Black Soldier Fly(Linnaeus 1758)
 |
| Fig. 1. Hermetia illucens (Linnaeus, 1758). Image from Samuel Ewing. Permission obtained and accredited under copyright. |
Overview
Table of Contents
The Black Soldier Fly (Hermetia illucens) is a cosmopolitan fly belonging to the Stratiomyidae family (1). It is a relatively large fly with wasp-like appearance. However, unlike wasps, H. illucens only possess one pair of wings and lacks a stinger. Moreover, the species exhibits a vast geographical range from America to South-east Asia (2) (3) (4). H. illucens is usually found in outdoor environments near livestock or decaying organic matter, including animal waste (5). H. illucens larvae are widely used in manure management, controlling of housefly populations and the conversion of organic waste into useful products such as compost (5). The larvae are also sold as important sources of food for reared amphibians and fish as they are high in calcium content (6). In forensic entomology, the life history and development of H. illucens in decaying corpses is used by medico-legal investigators for post-mortem analysis (7) (8).
Distribution
Locality
(Back to top)Hermetia illucens is a widespread cosmopolitan species found in various parts of the world (4). The species is hypothesized to originate from tropical, subtropical and temperate zones of America though this is widely contested (4) (9) (10). It is believed that international transport and commerce in the 20th century led to the introduction of H. illucens in other parts of the world. However, others hypothesize that the species is native to Palearctic regions of earth (3) (4) (11). In Europe, H. illucens was first recorded in Malta and has since been discovered and recorded in other parts of Europe and Asia (4); (11); (12). The widespread distribution of H. illucens is shown in the map below (4):
Habitat
Hermetia illucens are commonly found resting on garden plants, tree trunks and walls and windows in urban residential areas (13). The species also inhabit areas with livestock and decaying organic matter (13).Significance of Hermetia illucens
Environmental importance
(Back to top)Unlike the common housefly, Hermetia illucens is not considered a pest as it is not attracted to food or houses (14). In fact, their larvae play pivotal roles in both environmental and economic aspects of waste disposal and processing. H. illucens larvae have been documented to reduce dry manure biomass by 42-56%. Nitrogen concentration and mass were also reduced by 24% and 62% respectively (15) (16). This drastically reduces the amount of environmental pollutants. Moreover, food processed by larvae aerates and dries up organic waste, thereby reducing the production of gases such as methane, an important greenhouse gas (5). Other odours that may attract pests are also eliminated as the larvae feed on waste (5).
Feeding by H. illucens larvae greatly reduces the production and establishment of a pest housefly, Musca domestica L., near decaying organic matter (14) (15) (17). This occurs as H. illucens larvae inhibit the oviposition of M. domestica L. on food waste, possibly by overcrowding their food resource and producing short-lived interspecific chemical signals (18). H. illucens also alters the microflora composition of organic waste, reducing the growth of harmful bacteria such as Escherichia coli O157:H7 and Salmonella in manure (19) (20). Consequently, this reduces the spread of disease by reducing bacteria growth and disease vectors such as M. domestica L.
Vid. 1. Housefly larvae out-competed by Hermetia illucens larvae. Obtained from YouTube under fair use
Economic importance
Economically, Hermetia illucens reduces costs from waste collection and landfills. It is estimated that landfilling is three times more expensive than composting (21). By reducing the volume of waste by 50%, H. illucens drastically decreases the need and costs of landfill. Notably, organic waste composting by H. illucens allows for waste recycling and fertilizer production (20) (22). Harvested H. illucens larvae and pre-pupae can be sold as fish feed in various forms (see rearing and breeding in captivity), replacing more expensive conventional fish feed. H. illucens has also been used in the production of sugar and biodiesel from dairy manure (23). It is estimated that manure management using H. illucens could increase revenue by $25 000 per year per cage house of livestock (5). However, research into the industrialization of H. illucens is currently ongoing. For example, research on the use of H. illucens as a composting agent in campuses is currently conducted in Singapore and America (24) (25).
Hermetia illucens larvae or pre-pupae could be used as high fat and protein feed for fishes as many conventional fish feed lack essential amino acids or have low protein content (6) (26) (27) . Larvae and pre-pupae can be fed live, chopped, frozen or ground
(6) (26) (27). Studies have shown that a diet consisting of H. illucens larvae and pre-pupae does not negatively affect fish growth. It may thus serve as a valuable replacement for other more expensive sources of fish feed (28) (29). H. illucens larvae was so successful as a feeder insect that it became the first to be registered under a U.S trademark and is now marketed under the brand name “Phoenix Worms”. It is also sold under different brands such as "Reptiworms" (Canada) and "Calci Worms" (30) (31) (32).
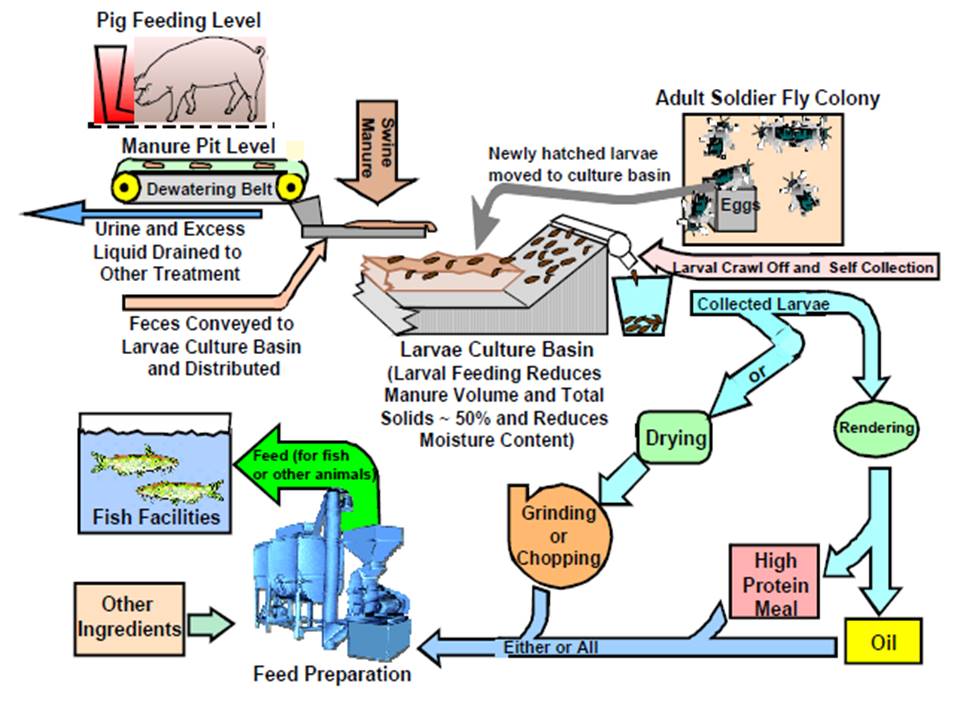 |
| Fig. 2. Schematic of an industrial composting process employing the use of Hermetia illucens larvae as a composting organism and harvesting for production of fish feed (Newton et al., 2005). Obtained and accredited under fair use |
Should Hermetia illucens be unavailable for composting, species such as the yellow soldier fly (Ptecticus trivittatus) are also known to degrade organic matter (33). However, unlike H. illucens, P. trivittatus prefers rotten fruit which attracts their females to lay eggs (34). P. trivittatus are usually found in compost along with H. illucens and can be distinguished by their smaller size, yellowish colouration, golden thorax and short yellow antennae (35). Little research has focused on the composting ability of P. trivittatus and its usefulness as a composting organism remains unknown.
 |
| Fig 3. Image of yellow soldier fly (Ptecticus trivittatus). Obtained from Sally G. Miller. Permission obtained and accredited under copyright. |
Morphology and Identification
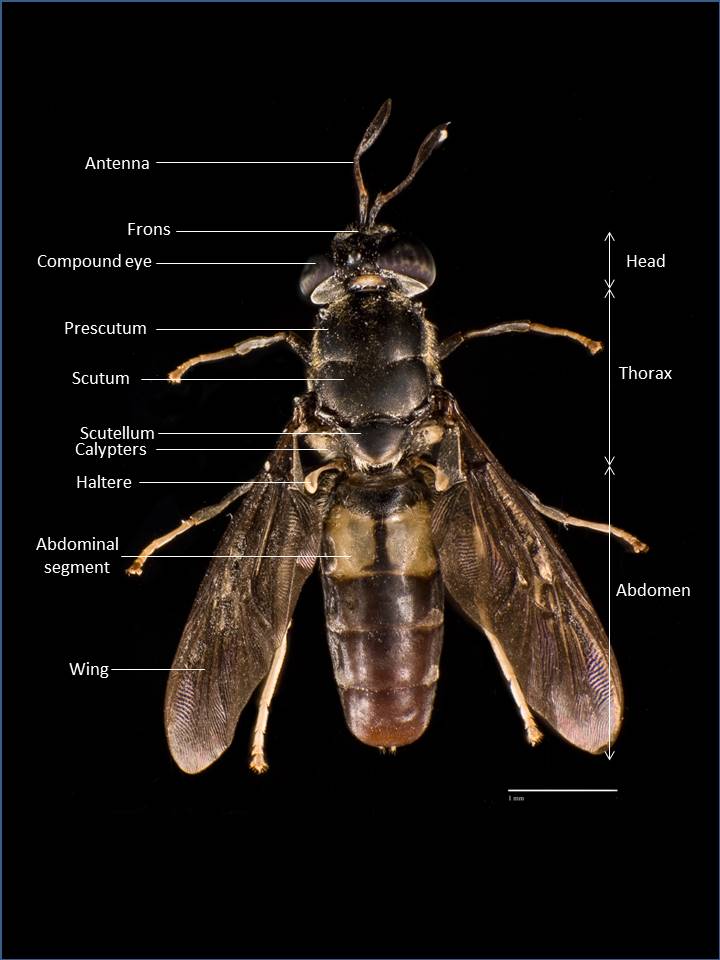
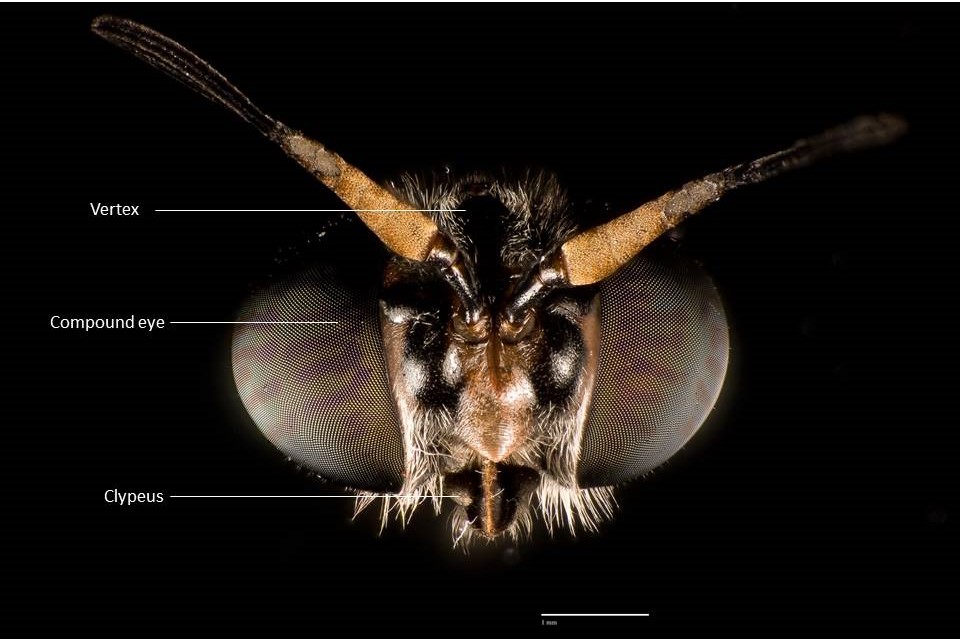
General anatomy
(Back to top)
|
|
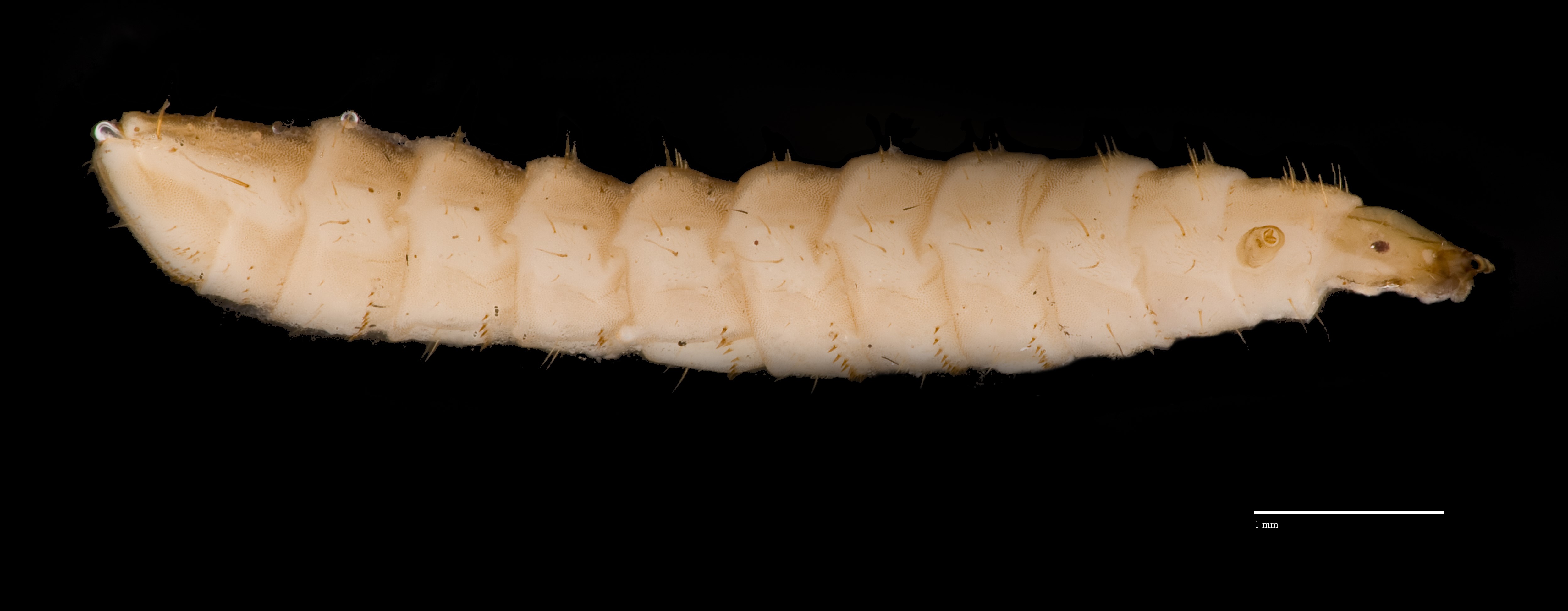
Larval and pupae
Larvae of Hermetia illucens are dull white in colour with golden-yellow setae and can grow up to 27mm in length (13). They have a small protruding yellowish-brown head with mouthparts for feeding. H. illucens larvae have light yellow ocular prominences on the lateral side of their head while the antennae are low and situated anterolaterally (4).
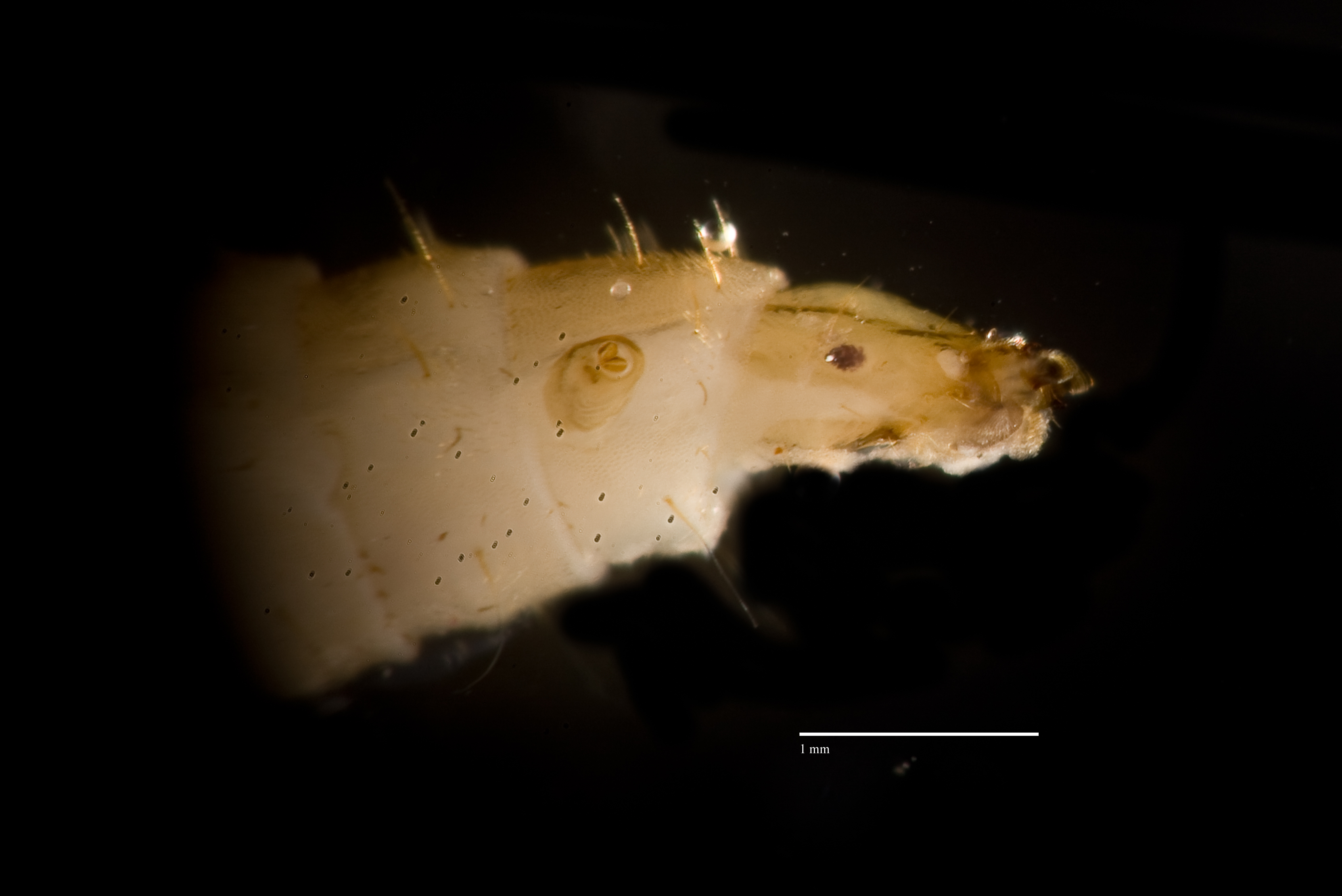
As Hermetia illucens larvae develops into pupae, they turn dark brown in colour. First instar larvae and pupae of H. illucens have not been imaged before. Thus, imaging of key features of H. illucens larvae will help to distinguish them from other fly larvae such as Musca domestica L. Moreover, images could also be used for identification. Mouthparts are also imaged to show morphological differences. Expertise description of larval and pupal images are extremely welcomed.
Adult characteristics and diagnostic features
| Trait |
Property |
Image |
||
| Anterior-posterior length |
|
See General Anatomy |
||
| Antennae |
|
|
||
| First two abdominal terga |
|
|
||
| Legs |
|
Sexual dimorphism
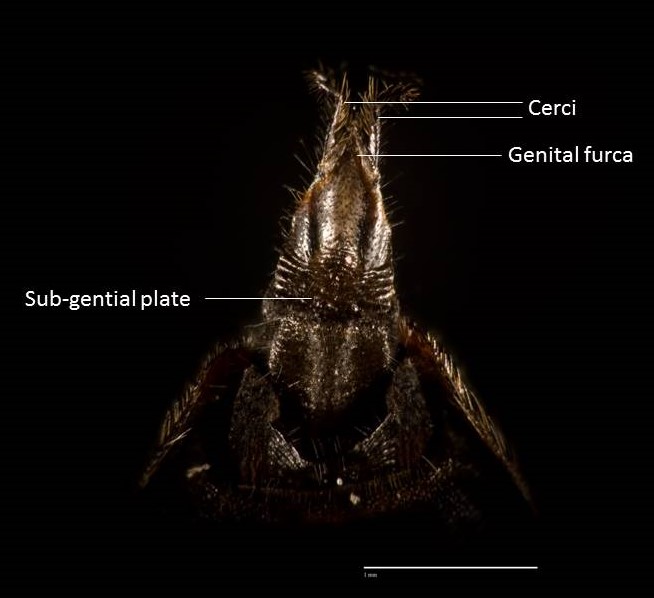
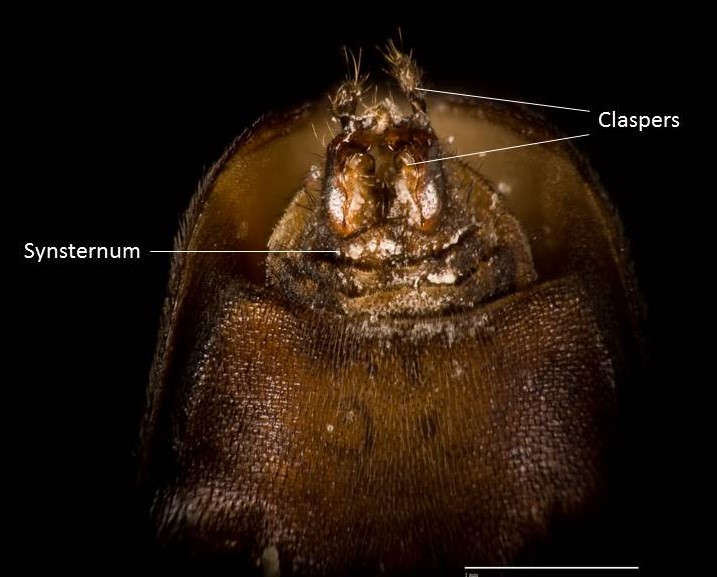
Hermetia illucens exhibit various sexually dimorphic traits such as body size, features on the frons and head, abdominal spots and the shape of their abdominal terminalia (4). The most common way of distinguishing them are the number of whitish hairs on the
face and shape of abdominal terminalia (4).
| Trait |
Female |
Male |
||||
Face
|
|
|
||||
| Abdominal terminalia Female:
|
|
|
Biology
Diet and Feeding
(Back to top)Hermetia illucens mainly feed during their larval stages. H. illucens larvae feed on a large variety of organic matter, including plant material, decaying animal matter and manure (5) (37) (38) (39). They are capable of converting large amounts of waste biomass into stored protein (≥40%) and fat (≥30%). Hence H. illucens larvae serve as highly proteinaceous animal feed with high energy content (5) (6). In contrast, H. illucens adults do not feed on solid material but drink liquids (36) (40). Thus most nutrient stores are accumulated during larval phases, reducing the need for feeding as an adult (5) (36).
Vid. 2: Time lapse of Hermetia illucens larvae finishing a banana. Obtained from YouTube under fair use
| Fig. 22. Image of Hermetia illucens adult feeding on sugar syrup. Image by Jonathan Tan. |
Mating behaviour and oviposition
Male Hermetia illucens exhibit territorial lekking behaviour (41). At forest edges, they were observed to be resting on plant leaves and waiting for potential female mates (41). Males tend to aggregate in large numbers at particular sites in the wild when mating (41). Aggressive behaviour was also reported when other male H. illucens invaders entered into the territory of the resting male. This usually occurs in the form of aerial spiralling in which one fly tries to fend off the other (41). At the end of this aerial display, the “victor” would return to its original resting place whilst the other leaves (41). When a male encounters a passing female, it would fly up towards her and grasp her (41). The pair then descends in copula. Mating activities usually occur in the day when sunlight intensity is high and decreases with diminishing light intensity (42). The presence of sunlight was correlated to mating and successful fertilization of H. illucens eggs (36) (42). However, additional research is required to determine the correlation between light wavelengths and mating behaviour.
Vid. 3. Initial courtship of Hermetia illucens individuals without in copula mating. Obtained from YouTube under fair use
 |
| Fig. 23. Successful mating of Hermetia illucens individuals in copulaImage. Obtained and use with permission from Foo Maosheng |
Once mating has been completed, the female departs in search of oviposition sites. H. illucens Females tend to lay their eggs on cracks and crevices such as the flukes of corrugated cardboard near decaying matter (13) (42) (43). Oviposition is affected by temperature and humidity. Females tend to deposit eggs at temperatures higher than 26 °C and at high humidity (≥60%). 80% of the eggs were deposited when environmental humidity exceeded 60% and this was almost twice the number of eggs laid at lower humidity (42).
Development and life cycle
 |
| Fig. 24. Hermetia illucens female laying eggs in corrugated cardboard. Image by Gee W. Obtained and accredited from Wikimedia Creative Commons |
A gravid Hermetia illucens female may deposit up to 500 oval-shaped, pale yellow eggs in a single clutch (13). After four days, larvae will hatch from these eggs (42). The entire developmental process from birth to adulthood takes approximately 40−43 days (14) (40) (42). A typical H. illucens larvae stage consists of 6 instars and last for 14−22 days during which they feed and accumulate biomass (13) (42). At the end of the larval stage, H. illucens larvae enter the pre-pupae stage. During the pre-pupal stage, H. illucens stop feeding and their mouthparts are modified into a climbing appendage (see Larvae and Pupae: Mouthparts). Pre-pupae then migrate away from the larval habitat and organic matter to pupate in a dry habitat using modified pupal mouth appendages (5). Adults then emerge approximately 14 days later (40). Pupal time may extend up to a few months and is highly variable (14). This depends on environmental conditions, particularly temperature and biotic conditions such as larval density and accumulated nutrients (40) (42) (44). Mating starts 2 days after adults emerge (45). The average life span of adults is approximately 10 days (40).
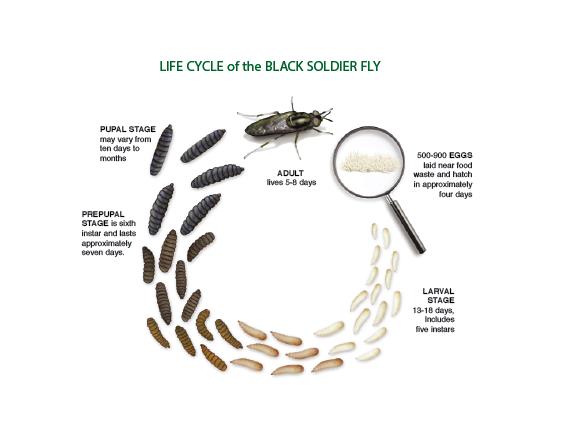 |
| Fig. 25. Diagram showing life cycle of Hermetia illucens. Obtained from YouTube under fair use |
 |
| Fig. 26. First five instars of Hermetia illucens followed by pre-pupae and pupae stages (from left to right). Image by Jonathan Tan |
Rearing and breeding in captivity
Hermetia illucens are able to process large quantities of organic matter and are consequently important composting organisms as manure and waste management tools (5) (46). Several substrates such as pig manure (5), poultry waste (46), food wastes (24) and various feed meals (40) have been used as raw material for composting. Rearing facilities usually consist of cage layered houses under the animal producing waste (5) (24) (46). Optimum temperature and humidity ranges are maintained in these facilities to ensure high yield of H. illucens larvae production. Temperatures are kept at 29–31 °C and humidity at 50–70% (24).
Rearing facilities operate by self-collection of H. illucens pre-pupae when they migrate away from their habitat into collection bins (5). This occurs as H. illucens larvae climb out of food bins onto a ramp that leads them into a container where they pupate (5) (47). Collected H. illucens pupae are then brought into a greenhouse where they will hatch into adults. The size of screen cages to effectively rear H. illucens adults vary. Sheppard et al. (2002) were able to successfully mate H. illucens in a 2 by 2 by 4m screen cage housed in a greenhouse (40). Space is required in order for the aerial mating process to take place (24). It is also recommended that adult H. illucens are kept in greenhouses or exposed to sunlight as sunlight intensity affects mating (42) (46). Optimal ranges of temperatures and humidity for mating and oviposition are 24–40°C and 30-90% respectively (36). Recommended minimum light intensity for mating to occur is 63 µmolm2s-1 and optimal light intensity at over 200 µmolm2s-1 (36). Exposure of the cage to sunlight is recommended though artificial lighting has also been shown to work. For successful oviposition, a moist container or corrugated cardboard tray (ie egg-holding tray) should be provided and placed near decaying organic matter which attracts gravid females (24).
Vid. 4. Home-made composting bin with Hermetia illucens larvae. Obtained from YouTube under fair use
Predation
Not much is known about predators of Hermetia illucens. However, animals such as birds, frogs and lizards are known predators of H. illucens larvae.
Defensive Adaptation
Hermetia illucens are often described as “wasp-like” due to two translucent patches on their second abdominal tergum, allowing them to appear to possess a thin slender, wasp-like waist (4) (36). H. illucens has an elongated antennae and black legs with white tarsi that are also features of wasp-like biomimicry to help deter potential predators (4) (36).
|
|
Conservation status
Hermetia illucens is listed as “extant” and does not face the threat of extinction due to their widespread and cosmopolitan distribution (1) (4).
Taxonomy and Systematics
Synonyms
(Back to top)Listed below are some of the synonyms for Hermetia illucens (Linnaeus, 1758) from the Integrated Taxonomic Information System (ITIS) (48):
Musca illucens Linnaeus, 1758
Musca leucopa Linnaeus, 1767
Hermetia rufiventris Fabricus, 1805
Hermetia nigrifacies Bigot, 1879
Hermetia pellucens Macquart, 1834
Hermetia mucens Riley & Howard, 1889
Hermetia illuscens Copello, 1926
Hermetia illucens var. nigritibia Enderlein, 1914
Type information
The holotype for Hermetia illucens is in the De Geer collection housed in the Swedish museum of Natural History (Naturhistoriska Riksmuseet, 4).
Taxonomic classification
The taxonomic classification shown below is referenced to UniProt Taxonomy (http://www.uniprot.org/taxonomy/343691). The classification reflects ranks of Hermetia illucens above the species level and is arranged in descending order from Kingdom to Species level:
› Eukaryota
› Opisthokonta
› Metazoa
› Eumetazoa
› Bilateria
› Protostomia
› Ecdysozoa
› Panarthropoda
› Arthropoda
› Mandibulata
› Pancrustacea
› Hexapoda
› Insecta
› Dicondylia
› Pterygota
› Neoptera
› Endopterygota
› Diptera
› Brachycera
› Stratiomyomorpha
› Stratiomyidae
› Hermetiinae
› Hermetia
Phylogeny
The phylogeny of soldier flies has been well-studied using molecular and morphological methods in phylogenetic tree construction (49) (50) (51) (52). Morphological characters include external and internal morphology of larvae, pupae and adults, such as wings and genitalia (11) (50) (51) (52). Molecular characters consist of nuclear protein-coding genes, ribosomal DNA (18S and 28S) and complete mitochondrial genomes (50) (51) (52).
Monophyly of the suborder Brachycera (>100,000 species, 40 families) which contains the family Stratiomyidae of solider flies is well supported (52). A comprehensive study of the fly tree of life by Wiegmann et al. (2011) places Stratiomyidae beside sister clades Xylomyidae and Pantophthalamidae, all of which comprise the infraorder Stratiomyomorpha or solider flies (52). However, relationships between the various subfamilies of Stratiomyidae are less apparent due to less well-supported monophyly and relationships between certain subfamilies such as Stratiomyinae and Clitellariinae (50).
| Fig. 29. Molecular phylogenetic tree for Diptera by Wiegmann et al. (2011). Circles on the tree indicate well-supported nodes with bootstrap values >80%. Nodes with stars indicate improvement of bootstrap values after postanalysis pruning of non-stable taxa. Coloured squares indicate certain ecological traits (listed at bottom left) present in at least one species of the family.Obtained and accredited under fair use |
Stratiomyidae encompass a family of cosmopolitan flies of suborder Brachycera and infraorder Stratiomyomorpha (11) (50). The monophyletic family comprises over 2800 species (50). Monophyly of the subfamily Hermetiinae which contains the genus Hermetia and species Hermetia illucens is well-supported both by morphological and molecular data (11) (50).
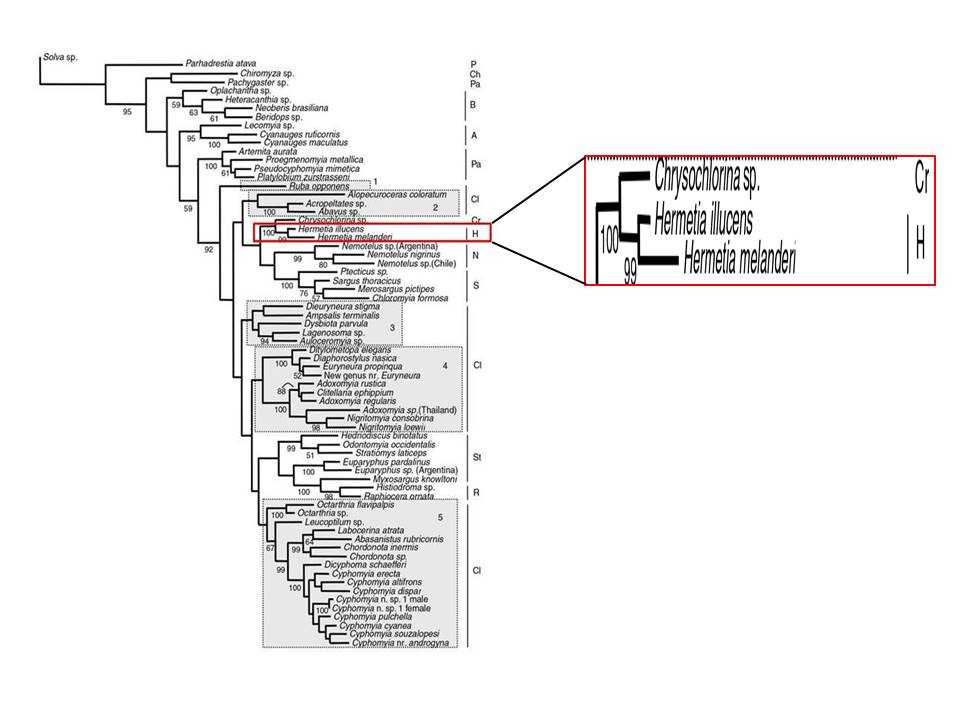 |
| Fig. 30. Molecular phylogenetic tree showing relationship of various sub-families of Stratiomyidae by Brammer & Dohlen (2007). The tree is one of the six most-parsimonious trees found with PAUP and NONA via combined EF-1-alpha and 28S sequences. Branch lengths reflect and are proportional to the number of base substitutions. Bootstrap values for nodes are indicated below the branches. Box in red indicates the sub-family Hermetiinae that contains Hermetia illucens. Abbreviations of sub-family names are as follows: P = Parhadrestiinae, Ch = Chiromyzinae, Pa = Pachygastrinae, B = Beridinae, A = Antissinae, Cl = Clitellariinae, Cr = Chrysoclorininae, H = Hermetiinae, N = Nemotelinae, S = Sarginae, St = Stratiomyinae, R = Raphiocerinae. Obtained and accredited under fair use |
It is hypothesized that the primary terrestrial lineages of the suborder Brachyera, including the infraorder Stratiomyomorpha, diversified in the early Jurassic (160 mya, 52). However, the common ancestor of the Stratiomyidae family originated more recently in a radiation event during the early Cretaceous period (around 129 mya, 50). The major radiation of clades in Stratiomyidae, such as Hermetiinae, is estimated to be around 60-80 mya using molecular data (50). The last common ancestor of H. illucens has been estimated to appear during the early Cenozoic (around 40 mya, 50).
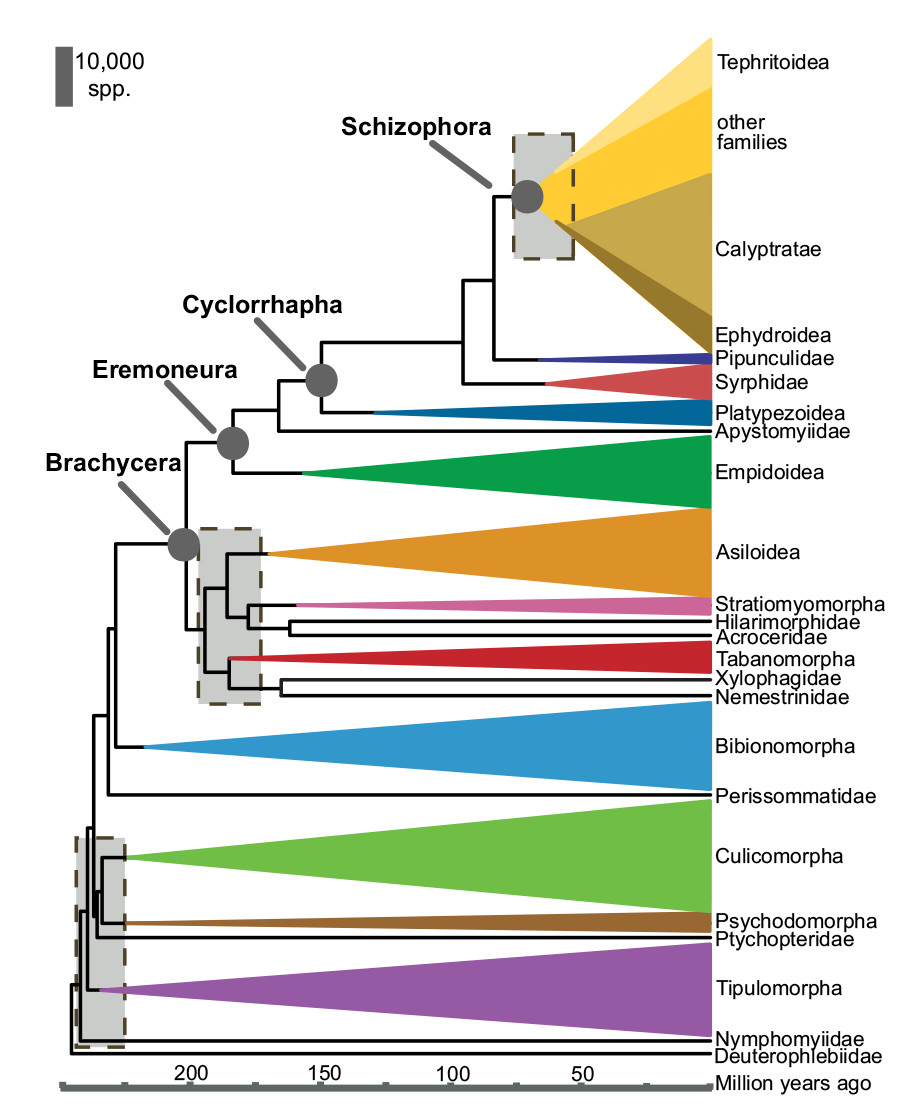 |
| Fig. 31. Chronogram showing the divergence of dipteran clades and estimated time of divergence by Wiegmann et al. (2011). Shaded boxes represent uncertainty in phylogenetic analysis and crucial periods of rapid diversification for diptera. The vertical height of the coloured triangles represents the estimated numbers of species described in each clade with Stratiomyomorpha indicated in pink. The corresponding height of the grey scale bar represents 10, 000 species. Timescale represents millions of years before present. Obtained and accredited under fair use. |
 |
| Fig. 32. Chronogram from r8s analysis of both EF-1-alpha and 28S sequences by Brammer & Dohlen (2007). Timescale represents millions of years before present. Nodes that had their age constrained due to fossil evidence are represented by filled circles with the corresponding age indicated. Branches with high posterior probability (>90%) and bootstrap support (>775%) are bolded. The bracket indicates the Cretaceous period. Sub-family Hermetiinae is highlighted via the red box. Abbreviations of sub-family names are as follows: P = Parhadrestiinae, Ch = Chiromyzinae, Pa = Pachygastrinae, B = Beridinae, A = Antissinae, Cl = Clitellariinae, Cr = Chrysoclorininae, H = Hermetiinae, N = Nemotelinae, S = Sarginae, St = Stratiomyinae, R = Raphiocerinae. Obtained and accredited under fair use |
References
(Back to top)
- Jacinto, V. & Coin, P. eds. (2015). "Hermetia illucens." Encyclopedia of Life. Retrieved 7 Nov 2015 from http://eol.org/pages/824054/overview.
- Hardouin, J. & Mahoux, G. (2003). Zootechnie d’insectes – Elevage et utilisation au bénéfice de l'homme et de certains animaux. Bureau pour l’Echange et la Distribution de l’Information sur le Mini-élevage (BEDIM), 164 p.
- Kim, J. I. (1997). Newly recording two exotic insects species from Korea. J. Kor. Biota, 2, 223−225.
- Rozkosný, R. (1983). Subfamily Hermetiinae. In A Biosystematic Study of the European Stratiomyidae (Diptera): Volume 2-Clitellariinae, Hermediinae, Pachygasterinae and Bibliography (Vol. 2). London: Springer Science & Business Media. pp.431.
- Newton, G. L., Sheppard, D. C., Watson, D. W., Burtle, G. J., Dove, C. R., Tomberlin, J. K., & Thelen, E. E. (2005). The black soldier fly, Hermetia illucens, as a manure management/resource recovery tool. In Symposium on the state of the science of Animal Manure and Waste Management. pp. 5−7.
- Kroeckel, S., Harjes, A. G., Roth, I., Katz, H., Wuertz, S., Susenbeth, A., & Schulz, C. (2012). When a turbot catches a fly: Evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute—Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture, 364, 345−352.
- Lord, W. D., Lee Goff, M., Adkins, T. R., & Haskell, N. H. (1994). The black soldier fly Hermetia illucens (Diptera: Stratiomyidae) as a potential measure of human postmortem interval: observations and case histories. Journal of Forensic Sciences, 39, 215−215.
- Pujol‐Luz, J. R., Francez, P. A. D. C., Ururahy‐Rodrigues, A., & Constantino, R. (2008). The Black Soldier‐fly, Hermetia illucens (Diptera, Stratiomyidae), Used to Estimate the Postmortem Interval in a Case in Amapá State, Brazil*.Journal of Forensic Sciences, 53(2), 476-478.
- Diener, S., Zurbrügg, C., Roa Gutiérrez, F., Nguyen, D. H., Morel, A., Koottatep, T., & Tockner, K. (2011). Black soldier fly larvae for organic waste treatment – prospects and constraints. WasteSafe 2011 – 2nd Int. Conf. on Solid Waste Management in the Developing Countries, Khulna, 13-15 February 2011, Bangladesh: ResearchGate.
- Benelli, G., Canale, A., Raspi, A., & Fornaciari, G. (2014). The death scenario of an Italian Renaissance princess can shed light on a zoological dilemma: did the black soldier fly reach Europe with Columbus?. Journal of Archaeological Science, 49, 203-205.
- Woodley, N. E., & Thompson, F. C. (2001). A world catalog of the Stratiomyidae (Insecta: Diptera) (Vol. 11). California: North American Dipterists' Society. pp 473.
- Roháček, J., & Hora, M. (2013). A northernmost European record of the alien black soldier fly Hermetia illucens (Linnaeus, 1758)(Diptera: Stratiomyidae)/Nejsevernější evropský výskyt nepůvodní bráněnky Hermetia illucens (Linnaeus, 1758)(Diptera: Stratiomyidae). Casopis slezskeho zemskeho muzea (A), 62(2), 101-106.
- Diclaro II, J. W. ; Kaufman, P. E. (2010). Black soldier fly Hermetia illucens Linnaeus (Insecta: Diptera: Stratiomyidae). EENY-461, Entomology and Nematology Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida.
- Furman, D. P., Young, R. D., & Catts, P. E. (1959). Hermetia illucens (Linnaeus) as a factor in the natural control of Musca domestica Linnaeus.Journal of Economic Entomology, 52(5), 917–921.
- Sheppard, C. (1983). House fly and lesser fly control utilizing the black soldier fly in manure management systems for caged laying hens. Environmental Entomology, 12(5), 1439–1442.
- Sheppard, D. C., Newton, G. L., Thompson, S., Davis, J., Gascho, G., & Bramwell, K. (1998). 1998 Annual Report of Sustainable Agriculture Research and Education: Using soldier flies as a manure management tool for volume reduction, house fly control and reduction, house fly control and feedstuff production. Retrieved 7 Nov 2015 from https://www.cals.ncsu.edu/waste_mgt/smithfield_projects/phase2report05/cd,web%20files/A2.pdf
- Axtell, R. C., & Arends, J. J. (1990). Ecology and management of arthropod pests of poultry. Annual review of entomology, 35(1), 101–126.
- Bradley, S. W., & Sheppard, D. C. (1984). House fly oviposition inhibition by larvae of Hermetia illucens, the black soldier fly. Journal of chemical ecology, 10(6), 853–859.
- Erickson, M. C., Islam, M., Sheppard, C., Liao, J., & Doyle, M. P. (2004). Reduction of Escherichia coli O157: H7 and Salmonella enterica serovar enteritidis in chicken manure by larvae of the black soldier fly. Journal of Food Protection®, 67(4), 685–690.
- Liu, Q., Tomberlin, J. K., Brady, J. A., Sanford, M. R., & Yu, Z. (2008). Black soldier fly (Diptera: Stratiomyidae) larvae reduce Escherichia coli in dairy manure. Environmental entomology, 37(6), 1525–1530.
- Kunzler, C., & Roe, R. (1995). Food service composting projects on the rise: Commercial & institutional organics. Biocycle, 36(4), 64–71.
- Diener, S., Zubrügg, C., Trockner, K. (2009). Conversion of organic material by black soldier fly larvae: establishing optimal feeding rates. Waste Management & Research, 27, 603–610.
- Li, Q., Zheng, L., Qiu, N., Cai, H., Tomberlin, J. K., & Yu, Z. (2011). Bioconversion of dairy manure by black soldier fly (Diptera: Stratiomyidae) for biodiesel and sugar production. Waste Management, 31(6), 1316-1320.
- Barry, T. (2004). Evaluation of the Economic, Social, and Biological Feasibility of Bioconverting Food Wastes with the Black Soldier Fly (Hermetia illucens). University of North Texas, Texas, United States of America.
- Chiam, A (2014). Composting Idea Take Flight in USP and UTown. National University of Singapore. Retrieved 7 Nov 2015 from http://www.usp.nus.edu.sg/highlights/2014/project-herme.html.
- Regost, C., Arzel, J., & Kaushik, S. J. (1999). Partial or total replacement of fish meal by corn gluten meal in diet for turbot (Psetta maxima). Aquaculture,180(1), 99–117.
- Slawski, H., Adem, H., Tressel, R.-P., Wysujack, K., Kotzamanis, & Y., Schulz, C. (2011). Austausch von Fischmehl durch Rapsproteinkonzentrat in Futtermitteln für Steinbutt (Psetta maxima L.). Zuchtungskunde, 83(6), 451–460.
- Bondari, K., & Sheppard, D. C. (1981). Soldier fly larvae as feed in commercial fish production. Aquaculture, 24, 103–109.
- Bondari, K., & Sheppard, D. C. (1987). Soldier fly, Hermetia illucens L., larvae as feed for channel catfish, Ictalurus punctatus (Rafinesque), and blue tilapia, Oreochromis aureus (Steindachner). Aquaculture Research, 18(3), 209–220.
- Calci-Worms (2015). Retrieved November 9, 2015 from http://www.silkwormstore.co.uk/calci-worms---explained.html.
- Phoenix Worm Store (2015). Retrieved 9 Nov 2015 from http://www.phoenixworm.com/.
- Welcome to ReptiWorms! - ReptiWorms (2015). Retrieved 9 Nov 2015 from http://www.reptiworms.com/index.php?index.
- Bentley. (2013). “Yellow Soldier Flies” – Revisited. Retrieved November 20, 2015, from http://www.redwormcomposting.com/bsfl/yellow-soldier-flies-revisited/
- Black vs. yellow soldier flies. (2014). Retrieved November 20, 2015, from http://www.waldeneffect.org/blog/Black_vs._yellow_soldier_flies/
- Sepulveda, T. (2013). Composting Using Black Soldier Flies (BSF) and Their Effect On Earth Worm Bins | Ed That Matters. Retrieved November 20, 2015, from http://www.edthatmatters.com/composing-using-black-soldier-flies-bsf-and-their-effect-on-earth-worm-bins/
- Sheppard, D. C., Tomberlin, J. K., Joyce, J. A., Kiser, B. C., & Sumner, S. M. (2002). Rearing methods for the black soldier fly (Diptera: Stratiomyidae) in a colony.Journal of Medical Entomology, 39(4), 695−698.
- St‐Hilaire, S., Cranfill, K., McGuire, M. A., Mosley, E. E., Tomberlin, J. K., Newton, L., Sealey, W., Sheppard, C., & Irving, S. (2007). Fish Offal Recycling by the Black Soldier Fly Produces a Foodstuff High in Omega‐3 Fatty Acids. Journal of the World Aquaculture Society, 38(2), 309–313.
- Hem, S., Toure, S., Sagbla, C., & Legendre, M. (2008). Bioconversion of palm kernel meal for aquaculture: Experiences from the forest region (Republic of Guinea). African Journal of Biotechnology, 7(8).
- Martínez-Sánchez, A., Magaña, C., Saloña, & M. & Rojo, S. (2011). First record of Hermetia illucens (Diptera: Stratiomyidae) on human corpses in Iberian Peninsula. Forensic Science International, 206(1), 76–78.
- Tomberlin, J. K., Sheppard, D. C., & Joyce, J. A. (2002). Selected life-history traits of black soldier flies (Diptera: Stratiomyidae) reared on three artificial diets. Annals of the Entomological Society of America, 95(3), 379–386.
- Tomberlin, J. K., & Sheppard, D. C. (2001). Lekking behavior of the black soldier fly (Diptera: Stratiomyidae). Florida Entomologist, 84(4), 729.
- Tomberlin, J. K., & Sheppard, D. C. (2002). Factors influencing mating and oviposition of black soldier flies (Diptera: Stratiomyidae) in a colony. Journal of Entomological Science, 37(4), 345–352.
- Booth, D. C., & Sheppard, C. (1984). Oviposition of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae): eggs, masses, timing, and site characteristics. Environmental entomology, 13(2), 421–423.
- Holmes, L. (2010). Role of Abiotic Factors on the Development and Life History of the Black Soldier Fly, Hermetia illucens (L.) (Diptera: Stratiomyidae) (Unpublished master’s thesis). University of Windsor, Ontario, Canada.
- Tomberlin, J. K. (2001). Biological, behavioral, and toxicological studies on the black soldier fly (Diptera: Stratiomyidae) (Unpublished doctoral dissertation). University of Georgia, Athens, United States of America.
- Sheppard, D. C., Newton, G. L., Thompson, S. A., & Savage, S. (1994). A value added manure management system using the black soldier fly. Bioresource Technology, 50(3), 275–279.
- Tran, G., Gnaedinger, C., & Mélin, C. (2015) Black soldier fly larvae (Hermetia illucens). Feedipedia, a programme by INRA, CIRAD, AFZ and FAO. Retrieved 7 Nov 2015 from http://www.feedipedia.org/node/16388.
- Hermetia illucens (2015). Integrated Taxonomic Information System on-line database. Retrieved 7 Nov 2015 from http://www.itis.gov.
- James, M. T. (1936). The Stratiomyidae of Colorado and Utah. Journal of the Kansas Entomological Society, 9(1), 33-36.
- Brammer, C. A., & von Dohlen, C. D. (2007). Evolutionary history of Stratiomyidae (Insecta: Diptera): The molecular phylogeny of a diverse family of flies. Molecular Phylogenetics and Evolution, 43(2), 660–673.
- Wiegmann, B. M., Yeates, D. K., Thorne, J. L., & Kishino, H. (2003). Time flies, a new molecular time-scale for brachyceran fly evolution without a clock.Systematic Biology, 52(6), 745–756.
- Wiegmann, B. M., Trautwein, M. D., Winkler, I. S., Barr, N. B., Kim, J. W., Lambkin, C., Bertone, M. A., Cassel, B. K., Bayless, K. M., Heimberg, A. M., Wheeler, B. M., Peterson, K. J., Pape, T., Sinclar, B. J., Skevington, J. H., Blagoderov, V., Caravas, J., Kutty, S. N., Schmidt-Ott, U., Kampmeier, G. E., Thompson, F. C., Grimaldi, D. A., Beckenbach, A. T., Courtney, G. W., Friedrich, M., Meier, R, & Yeates, D. K. (2011). Episodic radiations in the fly tree of life. Proceedings of the National Academy of Sciences, 108(14), 5690–5695.
Acknowledgement
I would like to express my heartfelt gratitude to the following individuals, without which the website would not have been made possible:
Mr. Ang Yuchen who gave invaluable advice and guidance in imaging.
Mr. Foo Maosheng who had allowed me to take specimens from his Black Soldier Fly culture for imaging and for sharing with me his own images and expertise in imaging.
Mr. James Hii who offered help and guidance in editing the images.
Mr. Theodore Lee who helped me in adult Black Soldier Fly Photography.
Mr Samuel Ewing who kindly allowed me to use his image of Hermetia illucens.
Mdm. Sally G. Miller who kindly allowed me to use her image of Ptecticus.
Mr. Stephen Cresswell who kindly allowed me to use his image of Trypoxylon politum.
Contact Me
Jonathan Tan is contactable at jonathan.twt@gmail.com