Asian Seabass

Table of Contents
(Photo credits: Mitch Ames, Licensed under Creative Commons Attribution- ShareAlike)
Name
Binomial: Lates calcarifer
Vernacular: Sea Bass (Asia), Barramundi (Australia), giant sea pearch (Papua New guinea), 尖吻鲈
Common names in Singapore market: 金目鲈 (jin1 mu4 lu2), Siakap, Kim Bak Lor
Synonyms
Meaning of synonyms click hereHolocentrus calcarifer (Bloch 1790), Type locality: Debated
Holocentrus heptadactylus (Lacepede, 1802), Type locality: unknown
Coius vacti (Hamilton, 1822), Type locality: Ganges River, India
Lates nobilis (Cuvier, 1828), Type locality: Pondicherry India)
Pseudolates cavifrons (Alleyne & Macleay, 1877), Type locality: Coast of New Guinea/ Torres Strait
Lates darwiniensis (Macleay, 1878), Type locality: Darwin Australia
Diagnosis
| Colour |
|
| Body |
|
| Fins |
|
| Lateral Scale Line |
|
| Size |
|
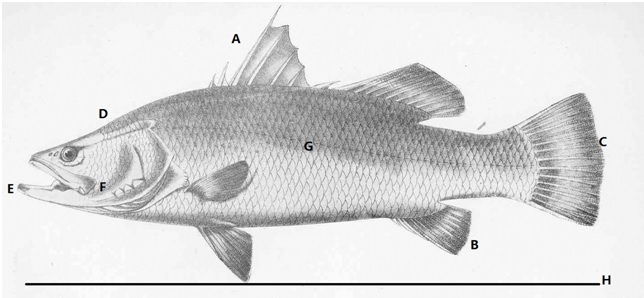
Figure 1. Sketch of L. calcarifer and important diagnosis features. A: 7-9 spines and 10-11 soft rays at dorsal fin. B: 3 spines and 7-8 soft rays at anal spines. C. Rounded claudal fin. D: Pointed head with concave forehead becoming convex towards the dorsal fin. E: Produced lower jaw. F: posterior edge of the maxilla falling distinctly behind the eyes G: 52-56 lateral line scale and 6 rows of scales between 3rd dorsal fin spine and lateral line. H: Common length is 1500mm and maximum length is 2000mm. (Photo credit: FAO, edited by Kwan Mei Yen, Licensed under Creative Commons Attribution- non commercial)
Try this!The first video below is a video of L. calcarifer while the second video below is a tank with several species including L. calcarifer. Try finding L. calcarifer in the second video if you can!Click here for answer
Video 1 (up): Video of L. calcarifer swimming in the tank.. Video 2 (down): Group of fishes swimming together in a tank, including L. calcarifer.
Introduction
Lates calcarifer is a popular aquaculture and sport fish species. It is not only well-known world-wide, but also has a long history with Singapore. As explained below, general information like biology and ecology regarding this species varies according to yearly conditions, geological conditions and physiological conditions. Highlight in blue under the respective topics, this page also highlight the relationship of this species with Singapore. Our nature population and escapees from aquaculture farms are closely related to local fishing enthusiasts while our aquaculture populations are generating incomes for us. On the other hand, our natural population are being affected by urbanization of Singapore, especially due to the damming of our rivers, which interrupts with the spawning of this species. Currently, the taxonomy identity this species is still highly debated and below, this page also reviewed some of the recent articles concerning this topic
Etymology
Meaning of etymology click hereThe word ‘calcarifer’ translates to ‘thorn carrier’ due to the preopercular spines and thorns present on the fish [1] (Figure 2) . The thorns on the dorsal fins would prevent predator from attacking it from the back. Stroking the fish from the back to the front would be painful as spines are pointed backwards.
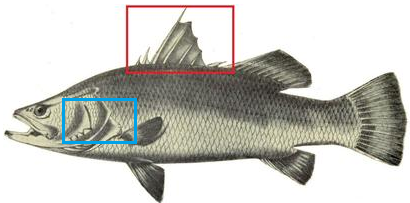
Figure 2. Preopercular spines as shown in blue box and thorns as shown in the red box. (Photo credits: Henry Sullivan Thomas, Edited by Kwan Mei Yen, Licensed under Public domain)
Distribution
“They are native to many places”
L. calcarifer are native to the Indo-West Pacific region from Arabian gulf to Southern China, Taiwan, Papua New Guinea and northern Australia. The eastern limit is at south east tip of Papua New Guinea mainland, northern limit at Amoy China (24o30”N), western limit at Persian Gulf and southern limit at Noosa river (26o30’s) and Ashburton River (22o30’S) of Australia mainland [1].
Figure 3. Distribution map of L. calcarifer, showing the western, eastern, northern and southern limits
Higher temperatures, species competition and higher salinities due to lack of freshwater inflow could have prevented L. calcarifer moving into the African continent [2].
Ecology and spawning
“They survive in various salinity levels!”
This species is euryhaline and thus are found in freshwater, estuarine, lagoons, brackish, rivers and coastal areas [3]. It is experimentally proven using isotope ratios in otoliths (ear bone of the fish) that some of the fish are catadromous while some are predominantly marine residents [4] (Figure 3).
Adults in freshwater usually migrate downstream, to spawn in water with higher salinity as eggs need saltwater to develop fully. Spawning usually occurs in brackish water like the river mouth and is seasonal, depending on geographical locations and yearly variation [5].
Generally, spawning is usually synchronized during the wet season, for juveniles to advantages of the larger aquatic habitat and move into off-stream nursery habitat [6,7]. Spawning is associated with incoming tides and lunar cycle, to transports the eggs or larvae to the estuarine or brackish water [5,7,8]. After hatching, the larvae move into coastal swamps, lagoons or other lotic habitats which act as the nursery grounds. Such nursery grounds are high in food abundance, provide protection and have less predators [5,9,10]. Surviving juveniles moves either into coastal water or ascend into freshwater at the coastal rivers and creeks. However, some alternate between fresh and salt water, at various ages or under various environment conditions [1]. The cues for such fish to move between fresh and salt water are complex and occur irregularly. The general life cycle is described in Figure 4.
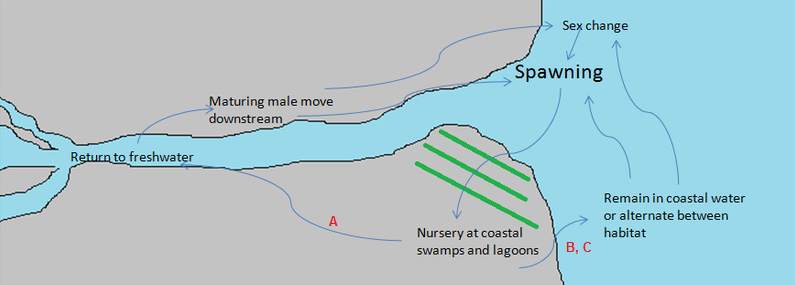
Figure 4. General life cycle of L. calcarifer. Begin from spawning & growth: After spawning at estuarine or brackish water near river mouth, the larvae and early juveniles move into lotic habitats, lagoons, coastal swamps as nursery swamps. As they grow older, they might A: migrate up to river, B: remain in coastal areas or C: alternate between the habitats. Mature males usually migrate back to coastal ares to spawn. Sex reversal occur when they reach certain age, where the age they sex reverse depends on the population and other cues. (Picture by Kwan Mei Yen)
In the Singapore context: L. calcarifer are observed in our estuaries and rivers like in Punggol, Singapore River and Serangoon. As rivers in Punggol and Serangoon are dammed to convert to reservoirs; L. calcarifer could not be observed using the same sampling techniques as compared to before and during damming, which is unexpected as L. calcarifer are known to be able to adapt to freshwater [11]. Some possible reasons could be due to the depletion of food caused by change in species composition. Alternatively, the sampling was not intensive enough. However, the damming of rivers in Singapore would prevent access to sea water and thus L. calcarifer that are trapped in freshwater might not be able to spawn successfully. As for the L. calcarifer in the coastal areas, with reduced input of nutrients from fresh water due to damming, the productivity of the estuarine/brackish water would also be reduced and affect the survivability of L. calcarifer and other estuarine associated species [12]. L. calcarifer are also found frequently around Singapore waters[13,14]. However, the fishes could belong to the natural population or escapees from local farms, which are located both in the northern and southern parts of Singapore [15].
Biology
Sexual Development
“Almost all are born male”
In most vertebrates, sexes are separated early due to sex differentiation (Figure 5). However, among vertebrates, teleosts represent almost all known types of sex determination systems [16]. It is stated that a third genome duplication occurred in the teleost lineage, giving genetic material for divergence and speciation [17,18,19]. Many evolved to present a huge diversity of sex determination and reproduction strategies.
Being a teleost, L. calcarifer evolved to be protandrous hermaphrodites [20], where they first mature as males and then sex inverse to become female later in life (Figure 5). The age where the males sex reverse varies and depends on various factors like environment and physiological environment. Generally, the fish takes about 3-5 years to mature as male, and sex inversion occurs at about 6-8 years in most parts of Australia but some fishes at in the Gulf of Carpentatia mature before the age of 2 and sex reverse earlier [21]. Fishes in aquaculture setting in Asia and Australia usually mature earlier, and sex reversing within 4 years [15,22]. Thus, sex change can occur between 4-8 years while male maturation can occur between 1-5 years. However, not all females are derived from males. Primary females were observed in both the natural and the aquaculture environment [20].
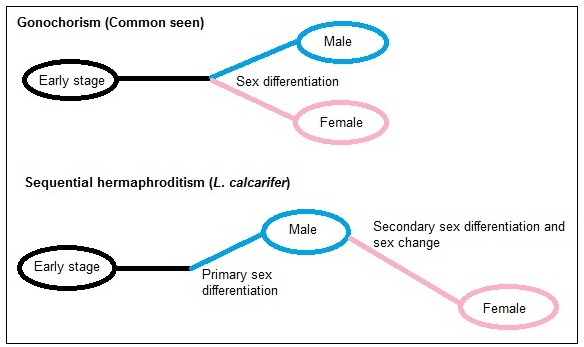
Figure 5. Sex differentiation commonly seen vs. Sex differentiation of L. calcarifer. Sex change can occur between 4-8 years for L. calcarifer. (Picture by Kwan Mei Yen)
The transition cannot be observed morphologically and require invasive method like dissection to collect gonards. Hence, information about sex inversion is not complete due to the need of invasive methods and around at least several years for sex inversion to occur. However, recent studies suggest the possibility of using hormones levels in body mucous to determine sex [Temasek Life Sciences laboratory, Laszlo Orban group, unpublished data].
In the Singapore context: Little research is done about the sexual development of L. calcarifer in our wild population. Generally for our aquaculture population, the male reach maturation within 2.5 years and sex reverse to female between 3-4 years [15]. However, since different farms use fishes origin from different location (i.e. Farms like Barramundi Asia uses Australia stock [23], while others uses Asia Stock), the sexual development might differs from farm to farm in Singapore.
Diet
"Diet varies based on size"
Lates calcarifer are carnivours, opportunistic predators and dermersal fish with diet changing according to their size. Wild populations feed on microcrustacea up to 50mm. The diet also transit into feeding on insects, fishes and crustaceans, depending on the availability of food, as they grow bigger [2,24,25].
In aquaculture setting, rotifers should be first given to larvae, and weaned to Artemia. Survival can be enhanced with highly-unsaturated fatty acid enhanced rotifers and brine shimp (Artemia) [26,27]. As brine shimp are expensive, other replacement can be cladoceran Moina macropora and Diaphanosoma celebensis [28,29]. As the fish grow older, they should be fed with trash fish. Alternatively, to reduce cost, plant based pellet food with enhanced essential nutrients can be used. Feeding should be done slowly to prevent overfeeding and water contamination. L. calcarifer are cannibalistic fishes and would feed on smaller ones that are smaller than 2/3 of their length. This sorting of the stock should be done every 4 days to reduce cannibalism [30].
In the Singapore context: Our wild population of L. calcarifer probably do not differ in diet compared to other populations. However, the feeding would still rely on food availability. In Singapore, selective breeding is on going for L. calcarifer. In terms of diet, experiments are carried out to better understand the feed and nutritional requirements of L. calcarifer, to increase nutritional value, health and growth rate for the aquaculture fish [31].
Economic Value
Aquaculture
Being a popular fish due to its dedicated fish flavor and nutritional value, L. calcarifer is highly valued throughout the Indo-Pacific region. It has been breed successfully in captivity and used for fisheries enhancement and aquaculture throughout Asia & Australia since 1973 [32]. Being a hardy species, L. calcarifer is relatively easy to culture in cages and tolerate freshwater to full strength seawater. Being fast growing, they reach marketable size within 8 months [33]. Even though mentioned earlier that they are usually catadromous, most aquaculture fishes in seacages can spawn regularly in pure seawater, producing large amount of larvae. If not, spawning can be induced using hormones or environmental stimuli [34]. Hence, due to these many reasons, it has been claimed to be the “next big fish” in terms of economics [35]. Much money has been channeled to the development of aquaculture technology and research on the biology of L. calcarifer, leading to increasing production from almost since 1970s to more than 70000 tonnes in 2012 (Figure 6).
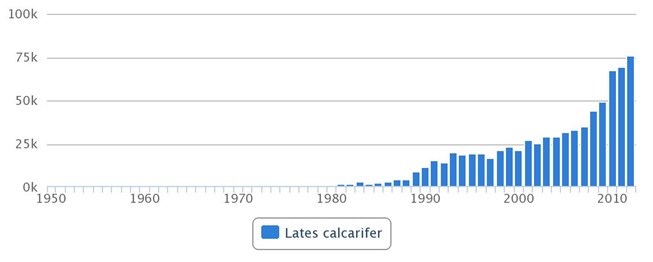
Figure 6. Global yearly aquaculture production in tonnes of L. calcarifer since 1950 to 2010. Data obtained from FAO - Fisheries and Aquaculture Information and Statistics Service – accessed on 10/11/2014.
In the Singapore context: Lates calcarifer is one of the main marine foodfish cultured in Singapore along with tilapia, milkfish, golden snapper (Lutjanus spp.) and estuarine grouper (Epinephelus tauvina) [15]. The development of sea cages intensified during mid 1981 when the government implemented a marine foodfish farming scheme to encourage people to set up floating farms in designated coastal areas along Northern parts of Singapore. In 1985, the total aquaculture production of L. calcarifer valued S$1.8 million, accounting 13% in terms quantity and 20% in terms of value [15]. The total production was 1284 tonnes in 1985 and around 4147 tonnes in 2011, accounting to 0.6% of the world’s production (Figure 7). Currently, we have fish farms in areas like the northern parts of Singapore, off Pasir Ris and southern parts of Singapore (e,g Palau Semakau) and even land-based closed aquaculture system which allows extensive water quality control by SIF technologies.
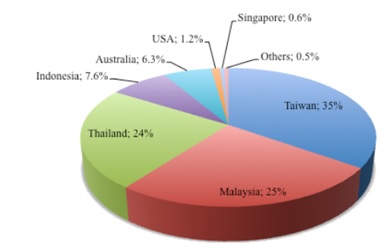
Figure 7. Global production of L. calcarifer according to countries in 2011. Data obtained from FAO - Fisheries and Aquaculture Information and Statistics Service – accessed on 19/02/2014.
Singapore government organization like National Research Foundation and Agri-food and Veterinary Authority of Singapore also provide research funding for selective breeding and genetic studies of L. calcarifer to produce faster growth and disease resistance fish, which are on going [37,38]. Local farms contribute up to about 15% of the total supply and we are planning to expand our aquaculture industry and greatly increasing our production. Currently, we have our local larvae supply of L. calcarifer and established our production cycle from broodstock, larval rearing to grow-out facilities. The geological location of Singapore (being surrounded by sea and sheltered from natural disasters) and funding channeled into research and development would help generate more economic growth in terms of Aquaculture in the near future.
With depletion of fish stock worldwide, aquaculture technology to increase productivity and fish quality have became a key solution to stabilize the supply of food fish for mass consumption. By owning the technology for fish production, this will also stabilize the fish supply for Singapore, who imports almost all of our food product. To prepare for the development of this industry, local polytechnics are coming up with new diplomas related to aquaculture for our students.
Recreational fishing
Lates calcarifer is also an important recreational fish, which is well loved among anglers who fish them using lines. Fishing of L. calcarifer has also become a tourism business in Australia. They are also stocked in ponds and lakes for recreational fishing. They grow up to around 1500mm and weight over 55kg and have good fighting ability [38]. As seen from the video below (2:06 - 3:00, 4:27 - 4:47, 5:12 - 6:35), skills and agility are required to catch L. calcarifer which are huge and strong. Catching them with a fishing rod indeed bring satisfaction to the anglers.
Video 3: Video of fishing trip in the wild, including L. calcarifer
In the Singapore context: Fishing companies in Singapore or individuals will travel out to Singapore waters by boats to fish for L. calcarifer (own interview). Also, L. calcarifer can be caught in some of Singapore's jetty, being a good pastime for some Singaporeans [39]. Below is a video of L. calcarifer being caught in Singapore's Woodland jetty. Visit FishingSpore Youtube page and local blog1 blog2 for more videos/stories regarding recreational fishing and fishing hotspot in Singapore! L. calcarifer indeed is commonly caught in Singapore water like Woodland jetty, Yishun Dam and Punggol Waterfront Jetty.
Video 4: Video of L. calcarifer caught in Woodland jetty
Taxonomy
History
Lates calcarifer was first described by Bloch (1790) using samples found at the Indo-Pacific region from Dutch merchants [1]. It was first named Holocentrus calcarifer and published at Naturgeschichte der ausländischen Fische. Morino, Berlin. The genus Lates (Cuvier and Valenciennes) was erected but in 1828, proposed to bring together other related species like the Nile perch (L. niloticus (Linnaeus, 1758)) for classification. The family to put L. calcarifer used to be highly debated and suggested to be put in Percinae, Centropomidae, Latidae and Serranidae [1]. Currently, it is placed in the family Latidae with three genera, Psammoperca, Hypopterus and Lates (excluding the extinct Eolates).
Clades
In descending order, with the clades below being a subset of the clades above:
>>Animalia
>> Chordata
>> Actinopterygii
>> Perciforms
>> Latidae
>> Lates
>> Lates calcarifer
Information Obtained from ITIS
Advance Diagnosis
Between Lates
It is challenging to distinguish species within the same genus as the morphological features are very similar. There are only several non-overlapping parameters to distinguish the fishes. The table below show L. niloticus being challenging to distinguish from L. calcarifer morphologically and how to distinguish L. calcarifer from three other Lates that can be found in the Indo-West Pacific region [40]. Figure 8. Photo of L. niloticus. (Photo credit: N Sloth, Licensed under Creative Commons Attribution- non commercial) Lates niloticus There are hardly any reliable ways or published to distinguish them apart, akin to many fish species where species identity is based solely on molecular data. The COI sequences of L. niloticus and L. calcarifer are available online on NCBI. Ecologically, L. niloticus is purely a freshwater fish as compared to L. calcarifer which is euryhaline. |
 Figure 9. Photo of L. japonicus. (Photo credit: OpenCage, Licensed under Creative Commons Attribution- ShareAlike) Lates japonicus It can be distinguished from L. calcarifer by having 7-8 (vs. 6) rows of scales between the base of the third dorsal-fin spine and lateral line; 58-63 (vs. 52-56) lateral-line scale; and having the 3rd anal-find spine shorter than the 2nd. This species is distributed at coastal areas off south-eastern shikolu and Kyushu Islands. |
 Figure 10. Holotype of L. Lakdiva stored in the Australian Museum Ichthyology Collection (AMS I.37516-001) © Australian Museum Lates lakdiva It can be distinguished from L. calcarifer by having 5 (vs. 6) rows of scales between the base of 3rd dorsal-fin spine and lateral line and having the 3rd dorsal fin spine 3.0 -3.5 (vs. 2.1-2.8) times the length of the second. This species is found in coastal seas off south-western Sri Lanka. |
 Figure 11. Holotype of L. uwisara stored in the Australian Museum Ichthyology collection (CSIRO H.6316-11) © Australian Museum Lates uwisara It can be distinguished from L. calcarifer by having 7 (vs. 6) scales between base of 3rd dorsal-fin spine and lateral line and having an eye diameter less than (v.s greater than) the depth of the maxilla. The length of the dorsal spine is equal or larger than the length of the pelvic spine. This species is found in estuaries between Sittang and Yangon, Burma. |
Between Latidae
As mentioned, in the family Latidae, there are three genera named Psammoperca, Hypopterus and Lates. The first two genera only have one species each, P. waigiensis and H. macropterus respectively. Lates can be mainly distinguished from Psammoperca and Hypopterus as 1) lower edge of the preopercle is serrated; 2) maxilla falling distinctly behind the eyes and; 3)produced lower jaw. In addition, P. waigiensis and H. macropterus are strictly coastal fish that would not enter freshwater unlike Lates. L. calcarifer average length is 1500 mm while P. waigiensis and H. macropterus rarely grow bigger than 400mm and 140mm respectively [41].In the Singapore context: Psammoperca waigiensis is distributed across the Indo-West Pacific region and thus, is commonly found in Singapore’s coastal water and fishery ports together with L. calcarifer [13]. There are several morphological features to tell them apart easily. P. waigiensis has maxilla that fall under the eyes (vs. maxilla falling distinctly behind the eyes), no produced lower jaw, wide apart nostrils and being smaller than 40cm (Figure 12).

Figure 12. Photo of Psammoperca waigiensis. Can be differentiated from L. calcarifer because A: maxilla does not fall behind the eyes but under. B: lower jaw is not in advance of upper jaw. C: nostrils are set wider apart. D: rarely exceed 40cm. (Photo credit: Randell, J.E., edited by Kwan Mei Yen, Licensed under Creative Commons Attribution- non commercial)
Problems in Taxonomy
All the Lates populations in the Indo-Pacific region were named L. calcarifer. However, only in 2012, two new species L. lakdiva and L. uwisara were recognized from within the range of L. calcarifer based on to morphological analysis [42]. Also karyotype studies done on Austraria and India population showed that the chromosome formulae are different between the two population despite having the same number of chromosome (48) [43,44].
In addition, published recently in 2014, Vij et al. showed that there are 2 distinct species of L. calcaifer across its geographical range, based on sequencing the COI, 16S rDNA and highly variable D-loop region of the mitochondria. India subcontinent and Myanmar group clearly form a well supported clade away from the Asia plus Australia group [45]. Also according to Yue et al. wild caught L. calcarifer from Australia, India and central Indonesia showed strong level of genetic divergence using 7 microsatellite loci [46]. Other researches also showed that there is a deep genetic divergence between Asian and Australian populations and even within South East Asia population [47, 48].
Thus, as more investigation is done on L. calcarifer, there might be change in species name as more information regarding hybridization, molecular markers and the extend of how the fishes are isolated from each other come in.
Type Locality & Type Information
Determining type locality is made challenging when Bloch’s holotype at the was misplaced and Bloch’s original description and illustration is somewhat inaccurate. A lectotype ZMB 13652 at Museum für Naturkunde, Berlin was recovered and believed to be Bloch's type [40]. The type locality has been changed from originally Japan to Java [40] and currently believed to be from Pattukottai, Tamil Nadu, India, based on interpretation of Bloch’s handwritten catalogue [40].
As mentioned earlier, all the Lates populations in the Indo-Pacific region were named L. calcarifer. However, the current L. calcarifer might be split up into other species based on molecular evidence. But, even if we want to establish the natural population of L. calcarifer at Pattukottai as the actual L. calcarifer, it would be challenging as other Lates are likely to be bought into the region for aquaculture purpose. Interbreeding would make identifying the ‘real’ L. calcarifer which Bloch described challenging, even with molecular methods.
Mitochondria sequences
The full mitochondrial sequence can be obtained from NCBI.
Genetic Linkage Map
The genetic linkage map of L. calcarifer is also mapped based on DNA sequences of L. calcarifer individuals obtained from a selection programme in Singapore. The first linkage map is published in 2007 while the high-resolution linkage map is published in 2011. Both are free-access articles.
Phylogenetic Tree
As mentioned earlier, the family to place L. calcarifer in is debated. Currently, most papers would place L. calcarifer in the family Latidae. Having two sub-families Latidae and Centropominae under the family Centropomidae is first proposed by Greenwood in 1976 [49]. However, this study by Otero provided morphological evidence to have Latidae as a family because Centropomidae is not monophyly group [50]. Even with molecular data, the closest family related to Latidae is still highly debated. with some claiming it to be Centropominae [51,52, 53] and some claiming it to be others [54,55]. Overall, it is generally agreed that Latidae is a monophyly group. The phylogenetic tree below shows the relationship of L. calcarifer and three other species in the family Latidae, one of the most comprehensive study to date, having including several species in the family Latidae. The closest family according to this study to Latidae is Centropominae based on maximum likelihood and Bayesian analysis with 12888bp aligned. It is also surprising to see that all the studies mentioned under this section exclude genus Hypopterus which is part of family Latidae.
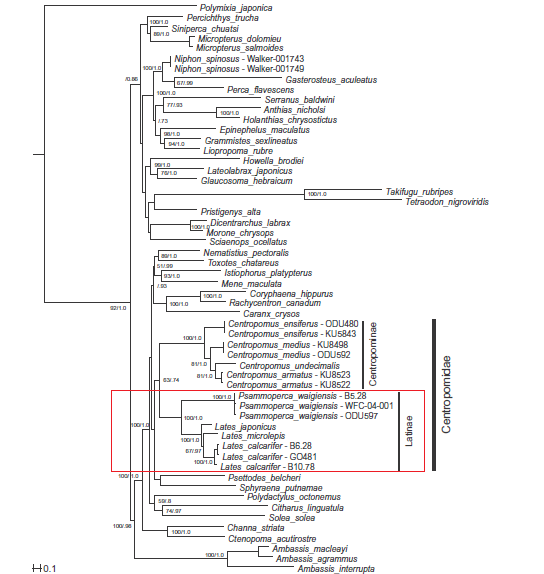
Figure 13. Phylogenetic relationship between L. calcarifer and related species in the same family. Although there are some species within Latidae are missing, this is currently one of the more comprehensive study using more genetic markers and species. (Image published by Li, et al in Molecular Phylogenetic and Evolution 60 (2011) 463-471, permission granted)
Conservation Issues
Lates calcarifer is not listed endangered by any conversational organisation.
In the Singapore context: However, it is surprising to see it listed "think twice" in the Singapore Seafood Guide accessed by WWF Singapore. This means L. calcarifer fisheries are at risk of becoming unsustainable, due to management, environmental or stock issues. Some reasons could be due to the environmental issues brought about by the aquaculture industry like pollution caused by over-feeding and increased nutrients input leading to algae bloom [56]. In 2009, a huge number of dead fish were found on the shores of Pasir Ris due to algae bloom and the link to pollution from aquaculture farms is till unknown [57, 58]. Currently, water quality studies and algae bloom management are ongoing. Another problem would be genetic pollution, when farmed fishes escape from their cages and breed with potentially distinct local wild populations. This is worrying as molecular studies are starting to show L. calcarifer might be made up of several potential species and interbreeding would prevent us from identifying the indigenous population. However, if good management and development of new technology are in place, balance between conservation and economics can be achieved.
Related Links
Fao, ////Lates calcarifer//// : Description on the detailed aquaculture techniques to rear L. calcarifer
Eol, ////Lates calcarifer//// : Information of L. calcarifer on Encyclopedia of life
FishBase, ////Lates calcarifer//// : Information of L. calcarifer on FishBase
ADW, ////Lates calcarifer////: Information of L. calcarifer on Animal Diversity
Wild Shores of Singapore: News and reviews about Singapore marine issues
Important Notes
The page is written on 13 Nov 2014 based on as much latest published paper and reviews as possible. The taxonomy and systematic is still pretty vague currently and as more data comes in, information on this page might not be relevant anymore. It is likely that there will be changes in the phylogenetic tree and L. calcarifer splitting into more species very soon.
Reference
[1] Grey, D.L. 1987. An overview of Lates calcarifer in Australia and Asia. J.W. Copland and D.L.Grey eds. Management of wild and cultured sea bass/barramundi (Lates calcarifer).
[2] Dunstan, D.J. 1959. The barramundi Lates calcarifer (Bloch) in Queensland waters. CSIRO Technical Paper No. 5. Commonwealth Scientific and Industrial Research Organisation.Melbourne, Australia 22 pp.
[3] Davis, T.L.O. 1986. Migration patterns in barramundi, Lates calcarifer (Bloch), in Van Diemen Gulf, Australia, with estimates of fishing mortality in specific areas. Fish. Res. 4: 243–258.
[4] Milton, D.A. & S.R. Chenery. 2005. Movement patterns of barramundi Lates calcarifer, inferred from Sr-87/Sr-86 and Sr/Ca ratios in otoliths, indicate non-participation in spawning. Mar. Ecol.: Prog. Ser. 301: 279–291.
[5] Moore, R. 1982. Spawning and early life history of barramundi, Lates calcarifer (Bloch), in Papua New Guinea. Aust. J. Marine Freshwater Res. 33: 647–661.
[6] Staunton-Smith, J. et al. 2004. Does the quantity and timing of fresh water fl owing into a dry tropical estuary affect year-class strength of barramundi (Lates calcarifer)? Mar. Freshwater Res. 55: 787–797.
[7] Ruangpanit, N. 1987. Biological characteristics of wild seabass (Lates calcarifer) in Thailand. J.W. Copland and D.L. Grey eds. Management of Wild and Cultured Seabass/Barramundi (Lates calcarifer). ACIAR, Darwin, Australia, pp. 55–56.
[8] Kungvankij, P. et al. 1986. Biology and Culture of Seabass (Lates calcarifer). Training Manual Series No. 3. Network of Aquaculture Centres in Asia, Bangkok, Thailand.
[9] Davis, T.L.O. 1985. Seasonal changes in gonad maturity, and abundance of larvae and early juveniles of barramundi, Lates calcarifer (Bloch), in Van Diemen Gulf and the Gulf of Carpentaria. Aust. J. Mar. Freshwater Res. 36: 177–190.
[10] Russell, D.J. & R.N. Garrett. 1985. Early life history of barramundi, Lates calcarifer (Bloch), in north-eastern Queensland. Aust. J. Mar. Freshwater Res. 36: 191–201.
[11] Ng, P. X., & Tan, H. H. 2013. FISH DIVERSITY BEFORE AND AFTER CONSTRUCTION OF THE PUNGGOL AND SERANGOON RESERVOIRS, SINGAPORE. NATURE IN SINGAPORE 2013. 6: 19–24.
[12] Robins, J. et al. 2006. Variable growth rates of the tropical estuarine fish barramundi Lates calcarifer (Bloch) under different freshwater flow conditions. Journal of Fish Biology. 69(2): 379-391.
[13] Kwik, J. T. 2012. CONTROLLED CULLING OF VENOMOUS MARINE FISHES ALONG SENTOSA ISLAND BEACHES: A CASE STUDY OF PUBLIC SAFETY MANAGEMENT IN THE MARINE ENVIRONMENT OF SINGAPORE.THE RAFFLES BULLETIN OF ZOOLOGY. 25: 93-99.
[14] Hui, T. H., Low, M. E., & Peng, K. L. K. 2010. Fishes of the Marina Basin, Singapore, before the erection of the Marina Barrage. The Raffles bulletin of zoology, 58(1), 137-144.
[15] Cheong, L. and L. Yeng. 1987. Status of seabass (Lates calcarifer) culture in Singapore. J.W. Copland and D.L. Grey eds. Management of wild and cultured seabass/barramundi (Lates calcarifer). Australian Centre for International Agricultural Research, Canberra, pp. 65–68
[16] Barske, L. A., & Capel, B. (2008). Blurring the edges in vertebrate sex determination. Current opinion in genetics & development, 18(6), 499-505.
[17] Meyer, A. & Schartl, M. (1999). Gene and genome duplications in vertebrates: the one-to-four (-to-eight in fish) rule and the evolution of novel gene functions.Current opinion in cell biology, 11(6), 699-704.
[18] Taylor, J. S. et al. (2003). Genome duplication, a trait shared by 22,000 species of ray-finned fish.Genome research, 13(3), 382-390.
[19] Jaillon, O. et al. (2004). Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate proto-karyotype. Nature, 431(7011), 946-957.
[20] Moore, R. 1979. Natural sex inversion in the giant perch (Lates calcarifer). Aust. J. Mar. Freshwater Res. 30: 803–813.
[21] Davis, T.L.O. 1984. A population of sexually precocious barramundi Lates calcarifer in the Gulf of Carpentaria, Australia. Copeia 1984: 144–149.
[22] Toledo, J.D. et al. 1991. Spontaneous maturation and spawning of seabass Lates calcarifer in fl oating net cages. J. Appl. Ichthyol. 7: 217–222.
[23] Leong, S. 2010. Meet the super sea bass, farmed-in-Singapore, Wild Singapore. Accessed on 29/10/2014. http://wildsingaporenews.blogspot.sg/2010/02/meet-super-sea-bass-farmed-in-singapore.html#.VFMf2vmUckB
[24] Patnaik, S. & S. Jena. 1976. Some aspects of biology of Lates calcarifer (Bloch) from Chilka Lake. Indian J. Fish. 23: 65–71.
[25] Davis, T.L.O. 1985. The food of barramundi, Lates calcarifer, in coastal and inland waters of van Diemen Gulf and the Gulf of Carpentaria. J. Fish Biol. 26: 669–682.
[26] Rimmer, M.A. & A. Reed. 1989. Effects of nutritional enhancement of live food organisms on growth and survival of barramundi/seabass Lates calcarifer (Bloch) larvae. Adv. Trop. Aquacult. AQUACOP IFREMER Actes de Colloque 9: 611–623.
[27] Dhert, P., P. Lavens, M. Duray and P. Sorgeloos. 1990. Improved larval survival at metamorphosis of Asian seabass (Lates calcarifer) using ω3-HUFA-enriched live food. Aquaculture 90: 63–74.
[28] Fermin, A.C. & M.E.C. Bolivar. 1994. Feeding live or frozen Moina macropora (Strauss) to Asian seabass Lates calcarifer (Bloch) larvae. Isr. J. Aquacult.—Bamidgeh 46: 132–139.
[29] de la Pena, M.R. 2006. Use of juvenile instar (Stingelin) in hatchery rearing of Asian seabass Lates calcarifer (Bloch). Isr. J. Aquacult.—Bamidgeh 53: 128– 138.
[30] Parazo, M., E. Avila and D. Reyes. 1991. Size and weight dependent cannibalism in hatcherybred seabass (Lates calcarifer Bloch). J. Appl. Ichthyol. 7: 1–7.
[31]under reviewed and to be published in later date
[32] Barnabe, G. 1995. The Sea Bass. In: C.E. Nash and A.J. Novotny (eds.). Production of aquatic animals: Fishes. World Animal Science C8, Elsevier Science B.V., Amsterdam, pp. 269–287.
[33] Boonyaratpalin, M. (1997). Nutrient requirements of marine food fish cultured in Southeast Asia. Aquaculture, 151(1), 283-313.
[34] Nacario, J.F. 1987. Releasing hormones as an effective agent in the induction of spawning in captivity of seabass (Lates calcarifer). J.W. and D.L. Grey eds. Management of wild and cultured seabass/barramundi (Lates calcarifer). Australian Centre for International Agricultural Research, Canberra, pp. 126–128.
[35] Pierce, C. (2006). The next big fish. The Boston Globe Magazine 26 Nov 2006.
[36] Wang, C., et al. 2007. A microsatellite linkage map of Barra-mundi, Lates calcarifer. Genetics 175.2: 907-915.
[37] Orban, L. n.d. Profile. Temasek Life Science Laboratory. Accessed on 14/11/2014. http://www.tll.org.sg/group-leaders/laszlo-orban/
[38] Martin F. Gomon & Dianne J. Bray, 2011, Barramundi, Lates calcarifer, in Fishes of Australia, accessed 15 Nov 2014, http://www.fishesofaustralia.net.au/home/species/4643
[39] Lim, A. 2013. Barramundi or Kim Bak Lor 金目鲈Caught At Woodland Jetty 2013. Woodland Jetty Fishing Spot. Accessed on 14/11/2014. http://woodland-jetty.blogspot.sg/2013/03/barramundi-or-kim-bak-lor-caught-at.html
[40] Pethiyagoda, R., & Gill, A. C. (2013). Taxonomy and Distribution of Indo-Pacific Lates. Biology and Culture of Asian Seabass Lates Calcarifer, Chapter 1. Jerry D. eds. Taylor and Francis Group. USA. 16pp.
[41] Allen, G.R., C.F. Allen and D.F. Hoese. 2006. Latidae. P.L. Beesley and A. Wells eds. Zoological Catalogue of Australia (vol. 35). ABRS and CSIRO Publishing, Canberra, pp. 966–968.
[42] Pethiyagoda, R. & A.C. Gill. 2012. Description of two new species of sea bass (Teleostei: Latidae: Lates) from Myanmar and Sri Lanka. Zootaxa 3314: 1–16.
[43] Sudhesh, P.S. et al. Chromosomes of Lates calcarifer. J. Inland Fish. Soc. India 24: 26–29.
[44] Carey, G. and P. Mather. 1999. Karyotypes of four Australian fish species Melanotaenia duboulayi, Bidyanus bidyanus, Macquaria novemaculeata and Lates calcarifer. Cytobios 100: 137–146.
[45] Vij, S. et al. 2014. Barcoding of Asian seabass across its geographic range provides evidence for its bifurcation into two distinct species. Marine Systematics and Taxonomy, 1, 30.
[46] Yue, G.H. et al. 2009. Genetic variation and population structure of Asian seabass (Lates calcarifer) in the Asia-Pacifi c region. Aquaculture 293: 22–28.
[47] Lin, G. et al. 2006. The complete mitochondrial genome sequence and characterization of single-nucleotide polymorphisms in the control region of the Asian seabass (Lates calcarifer). Mar. Biotechnol. 8: 71–79.
[48] Jerry, D. R., & Smith-Keune, C. (2013). The Genetics of Asian Seabass.Biology and Culture of Asian Seabass Lates Calcarifer, Chapter 7. Jerry D eds. Taylor and Francis Group. USA. 41pp.
[49] Greenwood, P.H. 1976. A review of the family Centropomidae (Pisces, Perciformes). Bull. Br. Mus. Nat. Hist. (Zool.) 29: 1–81.
[50] Otero, O. 2004. Anatomy, systematics and phylogeny of both recent and fossil latid fishes (Teleostei, Perciformes, Latidae). Zool. J. Linn. Soc. 141: 81–133.
[51] Li, C., B.-R. Ricardo, W.L. Smith and G. Orti. 2011. Monophyly and interrelationships of snook and barramundi (Centropomidae sensu Greenwood) and five new markers for fish phylogenetics. Mol. Phylogenet. Evol. 60: 463–471.
[52] Chen, W. J. et al. 2007. Relationships among four genera of mojarras (Teleostei: Perciformes: Gerreidae) from the western Atlantic and their tentative placement among percomorph fishes. Journal of Fish Biology, 70: 202-218.
[53] Near, T. et al. 2012. Nuclear gene-inferred phylogenies resolve the relationships of the enigmatic pygmy sunfi shes, Elassoma (Teleostei: Percomorpha). Mol. Phylogenet. Evol. 63: 388–395.
[54] Smith, W.L. and M.T. Craig. 2007. Casting the percomorph net widely: The importance of broad taxonomic sampling in the search for the placement of serranid and percidfishes. Copeia 2007: 35–55.
[55] Near, T.J. et al. 2013. Phylogeny and tempo of diversifi cation in the superradiation of spiny-rayed fi shes. Proc. Natl. Acad. Sci. USA.
www.pnas.org/cgi/doi/10.1073/pnas.1304661110.
[56] Wildshores. 2009. What goes on at Singapore's largest commercial fish farm?. Wildshores of Singapore. Accessed on 14/11/2014. http://wildshores.blogspot.sg/2009/12/what-goes-on-at-singapores-largest.html#.VHIKcouUckB
[57] Chen, K. 2010. Some thoughts of the fish kill in Pasir RIs. Water quality in Singapore. Accessed on 14/11/2014. http://waterqualityinsingapore.blogspot.sg/2010/01/some-thoughts-on-fish-kill-at-pasir-ris.html
[58] WIldshores. 2009. Why are there so many dead fish on Pasir RIs?. Wildshores of Singapore. Accessed on 14/11/2014. http://wildshores.blogspot.sg/2009/12/why-are-there-so-many-dead-fish-on.html#.VHILSYuUckA
Acknowledgement
I would like to express my gratitude to Prof. Rudolf Meier from National University of Singapore for his guidance and opportunity to complete this species page. I would also like to thank Prof. Laszlo Orban and Dr Liew Woei Chang from Temasek Life Science Laboratory for providing me with unpublished data regarding the status of this species in Singapore. Finally, I would like to thank people who have placed free-access photos for the public to use.