, M. K., & Short, F. (2009). A New Record of Seagrass Halophila beccarii Ascherson in Bangladesh. CMU. J. Nat. Sci, 8(2), 201–206.
- Muta Harah, Z., Japar Sidik, B., & Arshad, A. (2002). Flowering, Fruiting and Seedling of Annual Halophila beccarii Aschers in Peninsular Malaysia. Bulletin of Marine Science, 71(3), 1199–1205.
- Muta Harah, Z., Japar Sidik, B., & Hishamuddin, O. (1999). Flowering, fruiting and seedling of Halophila beccarii Aschers. (Hydrocharitaceae) from Malaysia. Aquatic Botany(65), 199–207.
- Abu Hena, M. K., Short, F. T., Sharifuzzaman, S. M., Hasan, M., Rezowan, M., & Ali, M. (2006). Salt marsh and seagrass communities of Bakkhali Estuary, Cox’s Bazar, Bangladesh. Estuarine, Coastal and Shelf Science(75), 72–78.
- Kendrick, G. A., Duarte, C. M., & Marbà, N. (2005). Clonality in seagrasses, emergent properties and seagrass landscapes. Marine Ecology Progress Series, 290, 291–296.
- Huang, X. P., Jiang, Z. J., Zhang, J. P., Shi, Z., Wang, F., Ye, F. & Li., L. (2010). Newly discovered seagrass beds in the coastal seas of Guangdong Province. Journal of Tropical Oceanography, 29(1), 132−135.
- Morton, B., & Lee., C. N. (2010). Spatial and temporal distributions of juvenile horseshoe crabs (Arthropoda: Chelicerata) approaching extirpation along the northwestern shoreline of the New Territories of Hong Kong SAR, China. Journal of Natural History, 45(3–4), 227–251.
- Waycott, M., Duarte, C. M., Carruthers, T. J. B., Orth, R. J., Dennison, W. C., Olyarnik, S., Calladine, A., Fourqurean, J. W., Heck, Jr., K. L., Hughes, A. R., Kendrick, G. A., Kenworthy, W. J., Short, F. T. & Williams, S. L. (2009). Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Paper presented at the Proceedings of the National Academy of Sciences of the United States of America.
- Davison, G. W. H., Ng, P. K. L., & Ho, H. C. (2008). The Singapore Red Data Book: Threatened plants and animals of Singapore. Singapore: Nature Society.
- Japar Sidik, B., Muta Harah, Z., & Kawaguchi, S. (2012). Historical review of seagrass research in Malaysia before 2001. Coastal Marine Science, 35(1), 157–168.
- 黄小平, 黄良民, 李颖虹, 许战洲, 方静威, 黄道建, 韩秋影, 黄晖, 谭烨辉 & 刘胜 (2006). 华南沿海主要海草床及其生境威胁. 科学通报, 51(2), 114–119.
- Duarte, C. M., Dennison, W. C., Orth, R. J., & Carruthers, T. J. B. (2008). The charisma of coastal ecosystems: addressing the imbalance. Estuaries and Coasts, 31(2), 233-238.
- den Hartog, C. (1970). The sea-grasses of the world. Amsterdam: North-Holland Pub. Co.
- Duarte, C. M., Fourqurean, J. W., Krause-Jensen, D., & Olesen, B. (2006). Dynamics of Seagrass Stability and Change. In A. W. D. Larkum, R. J. Orth & C. M. Duarte (Eds.), Seagrasses: Biology, Ecology and Conservation. The Netherlands: Springer.
- Jiang, K., Shi, Y.-S., Zhang, J., & Xu, N.-N. (2011). Microsatellite Primers for Vulnerable Seagrass Halophila beccarii (Hydrocharitaceae). American Journal of Botany, e155–e157.
- Japar Sidik, B., Muta Harah, Z., Arshad, A., Lam, S. L., & Ogawa, H. (2006). Flowers and sexes in Malaysian seagrasses. Coastal Marine Science, 30(1), 184–188.
- Ackerman, J. D. (2006). Sexual Reproduction of Seagrasses: Pollination in the Marine Context. In A. W. D. Larkum, R. J. Orth & C. M. Duarte (Eds.), Seagrass: Biology, Ecology and Conservation (pp. 89–109). The Netherlands: Springer.
- Merrill, E. D. (1912). A Flora of Manila. Manila: Bureau of Printing.
- den Hartog, C., & Kuo, J. (2006). Taxonomy and Biogeography of Seagrasses. In A. W. D. Larkum, R. J. Orth & C. M. Duarte (Eds.), Seagrass: Biology, Ecology and Conservation (pp. 1–23). The Netherlands: Springer.
- Yang, Y.-P., Fong, S.-C., & Liu, H.-Y. (2002). Taxonomy and Distribution of Seagrasses in Taiwan. Taiwania, 47(1), 54−61.
- den Hartog, C., & Yang, Z. (1990). A Catalogue of the Seagrasses of China. China Journal of Oceanology and Limnology, 8(1), 74−91.
- Lucas, C., Thangaradjou, T., & Papenbrock, J. (2012). Development of a DNA Barcoding System for Seagrasses: Successful but Not Simple. PLoS ONE, 7(1), 1−12.
- Tavares, E. S., & Baker, A. J. (2008). Single mitochondrial gene barcodes reliably identify sister-species in diverse clades of birds. BMC Evolutionary Biology, 8(81), 1−14.
- Sun, X.-Q., Zhu, Y.-J., Guo, J.-L., Peng, B., Bai, M.-M., & Hang, Y.-Y. (2012). DNA Barcoding the Dioscorea in China, a Vital Group in the Evolution of Monocotyledon: Use of matK Gene for Species Discrimination. PLoS ONE, 7(2), 1−7.