
Table of Contents
[1] Overview
The assassin bug (Reduviidae) Inara flavopicta, also known as the wasp-mimicking assassin bug, has a diet specialising on ants (1) and can be found in forests in the Southeast Asia region (1,2). The nymphs exhibit an interesting behaviour of piling ant corpses on their backs after feeding and are commonly known as ant-piling or ant-snatching nymphs. While the ant-piling nymph can look fearsome in photographs, it is in fact only a small insect of about 1 cm long.
Their "backpacks" function to deter predators and may also provide chemical camouflage from the ants (3,4). A recent phylogenetic study on assassin bugs revealed that Inara flavopicta is nested within the Acanthaspis clade of the polyphyletic subfamily Reduviinae (1), and there could be a name change in the future.
While much has been documented about the ant-piling behaviour in the speciose genus Acanthaspis (5), there is no formal documentation of such behaviour in Inara. The ant-piling behaviour observed in Inara flavopicta may be a first record for this genus.
[2] What are Assassin Bugs?
Assassin bugs (Hemiptera: Heteroptera: Reduviidae) is a diverse group of insects with more than 6800 described species worldwide (6). They are mainly predatory and carry out a wide range of prey capture methods (1,6) (Video 1 and 2).| Video 1. A feather-legged assassin bug (Holoptilinae) from Australia lures ants by waving its hindlegs. Video obtained from Youtube, under fair use policy. |
Video 2. An assassin bug feeding on bats in a cave. Video obtained from Youtube, under fair use policy. |
Besides their fascinating prey capture behaviour, assassin bugs also exhibit a wide diversity of morphology and life habits with some groups specialising on certain prey such as ants, termites and even millipedes (7). As with all hemipterans, they possess a sucking mouthpart (labium) which they use to pierce the prey and carry out extra-oral digestion (8). They also have a characteristic groove in their prosternum which is unique to this family of bugs (6). When the bugs feel threatened, they may rub their labium along the groove to produce sounds as a defensive mechanism or to reject copulatory advances by males (9).
[3] Distribution
[3.1] Global Distribution
Inara flavopicta was first described from Malaysia, Pulo Penang (2). Thereafter it has been recorded in other parts of Southeast Asia such as from Singapore, Sarawak, Thailand and Java (2,10).Map 1. Google map showing global distribution of Inara flavopicta. Permission obtained under permission guidelines for Google Maps and Google Earth.
[3.2] Local Distribution
The distribution of Inara flavopicta is based on the locality data from specimens deposited in the museum.Map 2. Google map showing local distribution of Inara flavopicta. Permission obtained under permission guidelines for Google Maps and Google Earth.
[3.3] Habitat
The adults of Inara flavopicta can be found on foliage in forested habitats (1). Based on personal observations in Singapore, the nymphs are commonly seen on the leaves Piper plants around the base of relatively large trees in the evening and at night (Fig. 1). However, the association with plants has not been tested.
Fig. 1. Inara flavopicta is commonly seen around Piper plants at the base of a tree such as this one. Photo by: Yeo Huiqing.
[4] Biology and Life History
[4.1] Diet
Inara flavopicta is an ant specialist (1) and is commonly seen feeding on ants about 5mm in length. The identification of the species is still in progress. Under laboratory conditions, both adults and nymphs tend to avoid bigger ants fed to them. Occasionally, the adults were seen predating on wasps.[4.2] Prey Capture and Feeding Behaviour of Nymphs
Video 3. Prey capture and feeding behaviour of ant-piling nymphs. Video by: Yeo Huiqing
The ant-piling nymphs detect moving prey quickly with their large eyes and orientate themselves with their head towards the prey to initiate a feeding response (Video 3). Once a prey gets close enough to the nymphs, it is grabbed within a few seconds with the fore and mid-legs and pierced with the labium. A highly effective cocktail of saliva and enzyme is then injected into the prey to digest the interior before it is sucked out by the assassin bug (11). The favoured site of injections are usually on the soft cuticle parts of the prey such as in between abdominal segments (12).
The assassin bug may readjust the prey using its fore and mid-legs and reinsert its labium in different parts of the prey when feeding to ensure that the contents are fully ingested.The prey’s tissue is then mixed with the saliva and thereafter the fluid is sucked out, a technique known as extra-oral digestion (8). While this method of feeding partially consumes the prey (leaving only the exoskeleton), ingestion and absorption of prey nutrients are highly effective (16).
Prey capture and feeding behaviour are mainly focused on the nymphs of Inara flavopicta due to their fascinating ant-piling behaviour and not much is known about the behaviour of the adults.
[4.3] Ant-piling Behaviour of Nymphs
The ant-piling nymph proceeds to pile the ant carcass after feeding (Video 4).Video 4. An ant-piling nymph carrying out the process of piling the ant corpse after it has finished feeding on it. Video by: Yeo Huiqing.
After the contents of the ant has been sucked dry, the carcass is then ‘compacted’ and placed on the ground. Subsequently, the nymph lifts itself up and walks over the carcass. Once the carcass is within reach of its hindlegs, it is then added onto its back by pushing the carcass underneath the existing corpses and onto the surface of the abdomen where dorsal glands are present (Fig. 2), thus the backpack of ant carcass is accumulated upwards (3). As the backpack of ants were noted to be held tightly by threads of sticky silk, it was postulated that the strategic placement of the newly acquired carcass under the backpack ensures that it is coated with enough secretions from the dorsal glands.

Fig. 2. Sticky secretion on dorsal gland of a nymph. Photo by: Yeo Huiqing.
The hindlegs of the ant-piling nymphs are remarkably dexterous and this is demonstrated by the precision in which the carcass was manipulated underneath the backpack. Besides that, the towering backpack as a whole is also readjusted using its hindlegs.
The nymphs take about 30 minutes in total from prey capture to feeding and piling the ant carcass onto its back, with a large proportion of time spent on feeding. During the process, the legs are essential for all the steps involved. The time taken for each step in the behavioural sequence and the respective combination of legs used are detailed in the flowchart (Fig. 3). The behavioural sequence shown in the flowchart is not exhaustive and is based on general observations of a few nymphs.
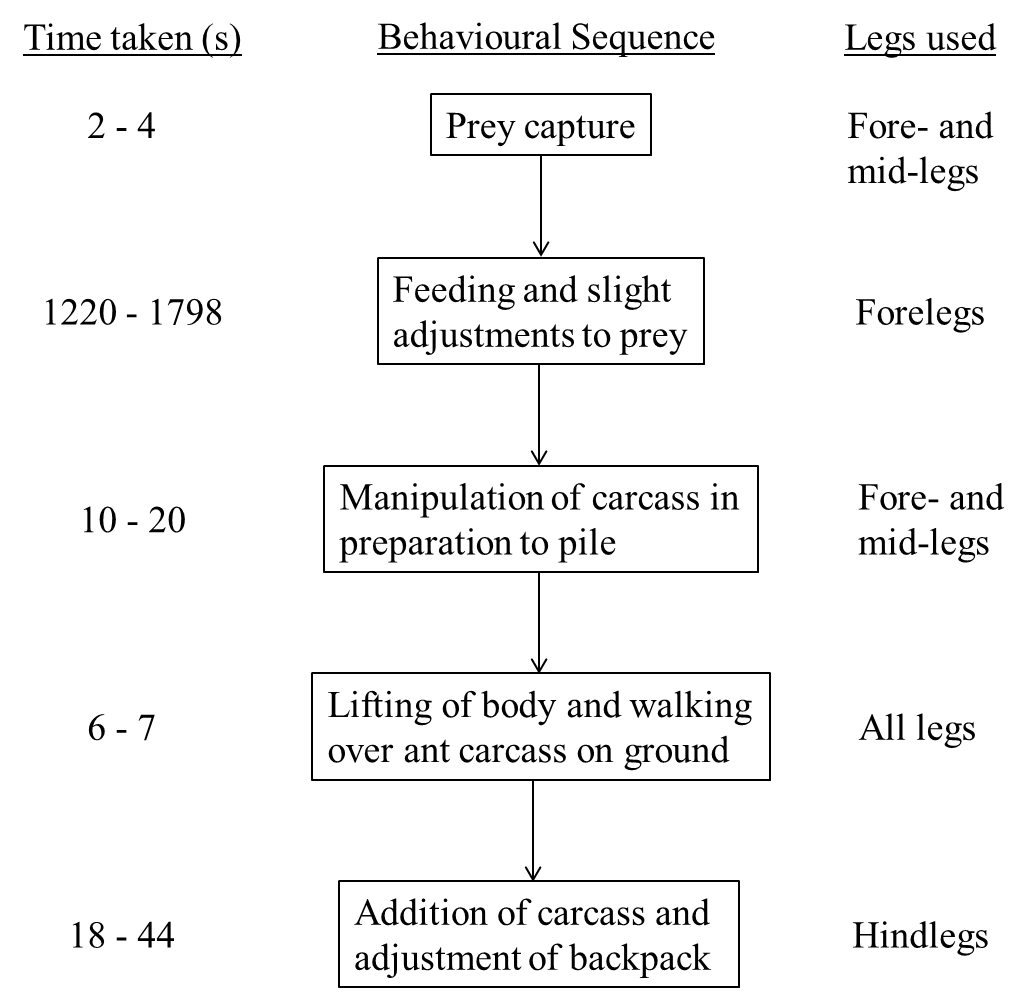
Fig. 3. Flowchart of ant-piling behaviour in nymphs showing time taken (seconds) and legs used for each action. Flowchart by: Yeo Huiqing
The ant-piling nymphs are also observed to transfer the existing backpack after moulting rather than starting a new one from scratch even when there is a fungal infection (see yellow coating on the moult and backpack) (Fig. 4A and B). This could suggest that the backpack is important in their survival (deterring predators and chemical camouflage from ants) and it is costly to expend energy to build a new one again.

Fig. 4A and B. Newly moulted ant-piling nymph which has transferred its existing backpack over from the old moult. The yellow coating is a result of fungal infection. Photos by: Yeo Huiqing
[4.4] Biochemistry of Saliva
The saliva of assassin bugs are toxic as it contains substances that are able to paralyse and digest prey within a short period of time (13). In general, the saliva consists of proteinase, phospholipase, and hyalurinidase among others and they aid in lysing the tissues of the prey (13,14). The enzyme profile varies between species, depending on their diet specialisation.The saliva can have a necrotic effect on humans, causing localised pain and swelling as well as a long-lasting blackened pit at the bitten area (11). Assassin bugs however, do not actively search for and bite humans (unlike mosquitoes for example) with the exception of the kissing bugs (Triatominae) (6). If you have been bitten by an assassin bug, please consult a doctor for treatment of pain and possible necrosis.
The potency of the assassin bug saliva may be due to the biologically active peptides present (15). Interestingly, they are relatively homologous to the calcium channel blockers in conotoxins from marine cone snails (17). However the findings are relatively new and the three dimensional structures as well as the biological targets in the prey’s cells has not yet been determined (17).
[4.5] Anti-predatory Mechanisms
The Inara flavopicta adult is thought to be a wasp mimic with its yellow and black colouration and constricted abdomen similar to a wasp (Fig. 5). Many species of insects tend to mimic members in the order Hymenoptera (ants, bees and wasps) as they tend to have stings and are more aggressive (18). The fear of being stung is effective in deterring potential predators (19).
Fig. 5. Lateral view of adult Inara flavopicta showing black and yellow colouration and constriction of the abdomen. Photo by: Yeo Huiqing
In the ant-piling nymphs, the backpack of corpses was hypothesized to confer an advantage in confusing their predators instead of serving as a form of camouflage. A study carried out on nymphs of three species (Paredocla cf. planquettei, Acanthaspis petax and Acanthaspis sulcipes) with similar ant-piling behaviour and their predators (Hemidactylus brooki(Fig. 6), Scolopendra morsitan (Fig. 7), wall spiders and jumping spiders) found that the absence of the backpack increases the susceptibility of the nymphs to predation (3, 4). This suggests that the mass of corpses disorientates the predators as it looks nothing like a normal prey, and having been perceived as a pile of debris instead of a potential prey item, the nymph is usually ignored (4).
It is to be noted that the species in the study are not found in Singapore and hence may have different predators.
 |
 |
| ig. 6. African house gecko (Hemidactylus brooki). Permission obtained under Creative Commons, Attribution Non-Commercial -Share-Alike 2.0 Generic as stated under some rights reserved in the original Flickr website. |
Fig. 7. Centipede (Scolopendra morsitan). Permission obtained from ARKive under terms of use for non-profit education. |
The backpack could also serve as a form of crypsis by masking the olfactory and tactile signals of the ant-piling nymphs, enabling them to approach ant trails for prey capture without being recognised as an intruder and attacked by the ants (3).
In addition, the ant corpses on the nymphs' backs were hypothesised to lure ants which are known to approach and ‘inspect freshly killed nestmates’ due to the presence of colony-specific volatile compounds (3, 4). However, the ant composition of the backpack may consist of a variety of species and colony-specific compounds in the corpses may degrade over time.
[5] Identification and Morphology
[5.1] General Morphology
The following images show the dorsal, lateral and ventral views (Fig 8 - 10) respectively of an adult Inara flavopicta with annotated morphological structures.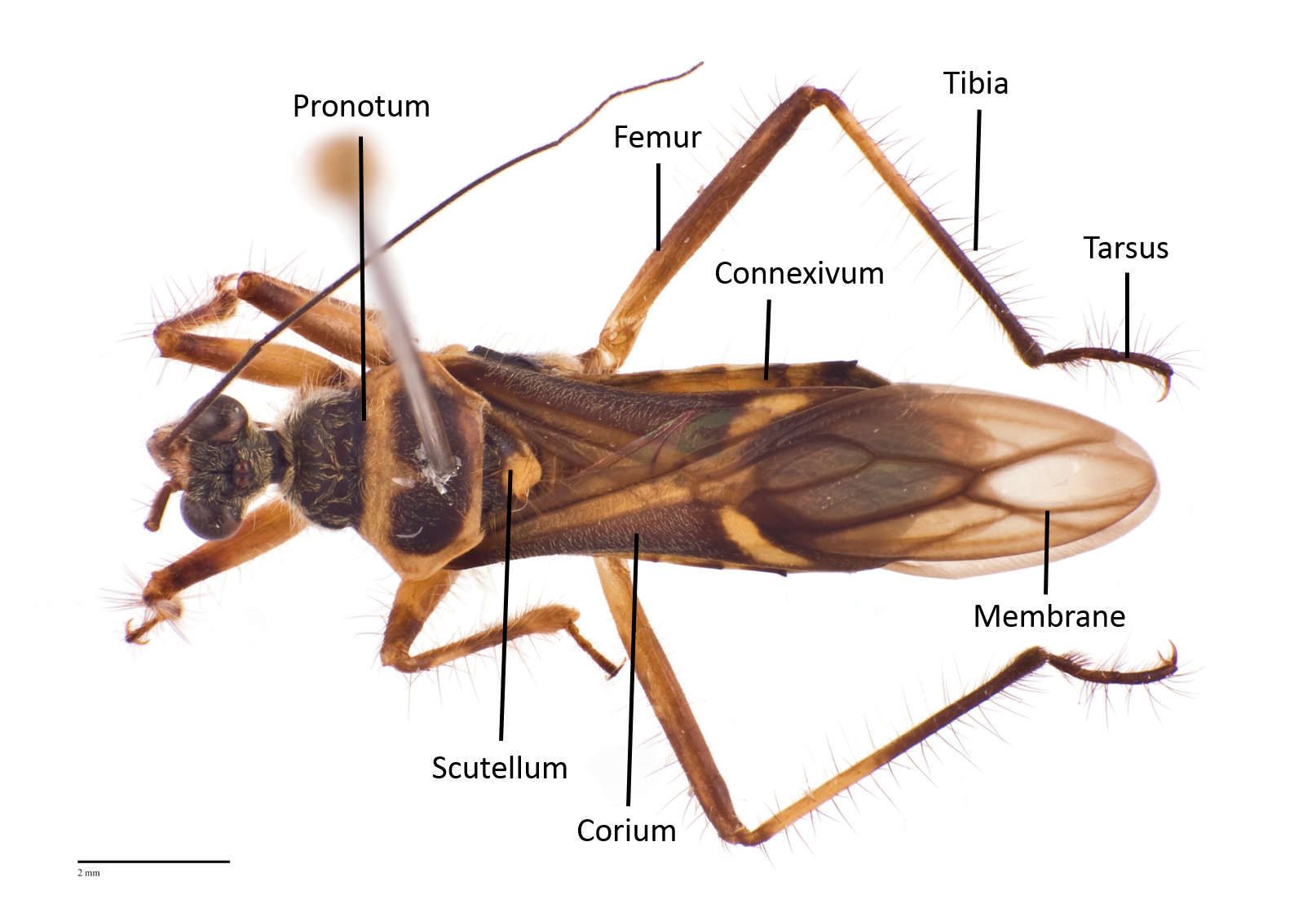
Fig. 8. Dorsal view of an adult male Inara flavopicta. Photo by: Yeo Huiqing.
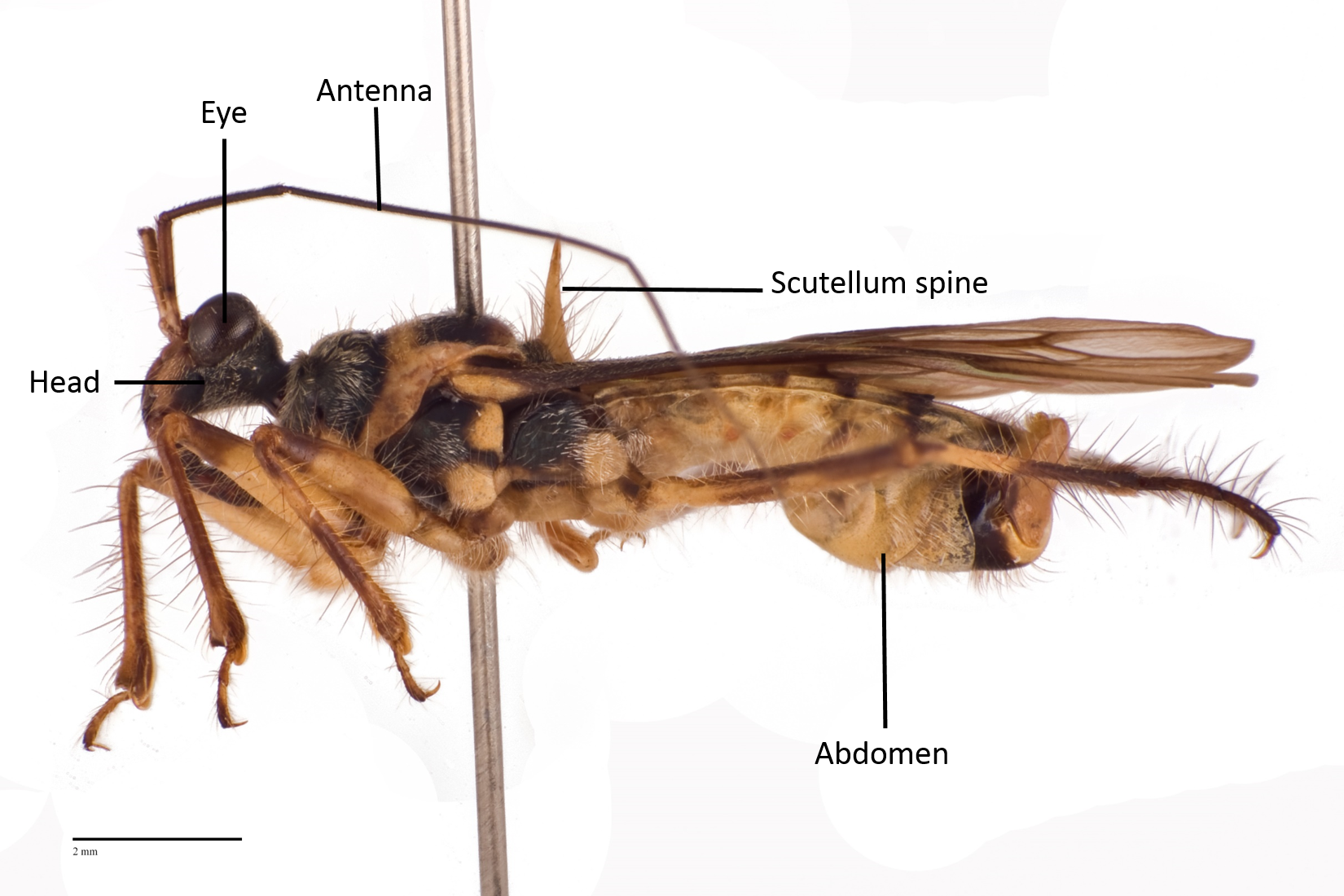
Fig. 9. Lateral view of an adult male Inara flavopicta. Photo by: Yeo Huiqing.
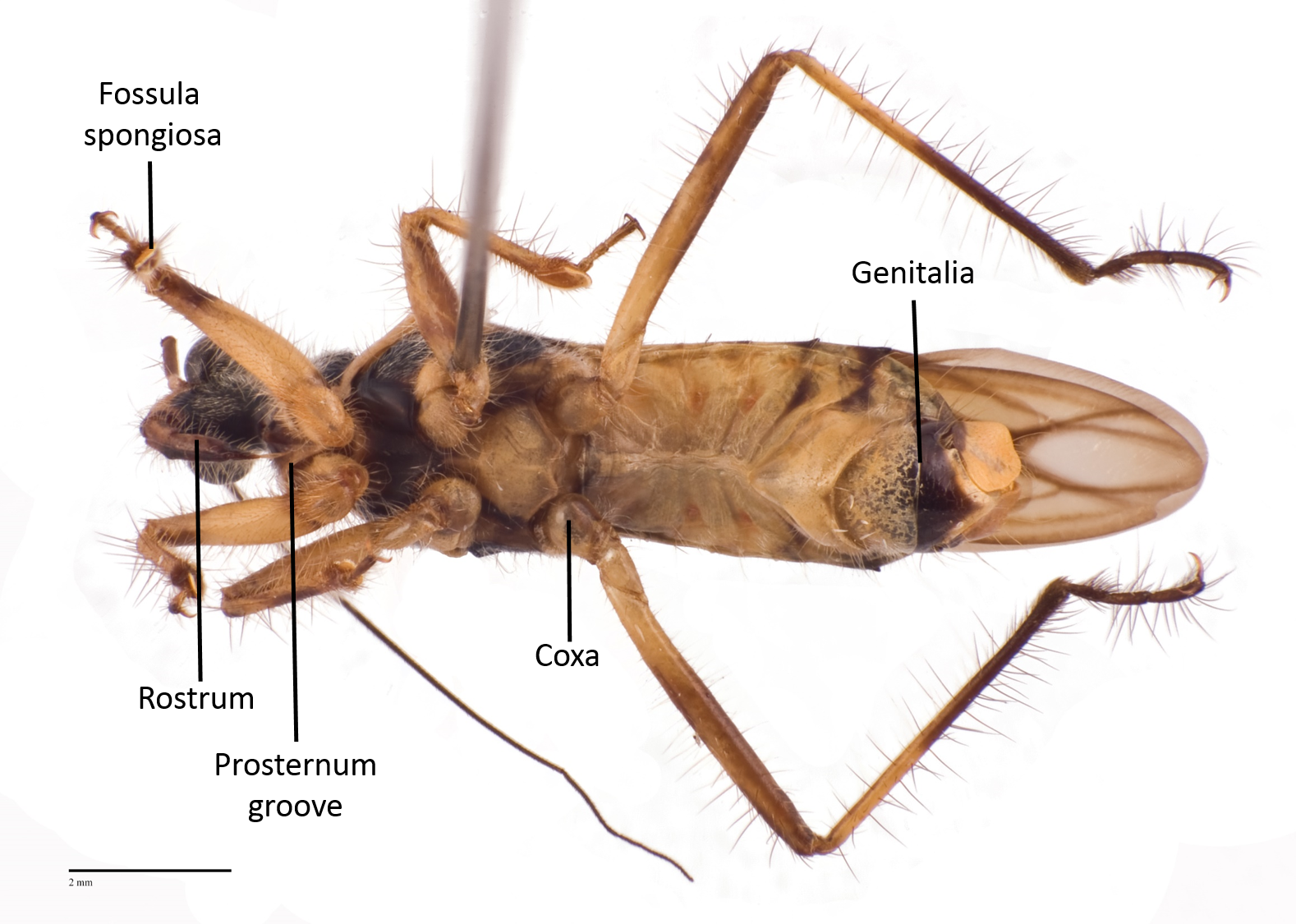
Fig. 10. Ventral view of an adult male Inara flavopicta. Photo by: Yeo Huiqing.
[5.2] Adult Description
The original description in Latin by Stål, 1859 in Till kännedomen om Reduvini (Fig. 11) (20).
Fig. 11. Original description in Latin by Stål. Screenshot obtained from Biodiversity Heritage Library under Creative Commons Attribution 2.0 Generic.
Using Google Translate, the Latin description roughly translates to:
"Black. Front part above the head, rostrum, the border of pronotum, scutellum spine, oblique streak subapical of corium, spots on the abdomen and legs yellow; legs with black bands; abdomen on both sides with dark purple markings; membrane slightly dusky in colour, with black veins."
As the original text is rather brief, a section on identifying features is added below to aid in the identification of Inara flavopicta.
[5.3] Identifying Features (Adults)
The following identifying features are adapted from descriptions from Stål, 1859 (20) and Walker, 1873 in Catalogue of the specimens of Hemiptera Heteroptera in the collection of the British Museum (21).| Characters |
Annotated Figures |
| Generally yellow and black in colour |
|
| Anteocular part of head as long as the postocular |
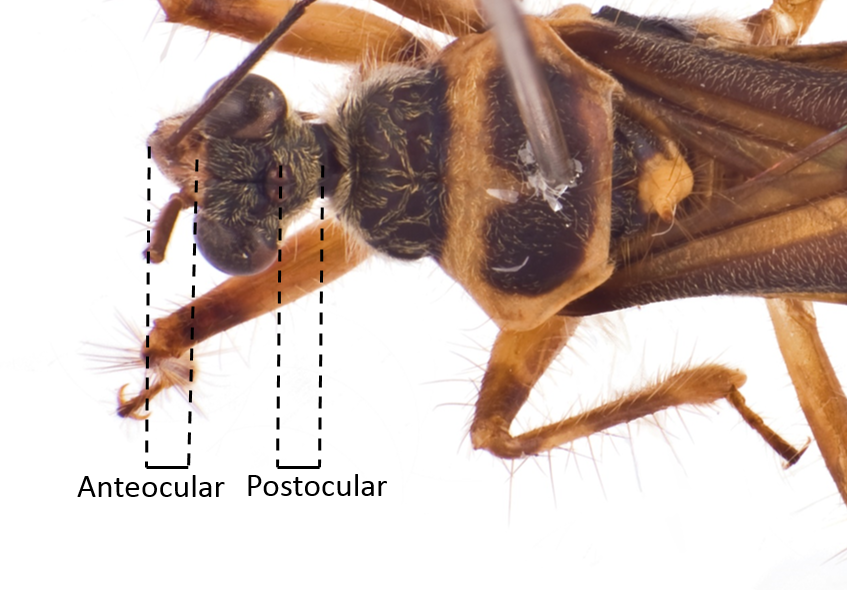 Fig. 12. Dorsal view of head. Image by: Yeo Huiqing. |
| Eyes are very large and prominent |
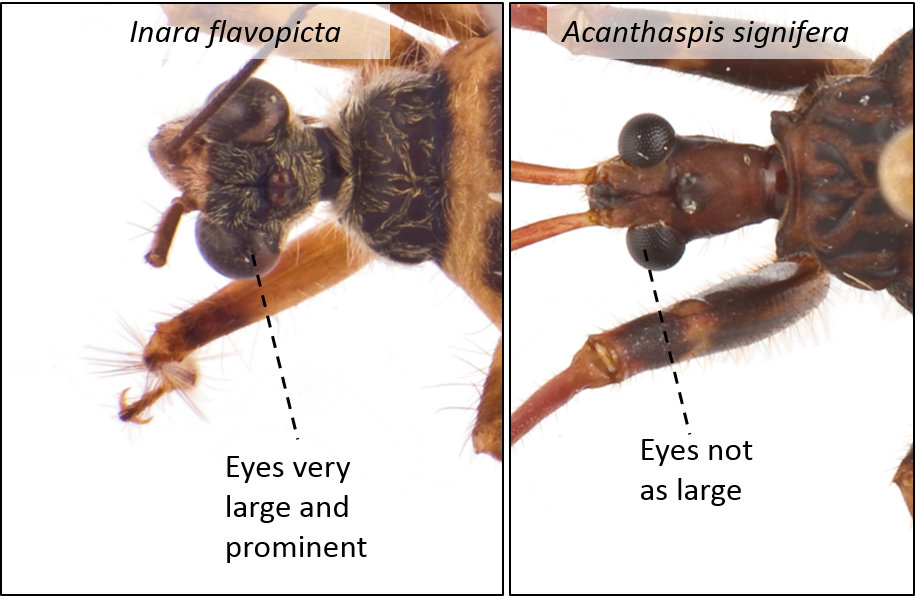 Fig. 13. Dorsal view of the eyes of Inara flavopicta (left) and Acanthaspis signifera (right). Image by: Yeo Huiqing. |
| Rostrum yellow except at the base and tip which are black |
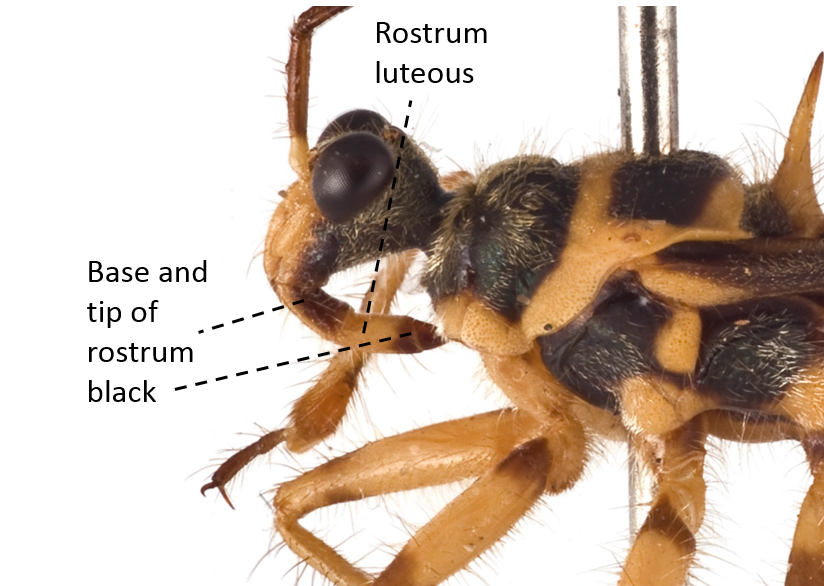 Fig. 14. Lateral view of rostrum. Image by: Yeo Huiqing. |
| Forelobe of the prothorax a little shorter than the hind lobe The hind lobe is bordered with yellow with a black tooth-shape on the center of the hind lobe |
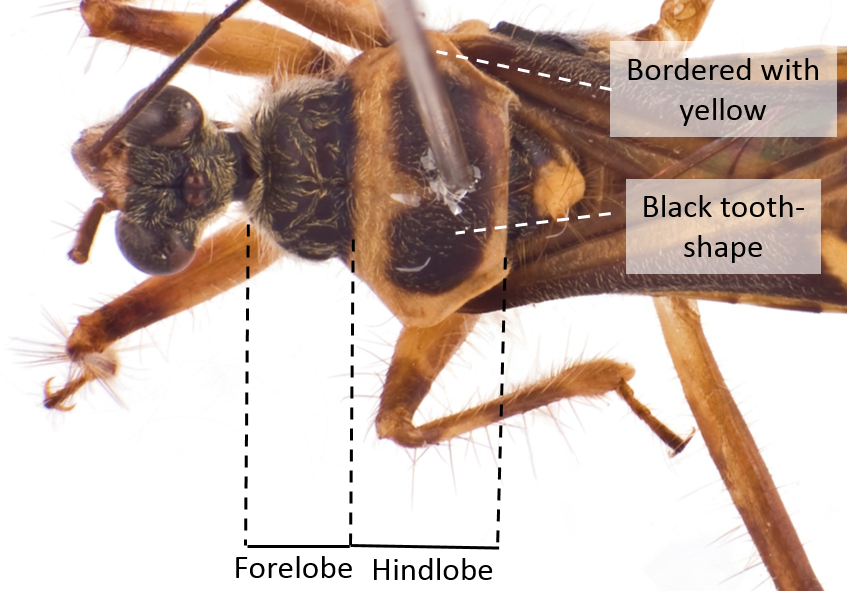 Fig. 15. Dorsal view of the pronotum. Image by: Yeo Huiqing. |
| Scutellum black, with a long yellow and erect spine ending with a black tip |
 Fig. 16. Dorsal (left) and lateral (right) view of the scutellum and scutellum spine. Image by: Yeo Huiqing. |
| Abdomen mostly yellow but also with black bands and patches of white setae |
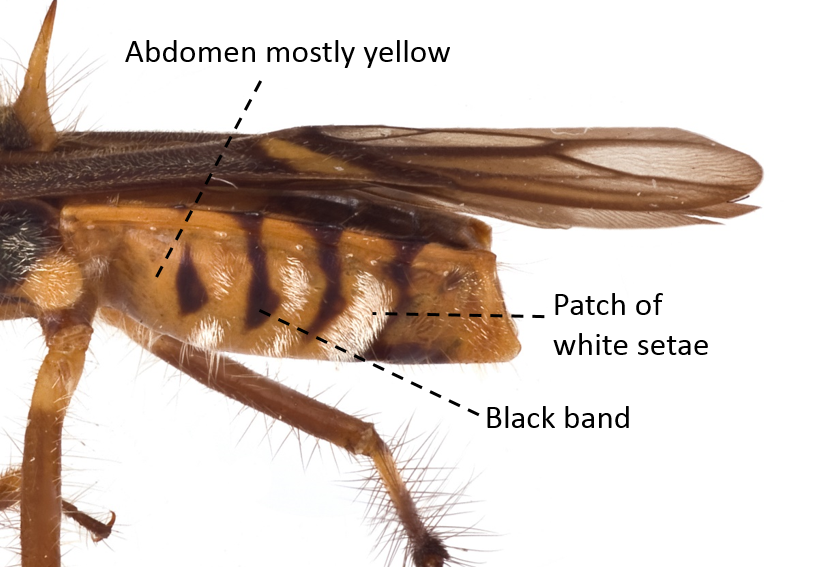 Fig. 17. Lateral view of abdomen. Image by: Yeo Huiqing. |
| Fore and mid-femora slightly thickened and all tibiae hairy |
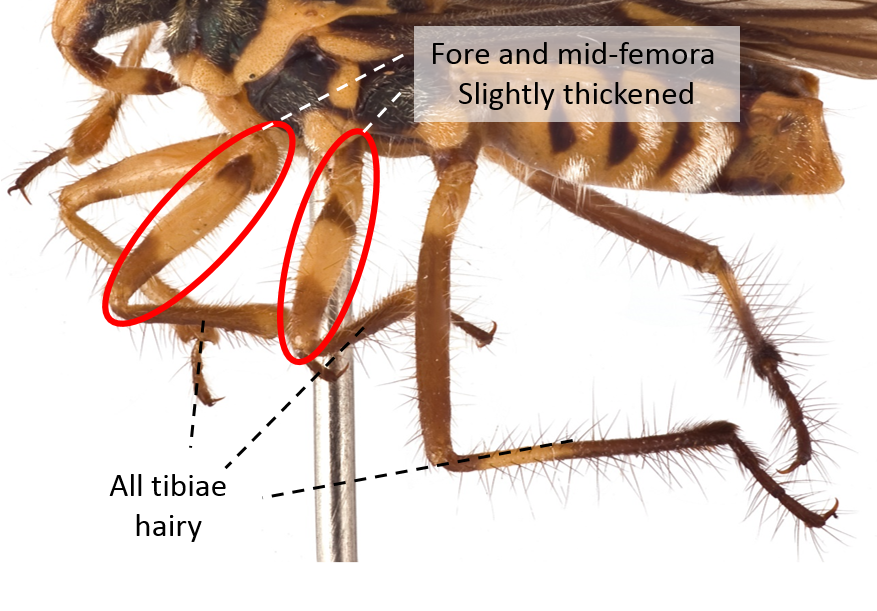 Fig. 18. Lateral view of legs. Image by: Yeo Huiqing. |
| Forewings with an oblique yellow streak between the corium and membrane Membrane dusky brown to black, with black veins |
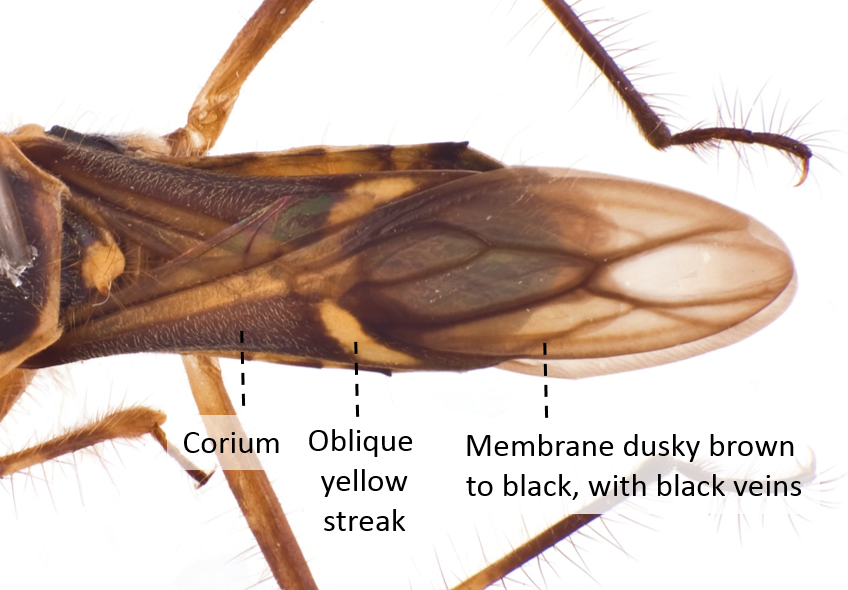 Fig. 19. Dorsal view of forewings. Image by: Yeo Huiqing. |
[5.4] Nymphal Stages
Currently there is no detailed description of the ant-piling nymph. As the various nymphs were caught at different instar stages in Singapore, the exact instar stage could not be determined although they can be categorized into early, middle and late instar stages by comparing the abdominal segments and development of wing buds (Fig. 20). Nymphs of later instars have abdominal segments which are harder and darker in colour (sclerotised) and a more developed wing buds. |
| Fig. 5. Ant-piling nymphs in the the early, middle and late instar stages (left to right). Photos by YeoHuiqing. |
Fig. 20. Ant-piling nymphs at various stages of development. Photos by: Yeo Huiqing.
[5.5] Comparison with Acanthaspis species in Singapore
Besides Inara flavopicta, the nymphs of Acanthaspis species are also known to carry out the unique piling behaviour (5). In Singapore, there are three other species of Acanthaspis (Acanthaspis nr. quadriannulata, Acanthaspis signifera and Acanthaspis inermis) and the adults appear distinct from Inara flavopicta (Fig. 21). However, as the nymphs of the Acanthaspis species in Singapore are not well-known, it is difficult to identify them and they may have very similar piling behaviour.
Fig. 21. A comparison of (A) Acanthaspis cf. quadriannulata, (B) Acanthaspis signifera, (C) Acanthaspis inermis and (D) Inara flavopicta. Photos by: Yeo Huiqing.
Based on personal observations in the field and subsequent attempts to rear them under laboratory conditions, the nymphs of Acanthaspis nr. quadriannulata are postulated to pile debris consisting of bark, soil and sometimes pieces of moss (Fig. 22 and Video 5). The piling behaviour of the nymphs of the other two species of Acanthaspis in Singapore are not known however. They may be similar to Inara flavopicta in that they pile ant corpses only, or they might pile debris only. It is also possible that both debris and ant corpses are use as material to build the backpack.
 |
|
| Fig. 22. Debris-piling nymph covered with a mixture of bark, soil and moss. Photo by Nicky Bay, permission obtained. |
Video 5. Two debris-piling nymphs are featured, the bigger nymph is actively gather debris and adding material to its existing backpack. Skip to 0:34 to see the piling action. Video by: Yeo Huiqing |
[6] Taxonomy and Systematics
[6.1] Classification
The following taxo-navigation system is obtained from UniProt showing classification from the genus Inara and above.› cellular organisms
› Eukaryota
› Opisthokonta
› Metazoa
› Eumetazoa
› Bilateria
› Protostomia
› Ecdysozoa
› Panarthropoda
› Arthropoda
› Mandibulata
› Pancrustacea
› Hexapoda
› Insecta
› Dicondylia
› Pterygota
› Neoptera
› Paraneoptera
› Hemiptera
› Euhemiptera
› Neohemiptera
› Prosorrhyncha
› Heteroptera
› Euheteroptera
› Neoheteroptera
› Panheteroptera
› Cimicomorpha
› Reduvioidea
› Reduviidae
› Reduviinae
› Inara
[6.2] Synonyms
The following names are synonymised by Distant, 1903 in Rhynchotal notes XVI, Heteroptera: Family Reduviidae, Apiomerinae, Harpactorinae, and Nabidae (22):Spiniger conficitus Walker, 1873
Spiniger limbifer Walker, 1873
[6.3] Type Information
The holotype (a specimen in which the scientific name is formally attached to and described from) was collected from Malaysia, Pulo Penang and deposited in the Swedish Museum of Natural History, Stockholm (20). However, no other information on the type specimen can be found.[6.4] Phylogeny
To date, the largest coverage of the phylogeny of assassin bugs consist of 178 species and 18 subfamilies (1). Assassin bugs are accepted as a monophyletic group; however the subfamily Reduviinae, in which Inara flavopicta belongs to, is polyphyletic and has been grouped into 11 clades (1, 23) (Fig. 23). Inara was found to be nested within the ‘Acanthaspis clade’ thus rendering the genus Acanthaspis non-monophyletic and could result in a name change for Inara flavopicta in the future when cladistics analysis inclusive of morphological data is carried out to further test the relationships.
Fig. 23. Maximum likelihood estimate for Reduviidae. The tree is based on RAxML analysis of 178 taxa using 5 gene regions (16S, 18S, 28SD2, 28SD3-D5, Wg). Bootstrap values are indicated on branches using colored triangles; Blue with >90% support, green with 70-90% support and red with 50-69% support. Red branch colour represents Reduviinae lineage, blue for the rest of the lineages and black for outgroups. The position of Inara flavopicta within the Acanthaspis clade is highlighted in yellow. (Source: Hwang & Weirauch, 2012, Under PLOS ONE Open-Access License)
Ancestral state reconstruction of microhabitats of assassin bugs revealed that foliage-living lifestyles evolved six times independently, one of which occurred in Inara among the largely bark-associated and ground-dwelling species in the Acanthaspis clade (3) (Fig. 24).
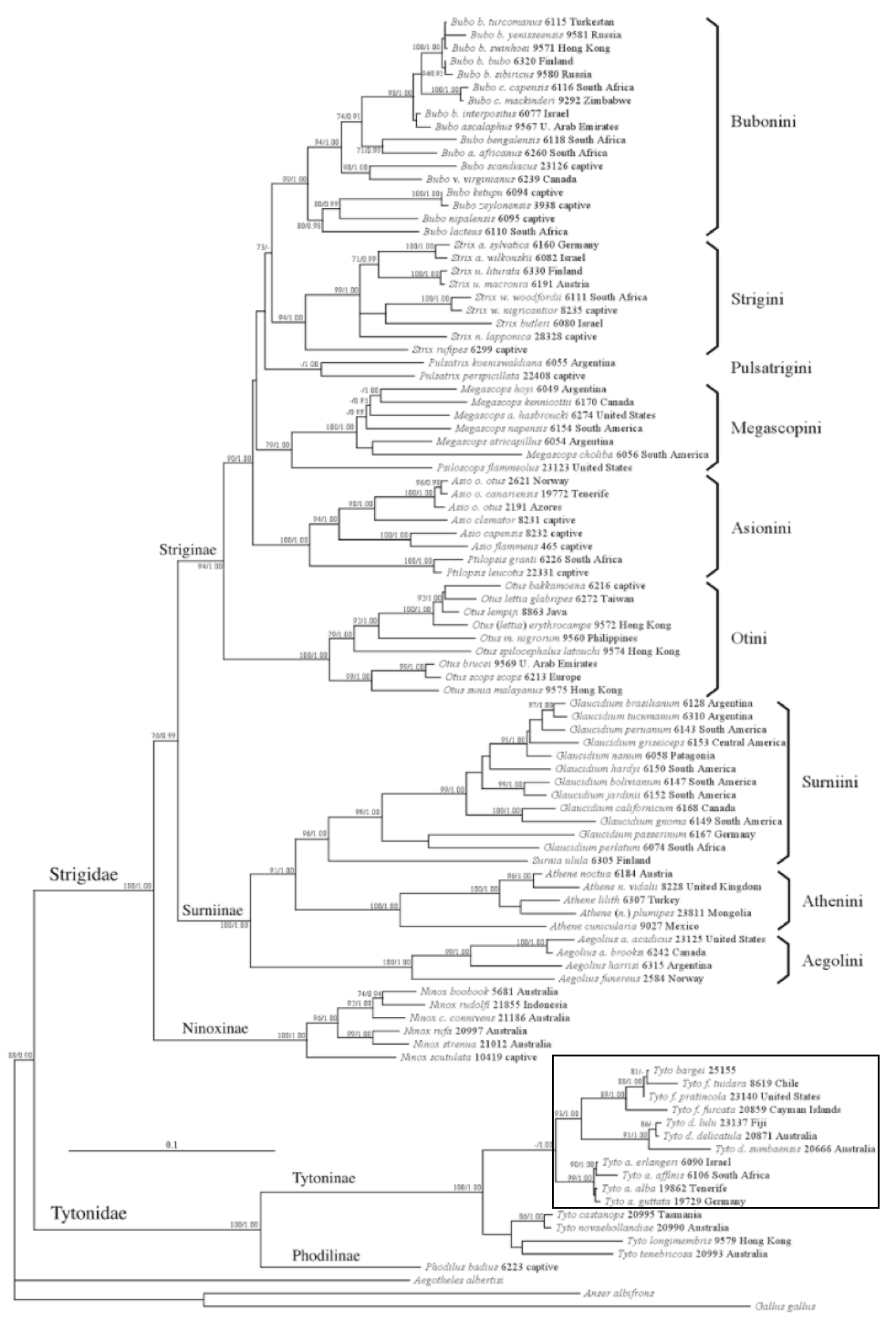
Fig. 24. Ancestral state reconstruction of microhabitats based on maximum likelihood tree. The branches are colour coded to represent different microhabitats (see legend in figure), and the pie-charts with similar colours represent probabilities generated from BayesTraits. Terminals without coloured squares indicated unknown microhabitats. Foliage-living Inara flavopicta (highlighted in yellow) evolved independently from the largely bark associated and ground-dwelling species in the Acanthaspis clade. (Source: Hwang & Weirauch, 2012; Under PLOS ONE Open-Access License)
In addition, ancestral state reconstruction for diet indicated that ant specialisation evolved at the base of the Acanthaspis clade (3) (Fig. 25) and all members of the group are predicted to prey mainly on ants.
Fig. 25. Ancetral state reconstruction of prey specialisation based on maximum likelihood tree. The branches are colour coded to represent different prey specialisation (see legend in figure), and the pie-charts with similar colours represent probabilities generated from BayesTraits. Terminals without coloured squares indicated unknown diet. Inara flavopicta (highlighted in yellow) is predicted to specialise on ants. (Source: Hwang & Weirauch, 2012; Under PLOS ONE Open-Access License)
The genes used in the phylogenetic analysis includes four ribosomal genes (16S rDNA, 18S rDNA, 28S D2 rDNA, 28S D3-D5 rDNA) and one nuclear protein coding gene (Wg; wingless) (1). The sequences are available in GenBank.
[7] Glossary
Ancestral state reconstruction – Also known as character mapping or character optimization. A method of mapping characters onto trees to understand the phenotypic and genetic states of organisms.
Biologically active – Use to describe a substance having an effect on living organisms in small amounts
Chemical camouflage – Use of chemicals which resembles that of ants to prevent the ants from detecting the ant-piling nymphs and attacking them
Crypsis – Ability of an organism to avoid detection by others. See Crypsis in Wikipedia.
Colony-specific volatile compounds – Compounds use by members in the same colony to communicate and which evaporates easily
Dorsal glands - Glands found on the dorsal surface of the abdomen of assassin bugs (See Fig. 2)
Dexterous – Skillful and competent; agile
Exoskeleton – A tough and rigid external covering in invertebrates
Extra-oral digestion – Digestion of food before it is taken into the insect’s body
Holotype – A specimen in which a scientific name is tied to and formally described
Homologous – Having the same relation, relative position or structure
Instar stage – A developmental stage of insects before adulthood (sexual maturity)
Labium – the long and thin mouthpart of bugs
Microhabitats – A small, localised habitat that supports an organism (for example a decaying tree stump)
Morphology – A particular form, shape or structure
Necrotic – A form of cell injury resulting in early death of cells by self-destruction (autolysis)
Prosternum – The first ventral segment of the thorax. (See Fig. 10)
Sclerotized – The hardening and darkening of an insect’s body
Toxic – Containing poisonous substances
[8] References
- Hwang, W. S., & Weirauch, C. (2012). Evolutionary history of assassin bugs (Insecta: Hemiptera: Reduviidae): insights from divergence dating and ancestral state reconstruction. PloS one, 7(9), e45523.
- Maldonado Capriles, J. (1990). Systematic catalogue of the Reduviidae of the world (Insecta: Heteroptera). Caribbean Journal of Science. Special Edition.
- Brandt, M., & Mahsberg, D. (2002). Bugs with a backpack: the function of nymphal camouflage in the West African assassin bugs Paredocla and Acanthaspis spp. Animal Behaviour, 63(2), 277-284.
- Jackson, R. R., & Pollard, S. D. (2007). Bugs with backpacks deter vision‐guided predation by jumping spiders. Journal of Zoology, 273(4), 358-363.
- Weirauch, C. (2006). Anatomy of disguise: camouflaging structures in nymphs of some Reduviidae (Heteroptera). American Museum Novitates, 1-18.
- Schuh, R. T., & Slater, J. A. (1995). True bugs of the world (Hemiptera: Heteroptera): classification and natural history. Cornell University Press.
- Weirauch, C., & Munro, J. B. (2009). Molecular phylogeny of the assassin bugs (Hemiptera: Reduviidae), based on mitochondrial and nuclear ribosomal genes. Molecular phylogenetics and evolution, 53(1), 287-299.
- Cohen, A. C. (1995). Extra-oral digestion in predaceous terrestrial Arthropoda. Annual review of Entomology, 40(1), 85-103.
- Schilman, P. E., Lazzari, C. R., & Manrique, G. (2001). Comparison of disturbance stridulations in five species of triatominae bugs. Acta tropica,79(2), 171-178.
- Discover Life, 2014. Inara flavopicta Stal 1859, http://www.discoverlife.org/mp/20q?search=Inara+flavopicta. Accessed on 12 November 2014.
- Edwards, J. S. (1961). The action and composition of the saliva of an assassin bug Platymeris rhadamanthus Gaerst.(Hemiptera, Reduviidae). Journal of Experimental Biology, 38(1), 61-77.
- Haridass, E. T., & Ananthakrishnan, T. N. (1980). Models for the predatory behaviour of some reduviids from Southern India (Insecta-Heteroptera-Reduviidae). In Proceedings of the Indian Academy of Sciences: Animal Sciences, 89(4), 387-402. Indian Academy of Sciences.
- Miles, P.W. (1972). The saliva of Hemiptera. Adv. Insect Physiol., 9:183-256
- Corzo, G., Adachi-Akahane, S., Nagao, T., Kusui, Y., & Nakajima, T. (2001). Novel peptides from assassin bugs (Hemiptera: Reduviidae): isolation, chemical and biological characterization. FEBS letters, 499(3), 256-261
- Evangelin, G., Horne, B., Muthupandi, M., & John William, S. (2014) Venomous saliva of non-haematophagous reduviid bugs (Heteroptera: Reduviidae): a review. Biolife 2(2), 615-626
- Cohen, A.C. (1989). Ingestion and food consumption efficiency in a predacious hemipteran. Ann. Entomol. Soc. Am, 82, 495-499
- Corzo, G., Adachi-Akahane, S., Nagao, T., Kusui, Y., & Nakajima, T. (2001). Novel peptides from assassin bugs (Hemiptera: Reduviidae): isolation, chemical and biological characterization. FEBS letters, 499(3), 256-261
- Penney, H. D., Hassall, C., Skevington, J. H., Abbott, K. R., & Sherratt, T. N. (2012). A comparative analysis of the evolution of imperfect mimicry. Nature, 483(7390), 461-464
- Ho¨lldobler, B. & Wilson, E. O. (1990). The Ants. Berlin: Springer Verlag
- Stål, C. (1859). Till kännedomen om Reduvini. Oefv. K. Vet.-Ak. Foerh, 16, 175-204
- Walker, F. (1973). Catalogue of the specimens of Hemiptera Heteroptera in the collection of the British Museum. Part VII. Printed for the Trustees, London. 7: 1–123
- Distant, W. L. (1903). Rhynchotal notes. XVI. Heteroptera: Family Reduviidae, Apiomerinae, Harpactorinae, and Nabidae. Ann. Mag. Nat. Hist., (7)11: 203-213, 245-258.Weirauch, C. (2008). Cladistic analysis of Reduviidae (Heteroptera: Cimicomorpha) based on morphological characters. Systematic Entomology,33(2), 229-274.
[9] Contact Information
If you have any suggestions or comments about the species page, you can leave a message in this website or contact me (Huiqing) at yeohuiqing92@gmail.com.