Macrobrachium malayanum (Roux, 1934)A common freshwater shrimp in Singapore's natural freshwater habitats

 Figures 1 & 2: Adult male M. malayanum (front and side view)
Figures 1 & 2: Adult male M. malayanum (front and side view)Photos by: Claudia Tan
Table of Contents
Freshwater shrimps of the Macrobrachium genus are probably more renowned for their value as food (the larger species such as M. rosernbergii), or less commonly, as pets. Species within this genus can be recognized by their long, robust second chelipeds (pincers), which its name suggests (Greek; Macro = Big, Brachium = Arm). They are especially important in tropical freshwater habitats due to their sheer abundance and macroconsumer diet. Their distinct fighting behaviour and success in colonizing freshwater environments also have implications to their populations and diversification respectively. However, like any other Southeast Asian non-aquaculture shrimps, little investment has been made to understand its ecology.
In Singapore, there are a few Macrobrachium species in our fresh waters. The most abundant freshwater shrimp in the remnant forest streams is M. malayanum (Roux,1934) [1], which is also common in acidic forest streams in tropical Southeast Asia [2-5]. This freshwater shrimp can be identified by its having one pincer larger than the other (Figures 1-2), especially in mature males. Individuals can grow over 60 mm [6], or 20 mm in carapace length (CL) [7]. Similar to some species of the genus, their abundance in many tropical streams and their shredder-predator habits allows them to play a prominent role in ecosystem functioning [5, 8, 9].
Hence, this webpage is designed to:
- Touch upon the geographical distribution, habitats and distinguishing features of the species
- Introduce the ecological importance of freshwater shrimps like M. malayanum in tropical freshwater streams via its diet
- Increase understanding of interesting agonistic behaviour and how it affects its population
- Describe its adaptation in freshwater habitats from its life history strategies
- Provide an overview of recent phylogenetic research of genus’ colonization into fresh waters
Next Section: Feeding and Ecological Role, Agonistic Behaviour, Life History Strategies, Shrimp Identification, Taxomony, Phylogeny
1) Geographic Distribution and Habitat
Habitat: Acidic fresh waters with natural bottom sediment; Often between leaf litter/ woody debris/root mats
Globally, M. malayanum is restricted to freshwater streams of tropical Southeast Asia, where it ranges as far up as Southern Thailand, to Malaysia, Sarawak and West Sumatra [2-4, 10, 11] (Figure 3). It is widespread in Singapore, common in the periphery and within forest streams of nature reserves and catchment areas [1, 4, 6, 7]. They are not known to occupy highly-artificial habitats like reservoirs, although they can be present in open rural-country streams [1].
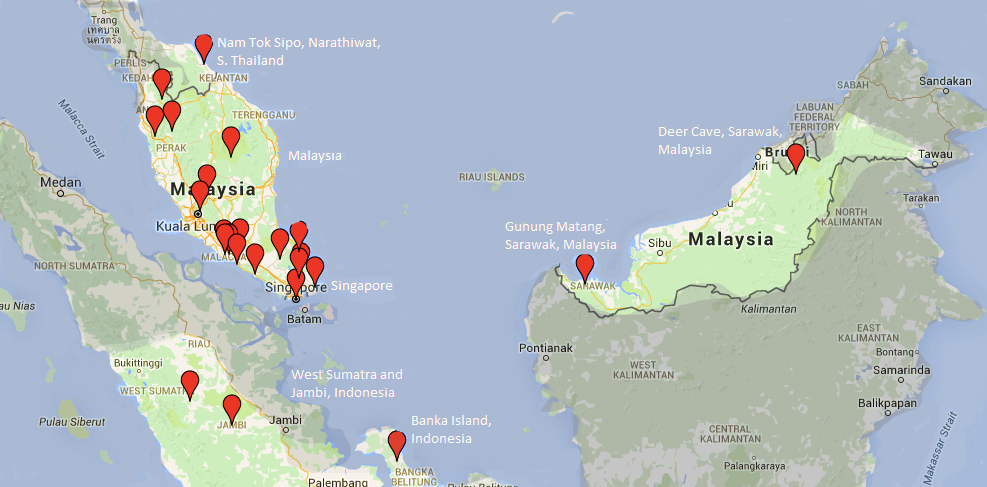
Figure 3: Global distribution of M. malayanum (Edited from Google Maps, 2015)
Macrobrachium malayanum is a benthic (bottom-living) shrimp, and is thus often found amongst leaf litter, between tree debris or roots/root mats [4, 12]. It prefers clear, unpolluted streams [3, 4]. All habitats (Figure 4) are characterized by natural bottom sediment, including sand, rocks, and leaf beds [4].This species is restricted to acidic forested streams, where pH can range from 4.90 to below 7.00 [9, 13, 14]. The shrimp does not appear to be affected by canopy cover [7] or flow rate [4, 15], although its morphology could be influenced by flow velocity [4]. Furthermore, M. malayanum is tolerant to low oxygen and calcium concentration levels [16]. Such wide tolerance to different environmental parameters probably contributed to its wide distribution.

Figure 4: One of forest stream habitats M. malayanum inhabits in Singapore
Photo by: Claudia Tan
Previous Section: Distribution and Habitat
Next Section: Agonistic Behaviour, Life History Strategies, Shrimp Identification, Taxomony, Phylogeny
2) Feeding and Ecological Role
Eats: Omnivorous - Leaf litter, algae, macroinvetebrates, dead and weakened animals
Eaten by: Fish and Birds
Ecological Role: Nutrient recycling and Benthic macroinvertebrate assemblage
2.1 What does it eat?
Videos 1-4 (Left to right, Top to bottom): Macrobrachium spp. (sold as Rusty macro) feeding on blood worms (Video Credit: YouTube user Dmytro Ko); M. meridionalis feeding on detritus (Video Credit: YouTube user 香港水生物愛護會/ 香港魚類愛護會); Macrobrachium spp. (sold as Indian whisker shrimp) feeding on smaller glass/ghost shrimp (Video Credit: YouTube user Zach Dewey); Macrobrachium spp. attacking conspecific that just molted (Video Credit: YouTube user Callatya)
Macrobrachium malayanum is known to be omnivorous [9], feeding on algae, detritus, leaf litter, and also on benthic macroinvertebrates such as insect larvae/ nymphs, worms, snails and smaller shrimp [7] (See Videos 1-4 for an idea of the different food items Macrobrachium spp. can eat). It is, however, not known if this species is more carnivorous [17], or is it more omnivorous-detritivorous [18, 19].

Figure 5: M. malayanum feeding on a forest halfbeak; Photo credit:Tan Heok Hui (permission obtained)
The shrimp is also a scavenger, and would attack and feed on weakened or dead animals like small fishes and other shrimps (e.g., in Video 3). A recent article has reported two M. malayanum individuals feeding on a forest halfbeak (Hemirhamphodon pogonognathus) approximately twice in size, probably when it was either weakened/ injured or cornered by the shrimps [20] (Figure 5). There have been shrimp hobbyists that have reported Macrobrachium species that attack and eat smaller fish as they grow bigger. They can also cannibalize on each other, especially when one has recently molted (pers. observ., Video 4).
2.2 What eats it?

Figure 6: M. malayanum being eaten by an Oriental dwarf kingfisher.
Photo credit: Johnny Wee (permission obtained)
There is no publication that has recorded the predators of M. malayanum. However, given that shrimps have been observed in the guts of fish, it can be assumed that some fish do prey on M. malayanum. Furthermore, birds are diurnal predators of the shrimp, as shown by a sighting of M. malayanum been fed on by an Oriental dwarf kingfisher (Ceyx erithaca)(Figure 5).
2.3 Key ecological role in the tropics
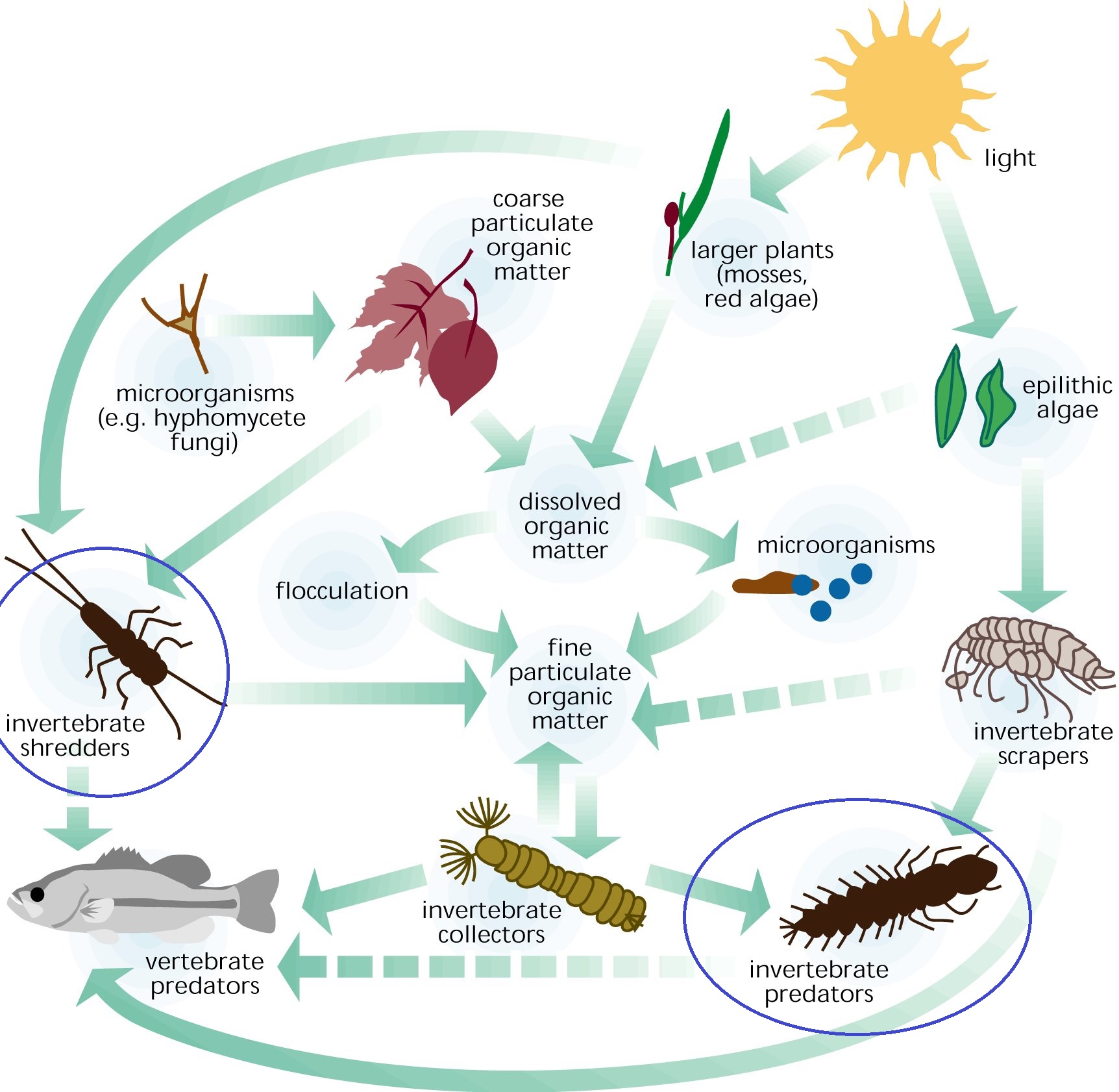
Figure 7: Illustration of temperate freshwater food web, with ecological roles served by M. malayanum circled in blue. Credit: Federal Interagency Stream Restoration Working Group (1998), In: Stream Corridor Restoration: Principles, Processes, and Practices
Tropical Macrobrachium freshwater shrimps are generalist ‘macroconsumers’— they facilitate macro-decomposition by scavenging and shredding debris and leaf litter [21, 22]. They may also regulate benthic macroinvertebrate populations [23, 24]. In addition, they provide plentiful food supply for larger invertebrate and vertebrate predators. They play multiple ecological roles served by various ‘specialist’ taxa in temperate inland waters [24, 25] (Figure 7). Combined with their high abundance [5], such roles can have significant impacts on ecosystem functioning, making them an important component in tropical stream ecosystems.
Previous Section: Distribution and Habitat, Feeding and Ecological Role
Next Section: Life History Strategies, Shrimp Identification, Taxomony, Phylogeny
3) Agonistic Behaviour
Characterization: 3 main stages - Investigation, Threat Display, and Physical Contact. Fights decrease in intensity and duration with more interactions
Dominance Hierarchies: Cost-minimization strategy to attain resources by reducing subsequent fight intensity and duration. Distinct behavioural differences between dominant and subordinate shrimp
Consequences: Spatial segregation between individual, survival, growth and reproductive difference, effects may be less drastic in the wild
An interesting feature of the Macrobrachium genus is their agonistic behaviour (e.g, Video 5 shows a different Macrobrachium species fighting, Video 6 onwards shows M. malayanum individuals fighting), which is defined as any actions or behaviour related to fighting, inclusive of non-contact threat displays, chases and retreats. Agonistic behaviour is very distinctive in the Macrobrachium genus, probably due to their enlarged chela that they use in bouts. This distinctive behaviour also makes some of the more attractive or larger species of this genus interesting pets. However, this agonistic behaviour also limits aquaria hobbyists’ choices in keeping smaller pets or more than a handful of them at one time, which may explain their rare appearances in aquarium shops. This section will showcase content based on unpublished observations of M. malayanum agonistic interactions (as part of an ongoing research project), although discussion material and terminology (e.g., behavioural categories) are adapted from published behavioural studies on other Macrobrachium spp.
Video 5: Macrobrachium formonense individuals fighting (Video Credit: YouTube user 香港水生物愛護會/ 香港魚類愛護會)
3.1 Characterization
Agonistic interactions usually start the moment both individuals detect/ investigate one another, characterized by individuals turning towards/ approaching one another slowly, and/or antennules pointing in the direction of / touching one another (see Video 6, till 0:20).Video 6: Two M. malayanum individuals' first interaction in an experimental setting. Interaction escalates from investigating (0:00-0:20), threat display (0:20-0:40) to physical contact and bouts between two (0:40-0:46), ending when one retreats (at 00:46).
Video credit: Claudia Tan
This is followed by threat display, such an antennule whipping back and forth, lifting their chelipeds off the substrate and/ or in front of their opponent, charging, amongst a few others (see Additional Info for details of their agonistic behaviour; see Video 6 - 00:20-00:40, or Video 7 - 0:05-0:30 for an idea of their threat displays).
Video 7: Another pair of M. malayanum individuals' first interaction in an experimental setting, with a shorter investigation period than that in Video 6. Interaction escalates to threat display (00:05-00:30) to physical contact and bouts between two, ending when one retreats (at 00:55). Video credit: Claudia Tan
The first interaction usually escalates to one involving physical contact (i.e. contact with chelae to determine the stronger individual. The duration and intensity of the interaction would vary according to the difference in dominance between two individuals (e.g., in terms of size difference; compare Video 6, where both shrimps are similar in size, and Video 8, which features one distinctly larger shrimp and thus, fight duration was shorter than among the two similarly-size individuals in Video 6). After the first few interactions, fight intensity and duration usually decreases, as a dominance subordinate hierarchy would have been established.
Video 8: Distinctly larger M. malayanum individual approaching and fighting with a smaller individual for shelter. Fight concluded within 22 seconds. Video credit: Claudia Tan
3.2 Winner gets priority access: Dominance Hierarchies
Despite their agonistic behaviour, Macrobrachium species probably do not fight intensely all the time. Preliminary studies between M. malayanum individuals show that fight intensity usually peaks in the first 10–20 minutes of contact, before a dominant-subordinate relationship is established.Video 9: Winner from video 8 defends its shelter effectively with just an attempted chela nip/ charge, ending the interaction quickly.
Once the hierarchy is established, the subordinate individual becomes submissive and acts distinctly differently from that of the dominant individual (Video 9; see Additional Info for more details of behaviour). Furthermore, subsequent interactions tend to involve little or low-level physical contact (more threat displays or chases; chela push). Some level of physical contact can occur even after the first few interactions, but these are few relative to non-physical interactions and lower in intensity (e.g., chela push by dominant shrimp, or attempted chela nip).
In general, fighting occurs for a longer time when the two shrimps are similarly-matched [26], be it in size, cheliped length/size/presence, molt stage, sex, or aggression levels. The more dominant the individual is, the less intense behaviour is required to elicit a submissive response by the subordinate [26, 27]. Such dominance hierarchies are also witnessed in other decapod crustaceans [28, 29].
3.3 Hierarchies minimize costs
Agonistic behaviour plays a determining factor in attaining scarce resources, including refuges, food, and mates [30]. The establishment of dominance hierarchies may be a strategy to minimize costs in either individual [31].Fighting often involves energy expenditure on both individuals, and may sometimes lead to exhaustion, injuries, and death. Should the value of the contested resource be lower than the perceived cost of bouts, most animals, tend not to choose to initiate prolonged fights, especially if one individual is much more dominant over the other [26-28, 31]. However, fights still ensue to decide who can access the usually limited resource. A dominance hierarchy establishment thus allows access of resources of the stronger, dominant individual within a few bouts, and also reduces the potential injuries inflicted on the subordinate individual, at the expense of less preferable resource accessibility [31].3.4 Ecological consequences
Agonistic interactions would often segregates dominant and subordinate individuals. Subordinate individuals tend to be forced into less preferable sites [32, 33], attain less optimal resources [30, 34], and thus may experience slower growth, reproductive maturity or lower survival as compared to dominant individuals [35, 36]. The extent varies from species to species, with some species being able to be kept at higher densities than others [e.g., 37, 38].One would thus expect that wild population densities would be kept low. This is not the case. Population studies reveal that many Macrobrachium spp., including M. malayanum, often reach high densities [14]. Behavioural observations in semi-natural conditions reveal that while aggressive interactions still take place over preferred resources and when a passing individual approaches within proximity of another, fight intensity and frequency is not as high as that observed in experimental and aquaculture conditions [32, 39]. This suggests a presence of a strong dominance hierarchy in the wild, where social status is a key regulator of resource distribution.
The cause for this stronger dominance hierarchy is not clear, although greater habitat/ shelter complexity in the wild could contribute to reduced resource competition with spatial segregation [e.g., 40]. This is seen in M. rosernbergii of varying molt stages, where individuals that are about to molt segregate themselves from the stronger inter-molt individuals, swimming towards the shallower, but more predator-susceptible waters [32]. Hence, while the effects of intraspecific agonism may not be as obvious in the wild, it probably still affects the spatial distribution, and hence the vital survival and growth of dominant and subordinate individuals.
Previous Section: Distribution and Habitat, Feeding and Ecological Role, Agonistic Behaviour
Next Section: Shrimp Identification, Taxomony, Phylogeny
4) Life History Strategies: Adaptations to freshwater life
Life History Strategies: Small but large eggs, abbreviated larval development, and parental care increases survival of larvae in harsh freshwater habitat
Implications in evolution: Adaptive convergence to freshwater habitat
4.1 Strategies
Many species within the Macrobrachium genus are estuarine or amphidromous (i.e. juveniles live in brackish or marine waters, before migrating to fresh waters to spawn), producing a large number of small eggs. The lower investment per egg results in less developed, free-swimming larvae, requiring 10–13 larval stages stages before they develop to juveniles [41, 42]. Such may be disadvantageous in freshwater environments, which are characterized by as hypertonic [43], food-scarce [44, 45], torrential (in some areas) and prone to drying in the shallower or ephemeral streams [45]. There is also less need for large-scale dispersal as observed in oceanic crustaceans given the isolation of most freshwater habitats [41]. Hence, freshwater decapods, including M. malayanum, have developed a few life history strategies to enable it to thrive in such ‘harsh’ habitats.Main Strategy 1: Smaller Number of Eggs, but Larger in Size

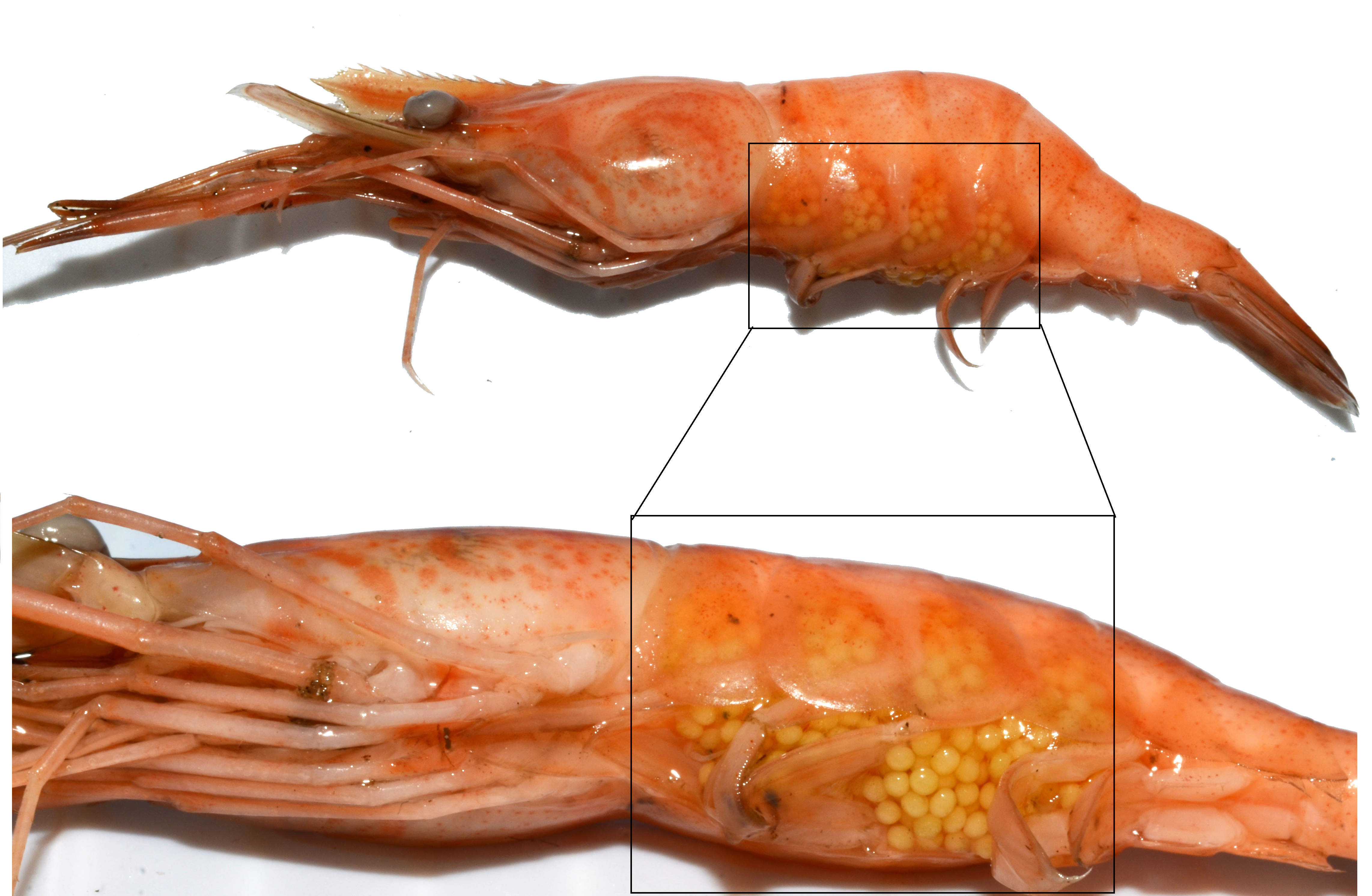
Figure 8: Gravid M. malayanum (left) compared to a larger gravid (but dead) M. nipponense (right). Notice the difference in size (relative to mother's body length) and number of the eggs between the two species, M. malayanum being a wholly freshwater shrimp while M. nipponense being a brackish-water and freshwater species.
Photos by: Claudia Tan
A general life history strategy adopted by freshwater animals is to produce less but larger eggs. Marine Macrobrachium spp. can lay hundreds of thousands of small eggs, while wholly freshwater shrimps like M. malayanum lay as little as 26 eggs (Table 1). While it is not known how many eggs M. malayanum produce, these eggs are considered large for the Macrobrachium genus, measuring 1.75–1.90 mm in length and 1.2–1.35 mm in diameter [42, 46], as compared to < 1 mm in length and diameter of brackish-water shrimps [42] (Figure 8).
Table 1: Comparison of life history strategies of selected Macrobrachium species. Macrobrachium lar and M. niphanae are not found in Singapore and are placed in the table due to the completeness of their data, allowing easier comparison. Citations beside species name refer to reference for life history data. Egg size refers to the volume of the eggs relative to the maximum carapace length of the ovigerous female.
| Species |
Lifestyle |
Clutch Size |
Egg Size |
Zoeal Stages |
| M. lar [47] |
Sea water |
up to 40 000 |
Small |
11+ |
| M. equidens [48] |
Brackish water |
1000–6000 |
Small |
10 |
| M. nipponense [49, 50] |
Brackish water |
4700–5600 |
Small |
9 |
| M. nipponense [50, 51] |
Freshwater |
1129–2600 |
Small (but about 2 times larger than brackish water population) |
? |
| M. lanchesteri [52] |
Freshwater |
59–393 |
Medium |
2+ |
| M. malayanum [46] |
Freshwater |
? |
Large |
2 |
| M. sundaicum [53] |
Freshwater |
? |
Large |
2 |
| M. niphanae [54] |
Freshwater |
32–81 |
Large |
2 |
Producing less but larger eggs increases energetic investment to each egg, allowing for more well-developed larvae. Marine crustacean larvae often lack functional organs/ appendages when just hatched, such as the mechanism to regulate osmo-ionic balance [44]. This will be detrimental in their hypertonic environment. Freshwater shrimp larvae thus tend to have more developed organs to cope with environmental stress.
In addition, larvae of M. malayanum and other freshwater shrimps are generally benthic [46] with functional pereiopods (walking limbs) (Figure 8; see Figure 13 for illustration of shrimp body parts). In contrast, marine crustacean larvae are small and free-swimming. Such features would be dangerous for freshwater crustacean larvae, given the unpredictable flow speeds and abundance of predators in the freshwater realm [45]. There is also no need for large dispersal within the usually isolated freshwater habitat [41]. Thus, the functional pereiopods allow freshwater larvae to hang onto substrate [46], seek optimal refuges and thus, have a higher chance of survival and development.
Main Strategy 2: Abbreviated Larval development
Another life history strategy that M. malayanum has adopted to cope with a freshwater lifestyle is the reduction of larval phases and time, also known as abbreviated larval development. Similar to the first strategy, the abbreviation of larval development means that zoea from freshwater decapods are more well-developed when they hatch, including the presence of functional pereiopods and yolk reserves to undergo metamorphosis without the need to feed on external sources [46, 55]. The shortening of larval development also reduces the time in which the larvae are vulnerable to predators and environmental conditions. Wholly freshwater shrimps such as M. malayanum tend to have about 2–3 zoea stages, as compared to > 10 stages in brackish water and marine Macrobrachium species (Table 1). They also spend less time in the phase (Figure 9).
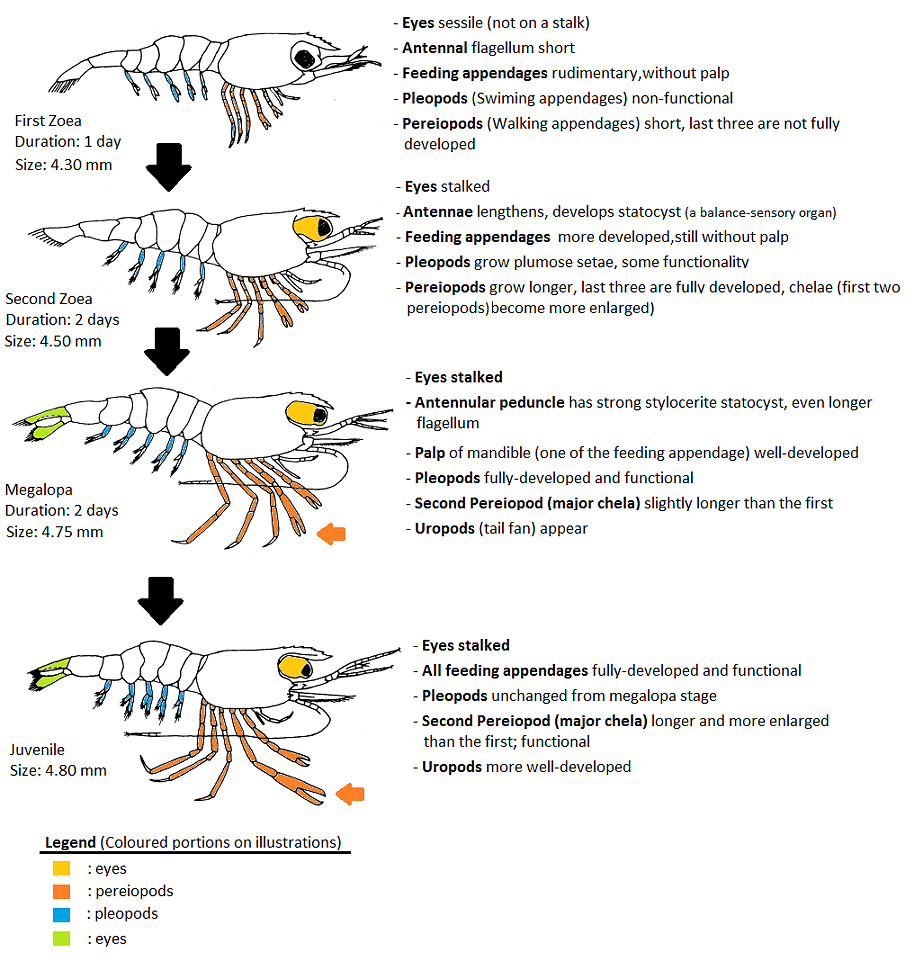
Figure 9: Larval development of M. malayanum and some morphological changes through its development. Adapted from Chong & Khoo (1987).
Parental care
Ovigerous caridean (i.e. the superfamily Macrobrachium spp. belongs to) shrimps including M. malayanum hold their eggs using pleopods (Figure 9). Until they are ready to hatch, the mother protects, grooms and aerates the eggs (Figure 10). This is in contrast to non-caridean shrimps that release their eggs immediately upon spawning.

Figure 10: Gravid M. malayanum female grooming eggs using her pereiopods. This involves her bending her body to reach the eggs. Re-adjustment of egg position and cleaning are usually done. Aeration is achieved by moving her pleopods up and down. Photo by: Claudia Tan
Parental investment in egg production is also observed in the larval stages of M. malayanum, where yolk reserves are still present in the larval stages [46]. This allows them to attain nutrition while their feeding appendages are still developing [46] while allowing them to tide over food-scarce conditions of freshwater environments better [56].
It is possible that blackwater or hillstream shrimps may have some extended parental care. This is illustrated by the ‘M. hendersoni complex’ (NOTE: M. malayanum is not part of this complex), where the mother continues to protect the larvae for a few days after hatching without feeding [57].
4.2 Relation to freshwater colonization in genus
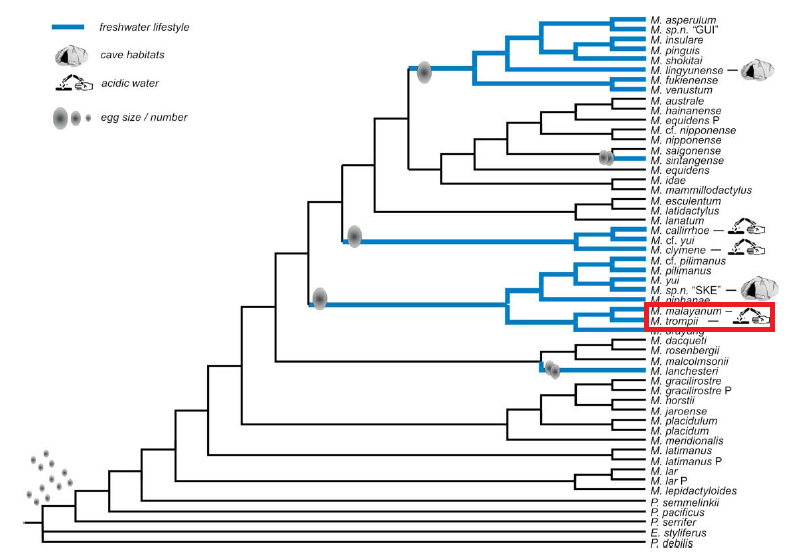
Figure 11: Illustration of the evolution of life history traits in Asian Macrobrachium. Bold (blue) branches are lineages with strict freshwater lifestyle derived from preferred optimization from. Relative egg size and numbers are mapped above branches. Figure taken from Worwor et al. (2009)/ Molecular Phylogenetics and Evolution 52: 340–350; with permission from corresponding author. Permission pending from publisher.
Some research have suggested that the Macrobrachium genus derived from a marine ancestor, which then diverged and colonized freshwater habitats (see Phylogeny for details). What makes it more intriguing is that life history traits like abbreviated larval development and large-intermediate egg size are characters found to be significantly correlated with freshwater life cycles [42, 58], making it possible that these traits may be used as a proxy to determine the evolution of the genus.
Unfortunately, these traits were likely to be gained by different freshwater shrimp clades independently [Figure 10, 58], even within the geographical location. One example to illustrate this would be between M. platycheles and M. malayanum, both of which can be found in Singapore. Although these two species are wholly freshwater species with abbreviated larval development, large egg size, and with overlapping distributions and populations, they do not share a common ancestry [42, 60]. This thus supports the hypothesis that more than one wave of freshwater invasion by Macrobrachium spp. occurred within the Southeast Asian region. These set of life history traits in different 'clades' of Macrobrachium spp. could thus be a phenomena of adaptive convergence [42, 58-60].
Previous Section: Distribution and Habitat, Feeding and Ecological Role, Agonistic Behaviour, Life History Strategies
Next Section: Taxomony, Phylogeny
5) Diagnosis: Which shrimp is which?
Shrimps from the Macrobrachium genus are the most ubiquitous decapod in many Southeast Asian tropical freshwater streams. However, they can be very similar in appearance. Macrobrachium malayanum can be identified by its hairy and robust main cheliped in mature males, conical carpus and generally short rostrum (see Figure 13 for shrimp anatomy illustration). However, there are morphologically-similar species in Singapore that co-occur with it, such as the critically endangered M. platycheles. Other species that one may encounter together with M. malayanum are M. sundaicum and introduced M. nipponense (Figure 12), and identification can be challenging due to their similar (or variable) colouration.
Figure 12: Freshwater Macrobrachium spp. with populations sympatric with M. malayanum: A) M. malayanum; B): M. platycheles; C): M. sundaicum. Photos credit: Kenny Chua, (permission obtained); D) M. nipponense (Photo by: Claudia Tan)
Distinguishing freshwater shrimps could help in identifying a common from a threatened native species, or a native from an introduced species. Furthermore, as mentioned earlier, different species may play varying ecological roles depending on their diets and feeding rates, something that is still not known in Singapore's shrimp populations. Identification would thus help increase understanding in species-specific ecology.
This section would thus cover the general morphological features between these species (Table 3). Please note that distinguishing them in the field can be a challenge, especially if they are not yet mature adults and thus, their chela and rostrum are not yet fully developed/well-defined. In the Macrobrachium genus, the main body parts that are key distinguishing features are their pincers (second pereiopods/ chelipeds) and their rostrum shape (see Figure 13 for anatomy illustration) . Colouration is also not a good indicator, although patterns may ease identification when present on the individual. Identification could require some technical knowledge, thus readers are suggested to look at the illustrated diagram of a shrimp’s body parts (Figure 13) and an example of some distinguishing morphology on Macrobrachium spp. (Figure 14) before proceeding.
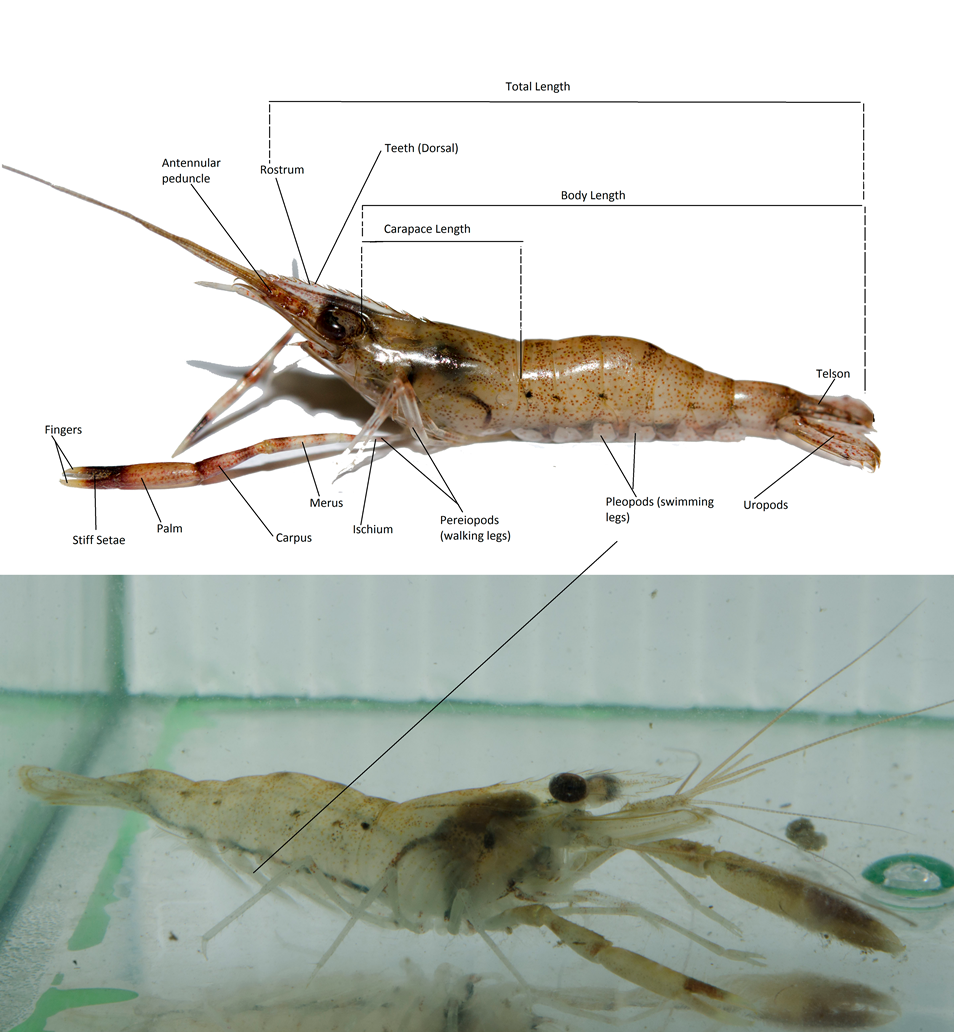
Figure 13: Illustration of shrimp body parts using M. malayanum photographs. Photo above captures a juvenile M. malayanum, hence its chelae are not yet as well-developed. Bottom photo shows the pleopods better since the shrimp is in water. Please note that colouration of this species ranges from pale yellow/brown to reddish-brown, and even black. Photos by: Claudia Tan
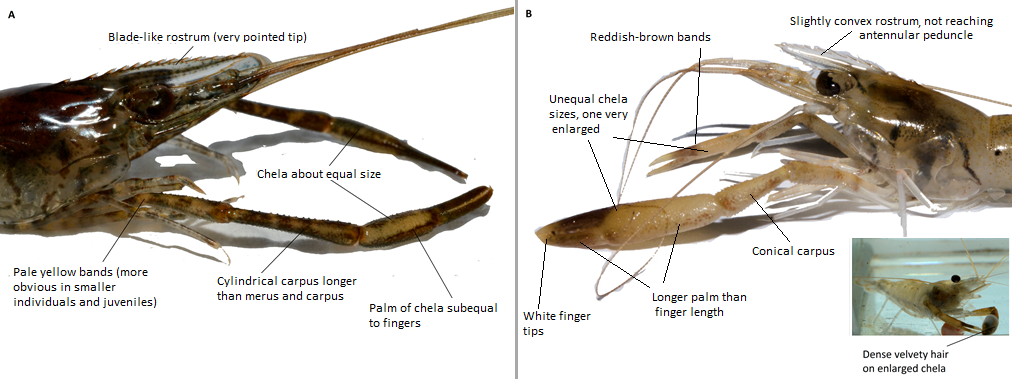
Figure 14: A) Headshot of M. nipponense and labels to depict distinguishing features of species. B) Headshot of M. malayanum with labels to depict distinguishing features of species and photo of individual submerged in water (to show hairs on chela).
Photos by: Claudia Tan
Table 3: Comparison of selected Singapore Macrobrachium species morphology. CL in 'max. size' row refers to carapace length, which is a more consistent way of measuring live shrimps in the field.
 |
 |
 |
 |
|
| Species |
M. malayanum |
M. platycheles(critically-endangered) |
M. sundaicum |
M. nipponense(introduced) |
| Sympatric at? |
- |
Nee Soon Swamp forest stream |
In most of M. malayanum local distribution, albeit in smaller numbers |
Forest stream-reservoir interfaces (e.g., Lorong Banir stream) |
| Max. Size (males) |
|
|
|
|
| Colour/ Pattern |
|
|
|
|
| Cheliped/Second Pereiopod |
||||
| Shape |
|
|
|
|
| Hairs/ Spines |
|
|
|
|
| Pattern |
|
- |
|
|
| Rostrum |
||||
| Shape |
|
|
|
|
| Relative length |
|
|
|
|
Previous Section: Distribution and Habitat, Feeding and Ecological Role, Agonistic Behaviour, Life History Strategies, Shrimp Identification
Next Section: Phylogeny
6) Taxonomy
6.1 What’s in the name?
Scientific name: Macrobrachium malayanum (Roux, 1934)
Common names:
“Malayan freshwater shrimp” and “Malayan river prawn” have been seen in literature, albeit infrequently. Such names may also be confused with the Malaysian giant prawn/ Giant freshwater prawn/ Giant river prawn, which is a different species, Macrobrachium rosernbergii, a famous large shrimp often harvested in aquaculture (but also sometimes seen in aquarium trade).
Junior Synonyms:
- Palaemon (Macrobrachium) pilimanus malayanus (Roux, 1934)
- Macrobrachium geron (Holthuis, 1950)
- Macrobrachium pilimanus (Holthuis, 1950)
- Cryphiops geron (Johnson, 1966)
Etymology: How did it get its name?
Macrobrachium malayanum was first described by Roux [11], whom identified the shrimp as a new sub-species of Palaemon (Macrobrachium) pilimanus due to their similar length and overlapping geographical distribution [11, Figure 15]. Macrobrachium pilimanus is known for its variable morphology and many sub-species have been grouped under the M. pilimanus complex [11]. The terms “malayanus” and “malayanum” were probably to refer to the species’ locality within Peninsula Malaysia, similar to other species such as Helarctos malaynus (Malayan sun bear).

Figure 15: Original description of M. malayanum (Roux, 1934). Original description available online at the Bulletin of the Raffles Museum Archives, Vol. 9
Holthuis (1950) collected a single adult male specimen and identified it as a new species, M. geron. The name geron is based on a Greek word that means “greyhead”, due to the long grey hairs on the major cheliped and the maturity of the specimen [10]. However, the use of only one specimen for species identifcation was erroneous. Later, Chong & Khoo (1987) synonymized M. pilimanus malayanus with M. geron after determining them as identical specimens. The authors also note that the species is markedly different from M. pilimanus (De Man, 1879), thereby promoting it to a full species status as M. malayanum (Roux, 1934).
6.2 Type Information
Type locality: Lasah, Plus Valley, East Perak, Malaysia
Type: Lectotype
Depository: Basel Museum of Natural History, Switzerland
Label: NHMB 711.Ia.i
Amongst the original collection of Tweedie, M.W.F. in 1933, one specimen from the first described collection was subsequently selected by Roux to serve as the single type specimen (lectotype). The specimen was the largest and most intact male in the collection, measuring 9.5 mm in carapace length [11].The remaining specimens among the set of syntypes (the original described collection of multiple individual of the species) are called paralectotypes (Table 2).
Table 2: Paralectotype information of Macrobrachium malayanum [4]
| Year of collection |
Number and sex of type specimens |
Type Locality |
Depository |
Label |
| 1933 |
|
Lasah, Plus Valley, East Perak, Malay Peninsula |
Basel Museum of Natural History, Switzerland |
NHMB 711.Iaii |
| 1971 |
|
Zoological Reference Collection, Singapore |
ZRC no. 1971.3.5.1–35 |
Previous Section: Distribution and Habitat, Feeding and Ecological Role, Agonistic Behaviour, Life History Strategies, Shrimp Identification, Taxomony
7) Phylogeny
Family and genus level: Some relationships yet to be resolved, may involve large changes to taxa in the future
Evolution within current genus: Studies indicate that genus may originate as far back as 20-30 million years ago, deriving from a marine ancestor that migrated in multiple, independent waves. Weak evidence for biogeographical patterns
7.1 Some relationships in Family Palaemonidae still unresolved
Before going in the phylogenetic relationships of the Macrobrachium genus, readers should take note that the phylogeny of the family which Macrobrachium belongs in, Palaemonidae Rafinesque, 1815, has yet to be resolved. Recent research findings have provided strong evidence of the collapse of the family Kakadcarididae Bruce, 1993 with the Palaemonidae, further enlarging the family [61, 62]. Despite this, Palaemonidae are still considered paraphyletic [63, 64], with families such as Hymenoceridae Ortmann, 1890 been nested within the family (Figure 16), and could be synonymised within Palaemonidae [64]. The systematic relationship between families Anchistiodidae and Palaemonidae is also still unresolved [63, 64]. Hence, the systematic relationship of the Palaemonidae may be subjected to changes in the future [63, 64].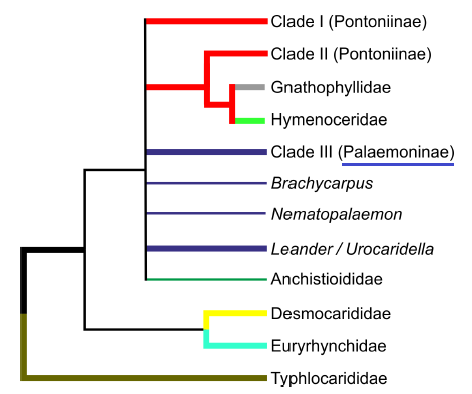
Figure 16: Derived cladogram of family relationships hypothesized within the Palaemonoidea ‘superfamily’ taxa using molecular analyses. Stronger evidence is indicated using thicker lines.Figure taken from De Grave et al. (2015)/ PeerJ 3: e1167. Article is distributed under the Creative Commons Attribution License.
7.2 Genus name could be changed?
Furthermore, even within the sub-family of Palaemoninae, re-assessment of a few genera, including Macrobrachium, is likely. An old confusion would be between the species-rich genus Palaemon and Macrobrachium. Many species in the current Macrobrachium genus was initially placed under the genus, Palaemon Weber, 1795, including M. malayanum (one of the junior synonyms is Palaemon pillimanus malayanus from the first descriptor of the species, Roux (1934)). Some species of Macrobrachium have also been found to be closer to the Palaemon genus, e.g., M. intermedium [60]. There could thus be further shifts between these genera as molecular data becomes more available to validate interspecific and intergeneric morphological comparisons, vice versa.To add to the confusion, there is a possibility of mergers between some genera and Macrobrachium [63]. One of which is between the genus Cryphiops Dana, 1852 and Macrobrachium Spence Bate, 1868 [63, 65]. The close morphological similarity between the two genera has made identification through morphology insufficient to some, with many species been placed and transferred between the two genera, including M. malayanum (i.e. Cryphiops geron by Johnson (1966)). If so, the genus would be re-named as the senior genus, Cryphiops [63]. Such issues may result in future changes of the Palaemondidae family and even the Macrobrachium genus with sufficient and reproducible molecular evidence.
7.3 Evolution within genus
The genus Macrobrachium is the most speciose in the family Palaemonidae, and one of the most diverse and widely-distributed crustacean genera [60]. This wide geographical distribution and great diversity of habitats it has colonized has garnered interest of the biogeographical and evolutionary relationships of this group. This is especially so given that the variety of life cycles and life history traits seem to be associated with their respective habitats, making one wonder if there is any evolutionary or phylogenetic significance behind these traits [42, 55, 60, see Section 4.2]. Furthermore, despite their widespread distribution, there seems to be a high degree of morphological similarity between species [10], challenging traditional taxonomic work. It is thus hoped that molecular and phylogenetic data could reinforce traditional morphological data in species identification. In particular, an increasing interest has been diverted to the Macrobrachium species in Asia [42, 58]. This is due to the bulk of Macrobrachium diversity occurring in the region (and in the Indian subcontinent) [66], commercial importance of some species [66] and their key ecological component in these habitats [5].Some studies have thus been conducted to understand the phylogenetic relationships of the genus in relation to their geographical distribution, lineage and life history traits. Various molecular markers, including genes from mitochondrial and nuclear genomes were used. Some progress has been made in this field, although findings still remain contentious or incomplete. Below are a summary of the findings made thus far:
(1) Macrobrachium could be an old lineage [60]
The large genetic divergence amongst species, even with the use of a highly conserved mtDNA sequence 16S rRnA, indicates that there was a significant time period for speciation to take place. A large portion of Macrobrachium fauna may have originated as far back as the late Oligocoene/ early Miocene, i.e. 20–30 million years ago, and diversified earlier in their evolutionary history rather than recently. Unfortunately, the lack of fossil records of the Macrobrachium genus has made it a challenge to give a more accurate age of the genus.
(2) Macrobrachium probably derived from a marine ancestor (contentious)
Macrobrachium is likely to have derived from a widespread marine ancestor that subsequently migrated towards freshwater habitats in multiple waves of migration [42, 58, 67]. This would explain the more widespread distribution of marine and estuarine Macrobrachium species, which would have the ability to overcome marine barriers in migration, before evolving and specializing in the more land-locked freshwater habitats across the subtropics and tropics. However, there are also studies that concluded that the genus is originally from a freshwater environment, before radiating to estuarine/marine habitats [60]. Thus, the ancestry of Macrobrachium is still in dispute.
(3) Macrobrachium have weak biogeographical patterns
There is little support for major endemic radiations, i.e. many sympatric species are not within the same sister group [60, 68, 69]. One local example would be the high genetic divergence between M. malayanum and M. playtycheles, which are both found in Singapore, Nee Soon Swamp, suggesting that they not derive from a single wave of ancestral migration and are not closely-related despite their overlapping distributions [60]
There may however been some influence of the Wallace’s Line on delineation, as demonstrated by the large genetic divergence between Eastern and Western populations of the widely distributed M. rosernbergii [60, 70]. This finding supports the following:
- Several waves of migration into fresh waters occurred within the genus
- The ancestor of Macrobrachium was probably marine, as oceanic biogeographical barriers did not affect its dispersal capacity
- The lineage of Macrobrachium predates at least 10 million years ago, before the collision of the Sunda and Sahul tectonic plates, hence explaining the wide distribution and regional non-monophyly in the Indo-West Pacific Macrobrachium species
Additional Info on Agonistic Behaviour
Behaviour Characterization

Dominant-Subordinate Behaviour Differences
After a dominance hierarchy is established, bouts rarely emerge. Detection of another individual after hierarchy formation usually results in either:
- A quick retreat by the subordinate shrimp as the dominant shrimp approaches, and the dominant shrimp does not pursue it
- Threat displays like charging by dominant shrimp and resultant retreat by the subordinate shrimp
Once the hierarchy is established (Video 9 and 10), the subordinate individual becomes submissive and acts differently from that of the dominant individual. With regard to M. malayanum, these are some of the differences in behaviour:
Dominant individuals tend to:
- patrol around the bottom of experimental tank more regularly if there is no shelter present (Note: M. malayanum is a benthic species)
- occupy or defend a shelter (if any)/ fixed area (e.g., Video 9), and
- initiate chases or attempted chela push/nips on the subordinate (Video 10)
Subordinate individuals tend to:
- swim to the surface and rest of the surfaces of the tank, swimming down at times and either 1) restrict themselves to an area where the dominant individual less frequents, or 2) swims back up when it encounters and gets chased by the dominant individual (Video 10)
- stays outside of shelter (although some individuals make failed attempts to enter shelter, e.g., Video 9)
- retreats almost immediately and does not initiate a fight with the dominant individual (Video 10)
Video 9: Winner from video 8 defends its shelter effectively with just an attempted chela nip/ charge, ending the interaction quickly.
Video 10: Dominant shrimp initiates chase and physical attacks on the subordinate shrimp, resulting in its swimming up (away from the bottom of the tank where the dominant shrimp is) and quick retreats (Video Credit: Claudia Tan)
References
1. Ng P.K.L. (1997) The conservation status of freshwater prawns and crabs in Singapore with emphasis on the nature reserves. The Gardens' Bulletin Singapore,49(1): 267–272.
2. Cai Y., Naiyanetr P. & Ng P.K.L.(2004) The freshwater prawns of the genus Macrobrachium Bate, 1868, of Thailand (Crustacea: Decapoda: Palaemonidae). Journal of Natural History, 38(5): 581–649.
3. Ng P.K.L. (1992) The freshwater crabs and palaemonid prawns (Crustacea: Decapoda) of Batam Island, Riau Archipelago, Indonesia, including descriptions of two new species. In: Proceedings of The Biological Society of Washington. (Ed C.B. Robbins), Allen Press Inc.: Lawrence, Kansas. p. 896.
4. Chong S.S.C. & Khoo H.W. (1987) Macrobrachium malayanum (Roux, 1934) stat. nov. (Decapoda, Palaemonidae) as a synonym of M. geron Holthuis, 1950, with notes on its distribution. Journal of Natural History, 21(4): 903–913.
5. Wowor D., Cai Y. & Ng P.K.L. (2004) Crustacea: Decapoda, Caridea. In: Freshwater Invertebrates of the Malaysian Region. (Eds C.M. Yule & H.S. Yong). Academy of Sciences of Malaysia: Malaysia. pp. 337–356.
6. Ng P.K.L. (1995) Freshwater Decapod Crustaceans. In: Rain forest in the city: Bukit Timah Nature Reserve, Singapore. (Ed S.C. Chin).Gardens' bulletin, Singapore, Vol. 3. National Parks Board, Singapore. pp. 151–157.
7. Ho J.K.I., Ramchunder S., Memory A., Meryl T., Li T.J., Yeo D.C.J., Esther C., Cai Y. & Tan H.H. (in press) A Guide to the Freshwater Fauna in the Nee Soon Swamp Forest, Singapore. p. 146.
8. Dudgeon D. (1999) The zoobenthos: a systematic review. In: Tropical Asian streams : zoobenthos, ecology and conservation, (Ed D. Dudgeon). Hong Kong University Press: Hong Kong. pp. 93–518.
9. Ng P.K.L. (1990) Freshwater Crabs and Prawns of Singapore. In: Essays in zoology : papers commemorating the 40th anniversary of the Department of Zoology, National University of Singapore (Ed.s L.M. Chou & P.K.L. Ng). Department of Zoology, National University of Singapore: Singapore. pp. 189–201.
10. Holthuis L.B. (1950) The Decapoda of the Siboga Expedition. Part X. The Palaemonidae collected by the Siboga Snellius Expeditions with remarks on other species. Subfamily Palaemoninae, Brill, E. J., Leiden, Holland. p. 268.
11.Roux J. (1934) New freshwater decapod crustaceans from the Malay Peninsula. The Bulletin of the Raffles Museum, 1934. 9:28–33.
12.Bishop J.E. (1973) The Invertebrate Fauna. In: Limnology of a Small Malayan River Sungai Gombak, (Ed J.E. Bishop). Springer: Netherlands. pp. 187–342.
13. Ng P.K.L. & Lim K.K.P. (1992) The conservation status of the Nee Soon freshwater swamp forest of Singapore. Aquatic Conservation: Marine and Freshwater Ecosystems, 2(3): 255–266.
14. Tok C.Q.Y. (2013) Population study on the freshwater shrimps in Nee Soon Swamp Forest, Singapore. Thesis. School of Biological Sciences, Nanyang Technological University, Singapore.
15. Johnson D. (1963) Distributional and other notes on some freshwater prawns (Atyidae and Palaemonidae) mainly from the Indo-West Pacific region. Bulletin of the National Museum of Singapore, 32:5–30.
16. Johnson D. (1967) Some factors influencing the distribution of freshwater prawns in Malaya. Proceedings of the Symposium on Crustacea, Marine Biological Association of India. 1:418–433.
17. Mantel S.K.& Dudgeon D. (2004) Dietary variation in a predatory shrimp Macrobrachium hainanense (Palaemonidae) in Hong Kong forest streams. Archiv für Hydrobiologie, 160(3): 305–328.
18. Jimoh A.A., Clarke E.O., Whenu O.O. & Adeoye H.B. (2011) Food and feeding habits of the African river prawn (Macrobrachium vollenhovenii, Herklots, 1857) in Epe Lagoon, southwest Nigeria. International Journal of Fisheries and Aquaculture, 3(1): 10–15.
19. Joseph I., Bassey A., George E. & Ubong G. (2013) Food and Feeding Habits of the Brackish River Prawn (Macrobrachium macrobrachion, Herklots, 1857) from Great Kwa River, Obufa Esuk Beach, Calabar, Cross River State, Nigeria. Journal of Natural Sciences Research, 3(9): 82–87.
20. Tan, H.H. (2014) Malayan freshwater shrimps attacking forest halfbeak. Singapore Biodiversity Records, 145.
21. Crowl T.A., Mcdowell W.H., Covich A.P. & Johnson S.L. (2001) Freshwater shrimp effects on detritial processing and nutrients in a topical headwater stream. Ecology, 82(3): 775–783.
22. Moulton T., Magalhães-Fraga S.P., Brito E. & Barbosa F. (2010) Macroconsumers are more important than specialist macroinvertebrate shredders in leaf processing in urban forest streams of Rio de Janeiro, Brazil. Hydrobiologia, 638(1): 55–66.
23.March J.G., Pringle C.M., Townsend M.J. & Wilson A.I. (2002) Effects of freshwater shrimp assemblages on benthic communities along an altitudinal gradient of a tropical island stream. Freshwater Biology, 47(3): 377–390.
24.Rosemond A.D., Pringle C.M. & Ramírez A. (1998) Macroconsumer effects on insect detritivores and detritus processing in a tropical stream. Freshwater Biology, 39(3): 515–523.
25. Boulton A.J., Boyero L., Covich A.P., Dobson M., Lake S. & Pearson R. (2008) Are Tropical Streams Ecologically Different from Temperate Streams? In: Tropical Stream Ecology (Ed D. Dudgeon). Academic Press: London. pp. 257–284.
26. Barki A., Karplus I. & Goren M. (1991) The Agonistic Behaviour of the Three Male Morphotypes of the Freshwater Prawn Macrobrachium Rosenbergii (Crustacea, Palaemonidae). Behaviour, 116(3): 252–276.
27. Lee C.L. & Fielder D.R. (1983) Agonistic behaviour and the development of dominance hierarchies in the freshwater prawn, Macrobrachium australiense Holthuis, 1950 (Crustacea: Palaemonidae). Behaviour, 83(1):1–16.
28. Goessmann C., Hemelrijk C. & Huber R. (2000) The formation and maintenance of crayfish hierarchies: behavioral and self-structuring properties. Behavioral Ecology and Sociobiology, 48(6): 418–428.
29. Vannini M. & Gherardi F. (1981) Dominance and individual recognition in Potamon fluviatile (decapoda, brachyura): possible role of visual cues. Marine Behaviour and Physiology, 8(1):13–20.
30.Fero K., Simon J.L., Jourdie V. & Moore P.A. (2007) Consequences of social dominance on crayfish resource use. Behaviour, 144(1):61–82.
31.Hock K. & Huber R. (2007) Effects of fighting decisions on formation and structure of dominance hierarchies. Marine and Freshwater Behaviour and Physiology, 40(1): 45–61.
32. Peebles J.B. (1980) Competition and habitat partitioning by the Giant freshwater prawn Macrobrachium rosenbergii (De Man) (Decapoda, Palaemonidae). Crustaceana, 38(1): 49–54.
33. Fero K. & Moore P. (2008) Social spacing of crayfish in natural habitats: what role does dominance play? Behavioral Ecology and Sociobiology, 62(7):1119–1125.
34. Gherardi F. & Cioni A. (2004) Agonism and interference competition in freshwater decapods. Behaviour, 141(10): 1297–1324.
35. Karplus I. (2005) Social control of growth in Macrobrachium rosenbergii (De Man): a review and prospects for future research. Aquaculture Research, 36(3): 238–254.
36. Kulesh V. (2009) Effect of biotic factors on growth and survival of the Oriental river prawn Macrobrachium nipponense (De Haan) in warm-water aquaculture. Russian journal of ecology, 40(6): 405–414.
37. Moraes-Valenti P.M.C. & Valenti W.C. (2007) Effect of Intensification on Grow Out of the Amazon River Prawn, Macrobrachium amazonicum. Journal of the World Aquaculture Society, 38(4): 516–526.
38. Lan L.M., Micha J.-C., Long D.N. & Yen P.T. (2006) Effect of densities and culture systems on growth, survival, yield, and economic return of freshwater prawn, Macrobrachium rosenbergii farming in the rice field in the Mekong Delta, Vietnam. Journal of Applied Aquaculture, 18(1): 43–62.
39. Karplus I. & Harpaz S. (1990) Preliminary observations on behavioral interactions and distribution patterns of freshwater prawns Macrobrachium Rosenbergii under semi-natural conditions (Decapoda, Caridea). Crustaceana, 59(2): 193–203.
40. Lammers J.H., Warburton K. & Cribb B.W. (2009) Diurnal Refuge Competition in the Freshwater Prawn. Journal of Crustacean Biology, 29(4): 476–483.
41. Vogt G. (2013) Abbreviation of larval development and extension of brood care as key features of the evolution of freshwater Decapoda. Biological Reviews, 88(1): 81–116.
42. Wowor D., Muthu V., Meier R., Balke M., Cai Y. & Ng P.K.L. (2009) Evolution of life history traits in Asian freshwater prawns of the genus Macrobrachium (Crustacea: Decapoda: Palaemonidae) based on multilocus molecular phylogenetic analysis. Molecular Phylogenetics and Evolution, 52(2): 340–350.
43. Wheatly M. (1993) Physiological Adaptations in Decapodan Crustaceans for Life in Fresh Water. In: Advances in Comparative and Environmental Physiology (Volume 15): Advances in Comparative and Environmental Physiology. Springer: Berlin Heidelberg. pp. 77–132.
44. Anger K. (1995) The conquest of freshwater and land by marine crabs: adaptations in life-history patterns and larval bioenergetics. Journal of Experimental Marine Biology and Ecology, 193(1):119–145.
45. Vernberg B.W. & Vernberg F.J. (1983) Freshwater Adaptations. In: The Biology of Crustacea (Volume 8): Environmental Adaptations (Ed.s F.J. Vernberg & B.W. Vernberg) Academic Press: London. pp. 335–359.
46. Chong S.S. & Khoo H. (1987) The abbreviated larval development of the freshwater prawn, Macrobrachium malayanum (Roux, 1934)(Decapoda, Palaemonidae), reared in the laboratory. Crustaceana, 53(1): p. 29–42.
47. Atkinson J.M. (1977) Larval development of a freshwater prawn, Macrobrachium lar (Decapoda, Palaemonidae), reared in the laboratory. Crustaceana, 33(2): 119–132.
48. Ngoc‐Ho N. (1976) The larval development of the prawns Macrobrachium equidens and Macrobrachium sp.(Decapoda: Palaemonidae), reared in the laboratory. Journal of Zoology, 178(1):15–55.
49. Kwon C. & Uno Y. (1969) The larval development of Macrobrachium nipponense (De Haan) reared in the laboratory. La mer, 7(4): 30–46.
50. Mashiko K. (1990) Diversified egg and clutch sizes among local populations of the fresh-water prawn Macrobrachium nipponense (de Haan). Journal of Crustacean Biology, 10(2): 306–314.
51. Mashiko K. (1983) Differences in the egg and clutch sizes of the prawn Macrobrachium nipponense (de Haan) between brackish and fresh waters of a river. 動物学雑誌, 92(1): 1–9.
52. Chong, S.S. & Khoo H. (1988) The identity of Macrobrachium lanchesteri (De Man, 1911) (Decapoda, Palaemonidae) from Peninsular Malaysia and Singapore, and a description of its first zoea. Crustaceana, 54(2): 196–206.
53. Ng P.K.L. & Chong S. (1992) A note on the taxonomy and larvae of the acid water prawn, Macrobrachium trompii (De Man, 1898)(Crustacea: Decapoda: Caridea: Palaemonidae). Malayan Nature Journal, 46: 119–129.
54. Shokita S., Takeda M., Sittilert S. & Polpakdee T. (1991) Abbreviated larval development of a fresh-water prawn, Macrobrachium niphanae Shokita and Takeda (Decapoda: Palaemonidae), from Thailand. Journal of Crustacean Biology, 11(1): 90–102.
55. Jalihal D.R., Sankolli K.N. &. Shenoy S. (1993) Evolution of Larval Developmental Patterns and the Process of Freshwaterization in the Prawn Genus Macrobrachium Bate, 1868 (Decapoda, Palaemonidae). Crustaceana, 65(3): 365–376.
56. Mashiko K. (1985) Comparison of Survival and Development between Large and Small Neonates of a Freshwater Prawn under Starvation Conditions (Behavior Biology and Ecology). Zoological Science, 2(3): 397–403.
57. Karge, A & Klotz W. (2008) Süßwassergarnelen aus aller Welt [Fresh water shrimps of the world], Vol. 2. Ettlingen: Auflage. 216 p.
58. Liu M.Y., Cai Y.X. & Tzeng C.S. (2007) Molecular systematics of the freshwater prawn genus Macrobrachium Bate, 1868 (Crustacea: Decapoda: Palaemonidae) inferred from mtDNA sequences, with emphasis on East Asian species. Zoological Studies, 46(3): 272–289.
59. Rabalais, N.H. & Gore R.H. (1985) Abbreviated development in decapods. In: Crustacean Issues (Volume 2): Larval Growth (Ed. A.M. Wenner). A. A. Balkema: Rotterdam. pp. 67–126.
60. Murphy N.P. & Austin C.M. (2005) Phylogenetic relationships of the globally distributed freshwater prawn genus Macrobrachium (Crustacea: Decapoda: Palaemonidae): biogeography, taxonomy and the convergent evolution of abbreviated larval development. Zoologica Scripta, 34(2):187–197.
61. Page T.J., Short J.W., Humphrey C.L., Hillyer M.J. & Hughes J.M. (2008) Molecular systematics of the Kakaducarididae (Crustacea: Decapoda: Caridea). Molecular Phylogenetics and Evolution, 46(3):1003–1014.
62. Short J.W., Humphrey C.L. & Page T.J. (2013) Systematic revision and reappraisal of the Kakaducarididae Bruce (Crustacea : Decapoda : Caridea) with the description of three new species of Leptopalaemon Bruce & Short. Invertebrate Systematics, 27(1):87–117.
63. Kou Q., Li X., Chan T.-Y., Chu K.H. & Gan Z. (2013) Molecular phylogeny of the superfamily Palaemonoidea (Crustacea : Decapoda : Caridea) based on mitochondrial and nuclear DNA reveals discrepancies with the current classification. Invertebrate Systematics, 27(5):502–514
64. De Grave S., Fransen C.H. & Page T.J. (2015) Let’s be pals again: major systematic changes in Palaemonidae (Crustacea: Decapoda). PeerJ, 3, e1167.
65. Pileggi L.G. & Mantelatto F.L. (2010) Molecular phylogeny of the freshwater prawn genus Macrobrachium (Decapoda, Palaemonidae), with emphasis on the relationships among selected American species. Invertebrate Systematics, 24(2):194–208.
66.De Grave S., Cai Y. & Anker A. (2008) Global diversity of shrimps (Crustacea: Decapoda: Caridea) in freshwater. In: Freshwater Animal Diversity Assessment (Ed.s E.V. Balian, C. Lévêque, H. Segers & K. Martens) Springer: The Netherlands. pp. 287–293.
67. Jalihal D.R., Sankolli K.N. & Shenoy S. (1993) Evolution of Larval Developmental Patterns and the Process of Freshwaterization in the Prawn Genus Macrobrachium Bate, 1868 (Decapoda, Palaemonidae). Crustaceana, 65(3):365–376.
68.Murphy N.P. & Austin C.M. (2004) Multiple origins of the endemic Australian Macrobrachium (Decapoda : Palaemonidae) based on 16S rRNA mitochondrial sequences. Australian Journal of Zoology, 52(5): 549–559.
69. Santini F. & Winterbottom R. (2002) Historical biogeography of Indo‐western Pacific coral reef biota: is the Indonesian region a centre of origin? Journal of Biogeography, 29(2):189–205.
70. De Bruyn M., Wilson J.A. & Mather P.B. (2004) Huxley’s line demarcates extensive genetic divergence between eastern and western forms of the giant freshwater prawn, Macrobrachium rosenbergii. Molecular Phylogenetics and Evolution, 30(1): 251–257.