Laticauda colubrina - Yellow-lipped sea krait
| Contents Identification Habitat Distribution Predator & Prey Reproduction Locomotion Venom Taxonomic Status Phylogeny Scientific Description Scientific Literature |
IDENTIFICATION
| Wu Weiyan |
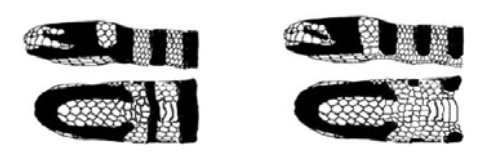
Laticauda colubrina is a species of marine snakes with distinctive morphological (physical) characters that allow for easy identification. They are one of few marine snake species with a flattened paddle-like tail that resembles the head. They have a distinctive band pattern of blue and black, yellow and black, or yellowish-blue and black depending on the location. They have a yellow upper-lip coloration, which can be used for identification from L. laticaudata (blue-lipped sea krait) with a blue or black upper-lip coloration. The lateral (horizontal) black head band across its eye is continuous with the first body band (the first ring of black around its neck). This is distinct from L. frontalis whose lateral head band is separated from the first body band. L. colubrina exhibits sexual dimorphism (the males and females look different), females being on average 40% larger than males. Males reach maturity at 70cm and attain an adult size of 90cm, whereas females reach maturity at 100cm and attain an adult size of 130cm. [1][2]
Lane & Shine 2011 [10]
HABITAT
L. colubrina are most commonly found in the coastal seas near islands, as well as on the islands. They spend about half their time in the marine environment foraging for food. The other half of their time they spend on land digesting their prey, sloughing (shedding) their skins, courting, mating and ovipositing (laying) eggs. [1][3]They spend on average about 10 days on either land or sea before moving to the other environment. This is related to the average digestion time of 8 days, and the average skin sloughing (shedding) time of 12 days. [3]
DISTRIBUTION
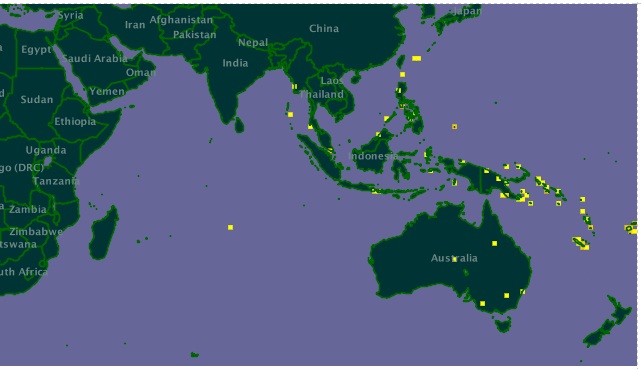
Found in tropical seas, mainly around the many islands in the South Pacific, the coral seas of South East Asia, and the Bay of Bengal.
Biodiversity occurrence data published by: Global Biodiversity Information Facility Open Geospatial Consortium services
(Accessed through GBIF Data Portal, data.gbif.org, 2011-10-31)
PREDATOR
| Wu Weiyan |
The natural predators of L. colubrina include sea eagles and sharks. In addition, some crabs are known to attack juveniles. [1]
FEEDING
Their preferred preys are moray eels (Family Muraenidae) and conger eels (Family Congridae). They are gape-limited predators that feed by ingesting (swallowing) the prey whole, hence the prey types are selected based on size. They exhibit intraspecific niche partitioning, hence, males, females and juveniles target different prey types. [2]
Juveniles of both sex feed almost exclusively on Moray eels which are small. Mature males continue feeding on moray eels, but target larger prey items, whereas mature females switch to feeding on the larger conger eels. Whereas juveniles and mature males tend to forage in the coastal seas nearer to land, mature females are known to travel further away from land, and dive deeper to seek out the larger prey. [2]
After feeding, all L. colubrina would return to land while digesting their prey. It has been suggested that having a prey item in their gut may reduce the capacity of their lungs, hence impairing respiration in an aquatic environment. Females most often return to land after obtaining a single large prey item, whereas males are able to return after obtaining multiple prey items since they target the smaller morays. [2]
REPRODUCTION
L. colubrina court, mate and lay their eggs on land. Females produce distinct chemical pheremone signals that control male courtship behavior. Males confirm the pheremone signal by tongue flicking upon contact with the female. Subsequently, male courtship behaviors include pressing of the chin against the females' dorsal surface, aligning of the body with the female, and body jerking. [4]L. colubrina form mating congregations like other terrestrial snakes. Males of this species do not exhibit aggression against other males even during mating. Therefore, no size benefit is observed for mating. In fact, smaller males may benefit from being more able to enter narrow crevices to find mates, being more mobile on land, and being able to stay on land without feeding for longer periods of time as compared to larger males. [4]
LOCOMOTION
L. colubrina possess several adaptations that allow for locomotion in the aquatic environment. They have flattened, paddle-like tails that aid in aquatic locomotion. They have valves on their nostrils that prevent water from entering the lungs when submerged. They also have salt glands that actively excrete excess salt. [1]
Sea kraits are a unique group of snakes that have invaded the aquatic environment, and yet retained a decent degree of terrestrial locomotion. Amongst the Laticaudidae, L. colubrina retains the best terrestrial locomotion. This may also reflect the fact that L. colubrina is the species that uses the terrestrial environment most intensively and frequently. They are able to climb steep rock cliffs, including overhangs, to get onto land. However, they tend to time their arrival on land to coincide with high tides, as well as actively choose sandy and gentle beaches. Mode of locomotion on land is similar to other land snakes, utilising the lateral undulating locomotion.[1][5]
L. colubrina also exhibit sexual dimorphism in terms of terrestrial locomotion. Males tend to spend longer periods of time and travel longer distances while on land to seek out mates, whereas females tend to be sedentary once on land while waiting for digestion or for mates. As a result, for individuals of the same length, males tended to be heavier, more muscular, and capable of stronger contractions. [1][5]
VENOM
L. colubrina are highly venomous. Their venom has a LD50of 45 μg/kg tested in mice. The venom is a neurotoxin that acts by attacking post-synaptic membranes of muscle tissues, and by inhibiting acetylcholine and carbochol. Victims of the venom die rapidly due to respiratory arrest and subsequent cardiovascular collapse due to diaphragm and heart muscle failures. Sublethal doses cause paralysis, and may still lead to death over a longer period of time.[6][7][8][9] Fortunately, L. colubrina are known to be docile, and are even tolerant to some degree of human handling. [3] Not all bites result in venom injection. [8]TAXONOMIC STATUS
| Wu Weiyan |
Laticauda colubrina was first described by Schneider in 1799 under the name of Hydrus colubrinus. Subsequently, it was placed under a different Genus by Boulgr in 1890, hence becoming Platurus colubrinus. Thereafter, the Genus Platurus was debated, reassessed, and the current Genus name of Laticauda was decided.
Currently, the name Laticauda colubrina is accepted by major online taxonomic and biodiversity databases. This binomial name is also the most widely used in the recent scientific literature. This species should be referred to as Laticauda colubrina (Schneider 1799). Several type specimens from Schneider are currently held by the Museum für Naturkunde, Berlin, Germany, although the existence of the holotype remains unclear.
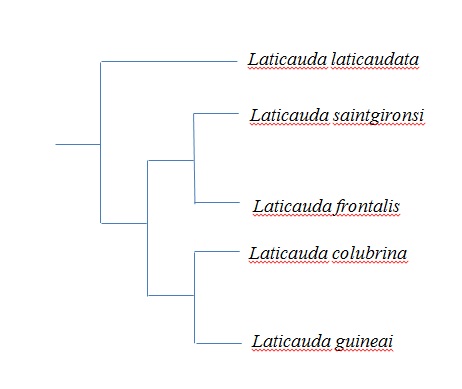
Nonetheless, recent studies have shown that what was once thought of as one species are in fact two reproductively isolated species. L. frontalis is morphologically similar to L. colubrina, with the only observable difference being a small gap between the lateral head band and the first body band. Another hint is a smaller adult size in L. frontalis. Reproductive isolation experiments demonstrate that L. colubrina and L. frontalis females produce different lipids on their scale surfaces, contact pheremones, which the males are able to distinguish and actively court only conspecifics (i.e. males only court females of the same species).
The species L. frontalis was proposed as early as 1905 by De Vis. However, between 1905 and 1987, many specimens were placed under L. colubrina which was better known due to their larger range. In 1987, L. frontalis as a distinct species was proposed again. As a result, many of the type specimens collected before 1987 may contain a mixture of both L. colubrina and L. frontalis.
PHYLOGENY
Apart from the reproductive isolation experiment, mitochondrial DNA analyses also provided evidence for the division of L. colubrina and L. frontalis.The most recent study of Genus Laticauda yielded the following outgroup rooted tree. However, the issue is unresolved as yet, since different authors have proposed the inclusion or exclusion of various species from other lineages. [10]
SCIENTIFIC DESCRIPTION
| Wu Weiyan |
Rostral - touches six shields; the rostro-labial sutures much the largest. Internasals - a pair. Praefrontals - three subsequent shields
in one transverse row; the outer touching no supralabial. Frontal - entire; touches seven shields; the fronto-parietal sutures largest. Supraoculars - half to about two-thirds as broad, and as long as the frontal. Parietals - entire. Nasals - lateral; in contact with the first three supralabials usually (sometimes only the first two). Praeoculars - one. Postoculars - two. Temporals, one (sometimes two). Supralabials - seven; the third and fourth touching the eye. Infralabials - the fourth is the largest of the series, and in contact with three of four scales behind. Marginals - a complete row after the second infralabials. Sublinguals - two well developed pairs, the fellows of each in contact. Costals, anteriorly 21 to 25, midbody 21 to 25, posteriorly usually 21 (rarely 23); imbricate; smooth. Ventrals - 195 to 240; three or more times as broad as the last costal row; the last one or two very frequently divided. Anal - divided. Colour - alternately banded with dark brown, and yellowish, or greyish. The bands well defined, and the dark rather broader; in old specimens the bands are often effaced ventrally, and converted into dorsal bars. Habitat - From the Bay of Bengal through the Malayan Region, China, Philipines to Australia and New Zealand. [11]
LITERATURE CITED
[1] Shine, R. and S. Shetty. 2001. Moving in two worlds: aquatic and terrestrial locomotion in sea snakes (Laticauda colubrina, Laticaudidae). J. Evol. Biol. 14:338-346[2] Shetty, S. and R. Shine. 2002. Sexual divergence in diets and morphology in Fijian sea snakes Laticauda colubrina (Laticaudidae). Austral. Ecol. 27: 77-84
[3] Shetty, S. and R. Shine. 2002. Activity patterns of yellow-lipped sea kraits (Laticauda colubrina) on a Fijian island. Copeia 2002: 77-85
[4] Shine, R., R.N. Reed, S. Shetty, M. Lemaster, and R.T. Mason. 2002. Reproductive isolating mechanisms between two sympatric sibling species of sea snakes. Evol. 56: 1655-1662
[5] Bonnett, X., I. Ineich, and R. Shine. 2005. Terrestrial locomotion in sea snakes: the effects of sex and species on cliff-climbing ability in sea kraits (Serpentes, Elapidae, Laticauda). Biol. J. Linnean Society 85:433-441
[6] Levey, H.A. 1969. Toxicity of the venom of the sea-snake, Laticauda colubrina, with observations on a Malay 'folk cure'. Toxicon 6: 269-276
[7] Sato, S., H. Yoshida, H. Abe and N. Tamiya. 1969. Properties and biosynthesis of a neurotoxic protein of the venoms of sea snakes Laticauda laticaudata and Laticauda colubrina. Biochem. J. 115: 85-90
[8] Takasaki, C., S. Kimura, Y. Kokubun and N. Tamiya. 1988. Isolation, properties and amino acid sequences of a phospholipase A2 and its homologue without activity from the venom of a sea snake, Laticauda colubrina, from the Solomon Islands. Biochem. J. 253:869-875
[9] Rowan, E.G., A.L. Harvey, C. Takasaki and N. Tamiya. 1989. Neuromuscular effects of a toxic phospholipase A2 and its nontoxic homologue from the venom of the sea snake, Laticauda colubrina. Toxicon 27(5):587-591
[10] Lane, A. and R. Shine. 2011. Phylogenetic relationships within laticaudine sea snakes (Elapidae). Molecular Phylogenetics and Evolution 59: 567-577
[11] Wall, M.F. 1909. Platurus colubrinus. In M.F. Wall (1909) A monograph of the sea snakes. Memoirs of the Asiatic Society of Bengal vol. 2(8).
OTHER REFERENCES
Schmidt, G.D. and R.E. Kuntz. 1973. Nematode parasites of Oceania. XX. Paraheterotyphlum ophiophagos (Heterocheilidae) from the Banded Yellow-lip sea snake, Laticauda colubrina. Am. Midland Naturalist 89: 481-484Heatwole, H., and N.S. Poran. 1995. Resistances of sympatric and allopatric eels to sea snake venoms. Copeia 1995: 136-147
Heatwole, H. and J. Powell. 1998. Resistance of eels (Gymnothorax) to the venom of sea kraits (Laticauda colubrina): a test of coevolution. Toxicon 36: 619-625
Heatwole, H., S. Busack, and H. Cogger. 2005. Geographic variation in sea kraits of the Laticauda colubrina complex (Serpentes: Elapidae: Hydrophiinae: Laticaudini). Herpetological Monographs 19: 1-136
Kharin, V.E., M. Rodel, and J. Hallermann. 2010. New records and distribution of a little-known sea krait Laticauda frontalis (de Vis, 1905) (Serpentes, Laticaudidae). Russian Journal of Herpetology 17: 285-289
Kim, H.S. and N. Tamiya. 1982. Amino acid sequences of two novel long-chain neurotoxins from the venom of the sea snake Laticauda colubrina. Biochem. J. 207: 215-223
Lading, E.A., R.B. Stuebing, and H.K. Voris. 1991. A population size estimate of the yellow-lipped sea krait, Laticauda colubrina, on Kalampunian Damit Island, Sabah, Malaysia. Copeia 1991: 1139-1142
Radcliffe, C.W., and D.A. Chiszar. 1980. A descriptive analysis of predatory behavior in the yellow lipped sea krait (Laticauda colubrina). J. Herpetology 14: 422-424
Rasmussen, A.R., and J. Elmberg. 2009. ‘Head for my tail’: a new hypothesis to explain how venomous sea snakes avoid becoming prey. Marine Ecol. 30: 385-390
Rioux, V., M. Gerbod, F. Bouet, A. Menez, and A. Galat. 1998. Divergent and common groups of proteins in glands of venomous snakes. Electrophoresis 19: 788-796
Schmidt, G.D. and R.E. Kuntz. 1973. Nematode parasites of Oceania. XX. Paraheterotyphlum ophiophagos (Heterocheilidae) from the Banded Yellow-lip sea snake, Laticauda colubrina. Am. Midland Naturalist 89: 481-484
Shetty, S. and R. Shine. 2002. Philopatry and homing behaviour of sea snakes (Laticauda colubrina) from two adjacent islands in Fiji. Conservation Biol. 16: 1422-1426
Shetty, S. and R. Shine. 2002. The mating system of yellow-lipped sea kraits (Laticauda colubrina: Laticaudidae). Herpetologica 58: 170-180
Shine, R., R.N. Reed, S. Shetty, and H.G. Cogger. 2002. Relationships between sexual dimorphism and niche partitioning within a clade of sea-snakes (Laticaudinae). Oecologia 133: 45-53
Shine, R. and S. Shetty. 2002. The influence of natural selection and sexual selection on the tails of sea-snakes (Laticauda colubrina). Biol. J. Linnean Soc. 74: 121-129
Shine, R., X. Bonnet, and H.G. Cogger. 2003. Antipredator tactics of amphibious sea-snakes (Serpentes, Laticaudidae). Ethology 109: 533-542
Shine, R., H.G. Cogger, R.R. Reed, S. Shetty, and X. Bonnet. 2003. Aquatic and terrestrial locomotor speeds of amphibious sea-snakes (Serpentes, Laticaudidae). J. Zool. Lond. 259: 261-268
MORE INFORMATION
Encyclopedia or Life (eol.org)
Global Biodiversity Information Facility (data.gbif.org)
Biodiversity Heritage Library (biodiversitylibrary.org)
World Register of Marine Species (marinespecies.org)
Museum für Naturkunde, Berlin (naturkundemuseum-berlin.de)