Perisesarma eumolpe
Last updated by Wong Jinfa on 25 November 2012.
Table of Contents
Vid. 01. Photograph slideshow of P. eumolpe. (Photos: Jinfa, Maosheng & Christopher©)
Name
|
 Fig. 2. Dorsal view of P. eumolpe. (Photo: Jinfa©) |
Introduction
Perisesarma eumolpe is a common crab that is found in the mangroves of the Indo-pacific region. [4] It has colourful facial bands that are usually iridescent blue, yellow, green or turquoise. [5] Unlike most other animals that can only see in black and white, these crabs can see in colour! [6] This allows them to recognize and communicate to one another with their facial bands. For example, bigger crabs often have blue bands which indicate that they are mature and "fitter", so weaker individuals would know not to mess with that individual. [7]
Colour is also good indication of fitness as the crabs have to obtain enough carotenoids in their diet to produce it. [8] This comes from eating leaves. [9] While foraging, crabs also disturb the muddy substrate and help aerate the anaerobic soil of the mangrove, such that mangrove plants can grow better. [10] Thus, the crabs are very important in the mangroves and can be easily spotted at Sungei Buloh Nature Reserve (Singapore) at low tide. Can you spot the crab in Fig. 3? Click on it after you find it.
Fig. 3. P. eumople in Mandai mangroves. (Animation: Jinfa©)
Did you notice the water on the face of the crab in Fig. 3? The crabs are able to stay out of water for long periods because of the net-like hairs on their face that trap water. Deoxygenated water that has passed through the gills can be directed to these hairs where they are aerated with oxygen before it is moved back. [11]
The crabs also communicate to one another by rubbing the bumps on their claws together to produce sound and listen to one another with their legs! [12]
When a male crab finds a potential mate, it often has to guard the female from other males before copulation. After successfully mating, the male would leave the female to take care of the fertilised eggs that are held in her abdomen until a full or new moon. During the high tide of such a night, the female will spawn the eggs that will hatch into planktonic larvae. [13]
For more detailed explanation on these interesting facts, refer to the Biology section,
Distribution
P. eumolpe is found in the Indo-pacific region [4]. Fig. 5 has the locations of where crabs were found or collected in some publications, in addition to locations indicated on Flickr. I have also included the locations and photographs of some crabs that I have found personally. The locations demarcated with an asterisk are from very old records, that might have changed such that they are no longer habitats for P. eumolpe.
Indo-Pacific [4]:
|
 Fig. 4. P. eumolpe hiding under roots (Photo: Jinfa©) |
View Perisesarma Eumolpe Distribution in a larger map
Fig. 5. Distribution of P. eumolpe
Diagnosis
There are 23 species of face-banded crabs (Perisesarma sp.) found in the world and six that are recorded in Singapore. [20][21] Two common, similar looking face-banded crabs that can be found living side by side are Perisesarma eumolpe and Perisesarma indiarum (Fig. 6) [5] .
Fig. 6. Photographs of P. eumolpe (left) and P. indiarium (right) (Animation: Jinfa© with photographs by Dr. Naruse, Jinfa and Christopher©)
It is often difficult to tell them apart in the wild. This is because when they are covered in mud, one can only see their red chelae (pincers) and iridescent blue, yellow or green facial bands (personal observation). There are ways though to tell the two crabs apart:
Tubercles
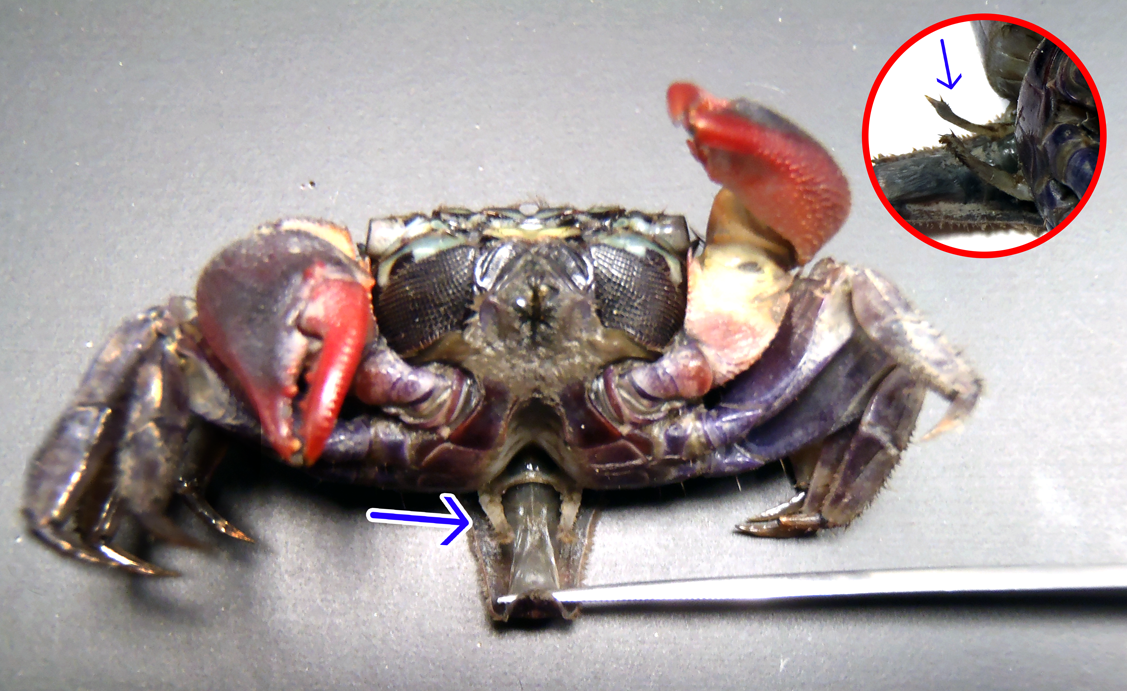
 Fig. 7. Tubercles on the dactyl of P. eumolpe (left) and P. indiarum (right). (Photo: Jinfa & Dr. Naruse©) > . 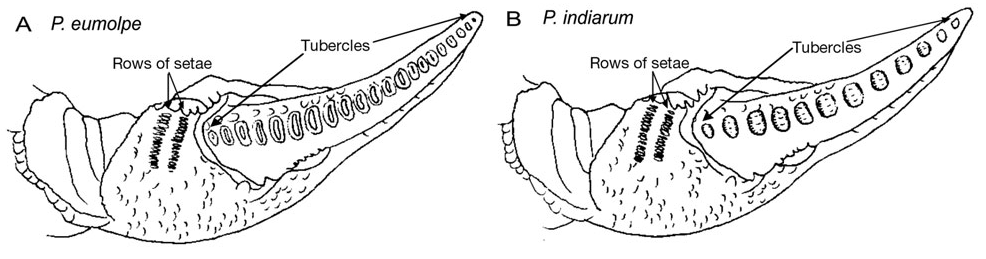 Fig. 8. Tubercle illustrations on the dactyl (Illustration reproduced with permission from Boon et al, 2009) To learn more on how P. eumolpe produces sound with its chelae, refer to the Biology (Stridulation) section. |
Third Maxillipeds
  Fig. 9. Comparison of the 3rd maxilliped of P. eumolpe (left) and P. indiarium (right) (Photo: Peiya©) To learn more on how to identify P. eumolpe, refer to the Anatomy and Description section. |
Anatomy
| The annotations (Fig. 10 & 11) were made with reference to Ng, 1988 [22] and help of Dr. Ng Ngan Kee. |
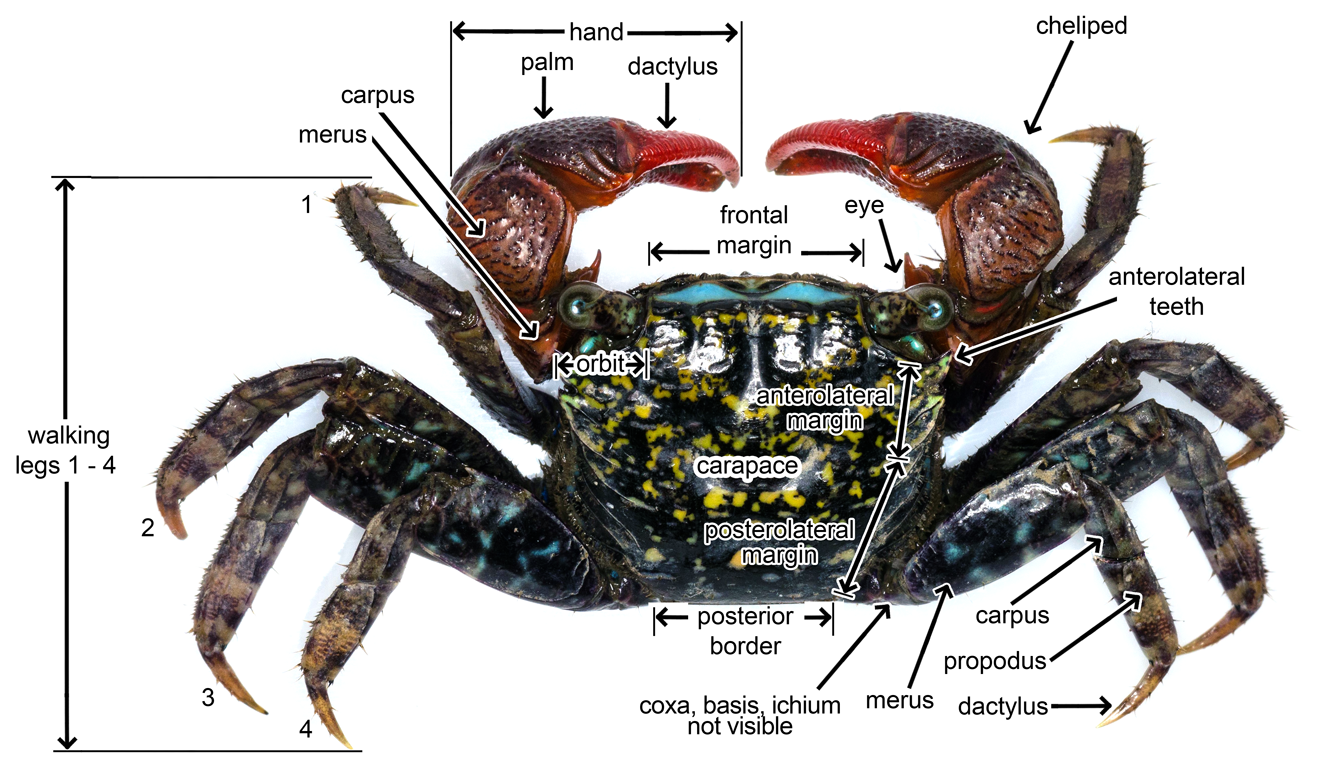 Fig. 10. Dorsal (top) view of P. eumolpe with annotations. (Photo: Jinfa©) |
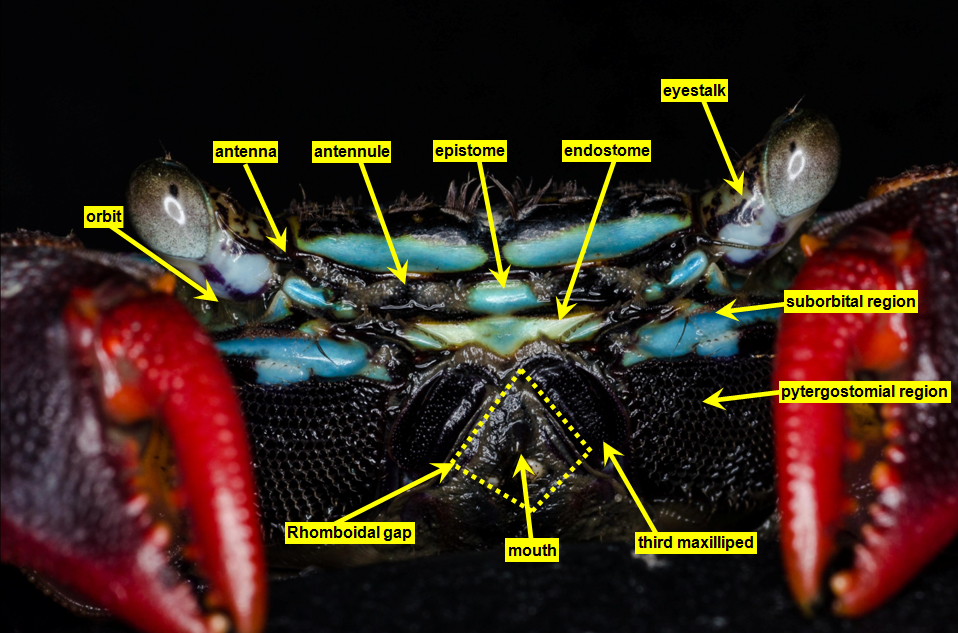 Fig. 11. Front view of P. eumolpe with annotations. (Photo: Jinfa©) |
| Male and female crabs can be identified by the differences in the shape of the abdomen on the ventral side (underside) of the crab. Females have a circular abdomen (Fig. 12) while males have a triangular abdomen (Fig. 13). >  >> >>  Fig. 12. Ventral (bottom) view of a P. eumolpe female (Photo: Jinfa & Christopher©)>>>>Fig. 13. Ventral (bottom) view of a P. eumolpe male (Photo: Jinfa & Christopher©) |
| The female's opening where sperm is injected into is called the vulva (Fig 14). Females have two vulvae. Upon fertilization, the eggs are held by the female in her abdominal cavity before she spawns (Fig. 15). >   Fig. 14. Location of vulva in a female (Photo: Jinfa & Maosheng©) >>>>Fig. 15. Eggs held in the abdominal segments of a female P. eumolpe (Photo: Jinfa & Maosheng©) |
| The male's reproductive organs used to insert into the female's two vulvae are called gonopods (Fig. 16) > Fig. 16. Male P. eumolpe have a pair of gonopods (Photo: Jinfa©) |
Description
The crab was named and first described by De Man (Fig. 17). [2] For more information on its taxonomy, please refer to the Taxonomy section.
 Fig. 17. Screenshot of De Man's description (Image: BioStor) |
Readers, please be advised that colour is not a good technique to identify a species as:
However, colour has been included in the subsequent descriptions as they are more practical to be applied in the field before more robust checks can be applied. If you come across any unfamiliar terms, please refer to the Anatomy section for reference.  Fig. 18. Front view of preserved P. eumolpe. (Photo: Jinfa©) |
|
Carapace
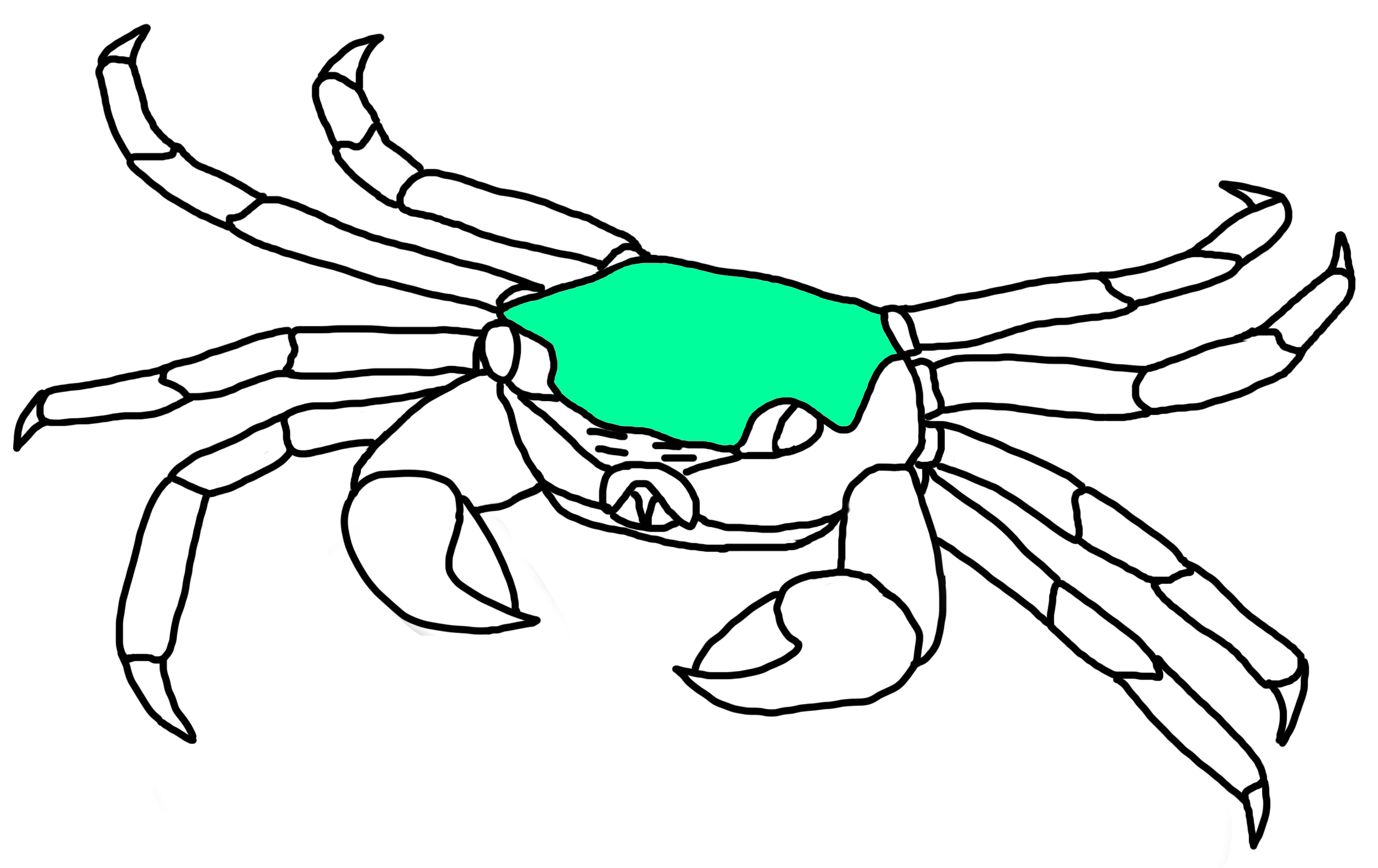 Fig. 19a. Carapace location on P. eumolpe (Illustration: Jinfa©) |
 Fig. 19b. Squarish carapace of P. eumolpe. (Photo: Jinfa©) |
|
Facial Bands
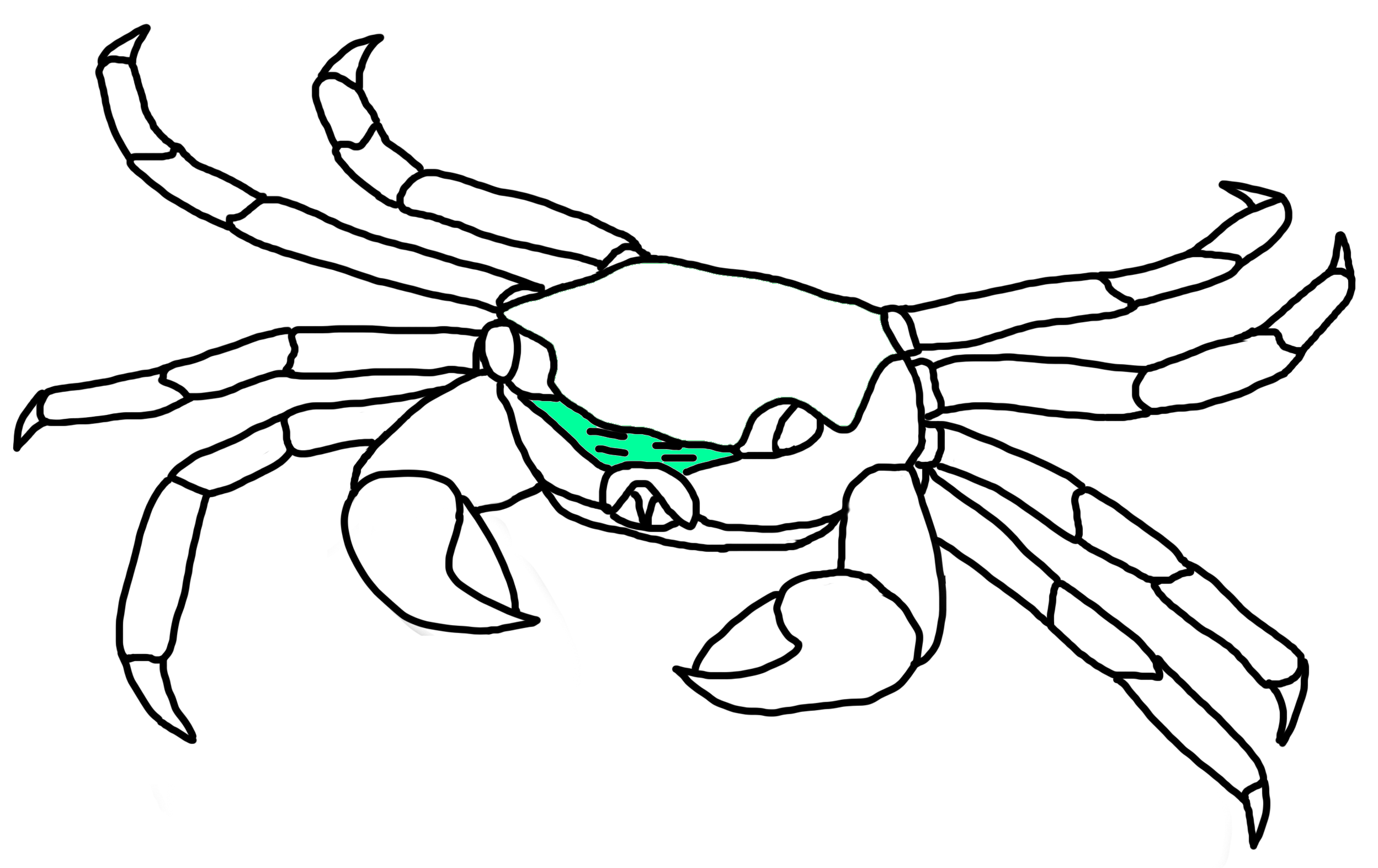 Fig. 20a. Facial band location on P. eumolpe (Illustration: Jinfa©) |
  Fig. 20b. P. eumolpe with different coloured facial bands.(Photos: Jinfa & Maosheng©) |
|
Third Maxillipeds
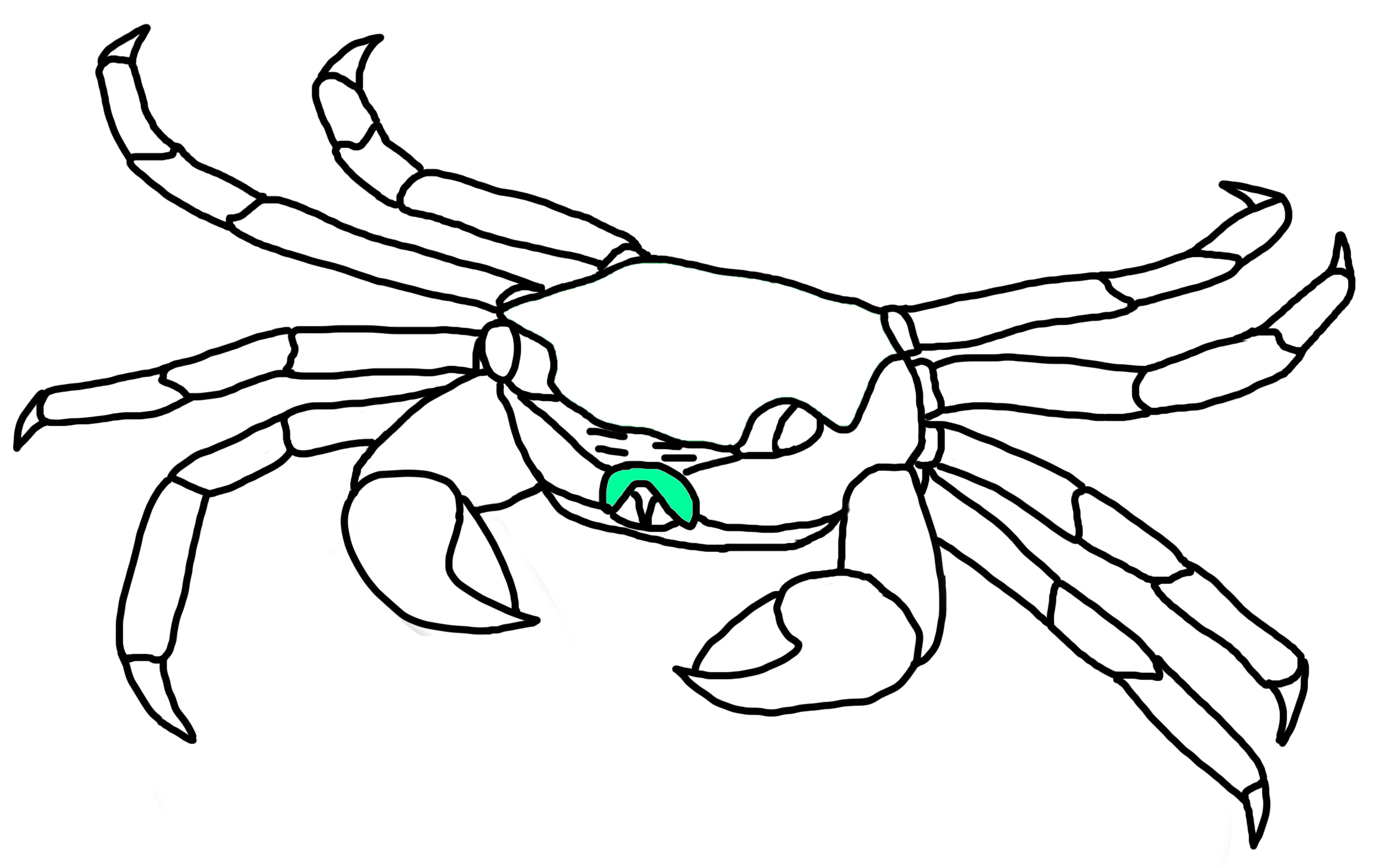 Fig. 21a. Third maxilliped locations on P. eumolpe (Illustration: Jinfa©) |
  Fig. 21b. Third maxillipeds of P. eumolpe tucked in (top left), stretched out (top right) & moving (bottom). (Photos & Animation: Jinfa©) |
|
Pterygostomial Region
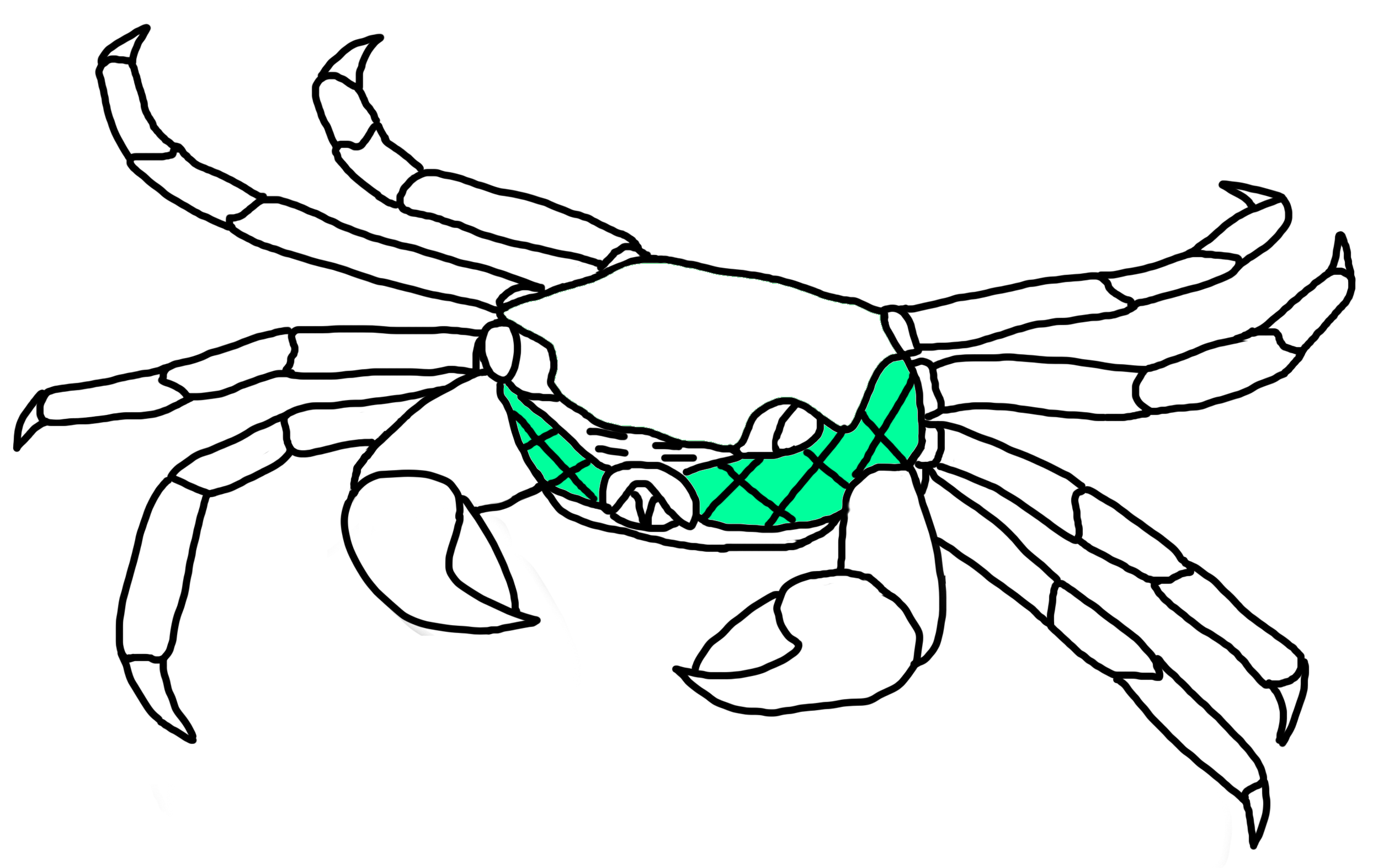 Fig. 22a. Pterygostomial region on P. eumolpe (Illustration: Jinfa) |
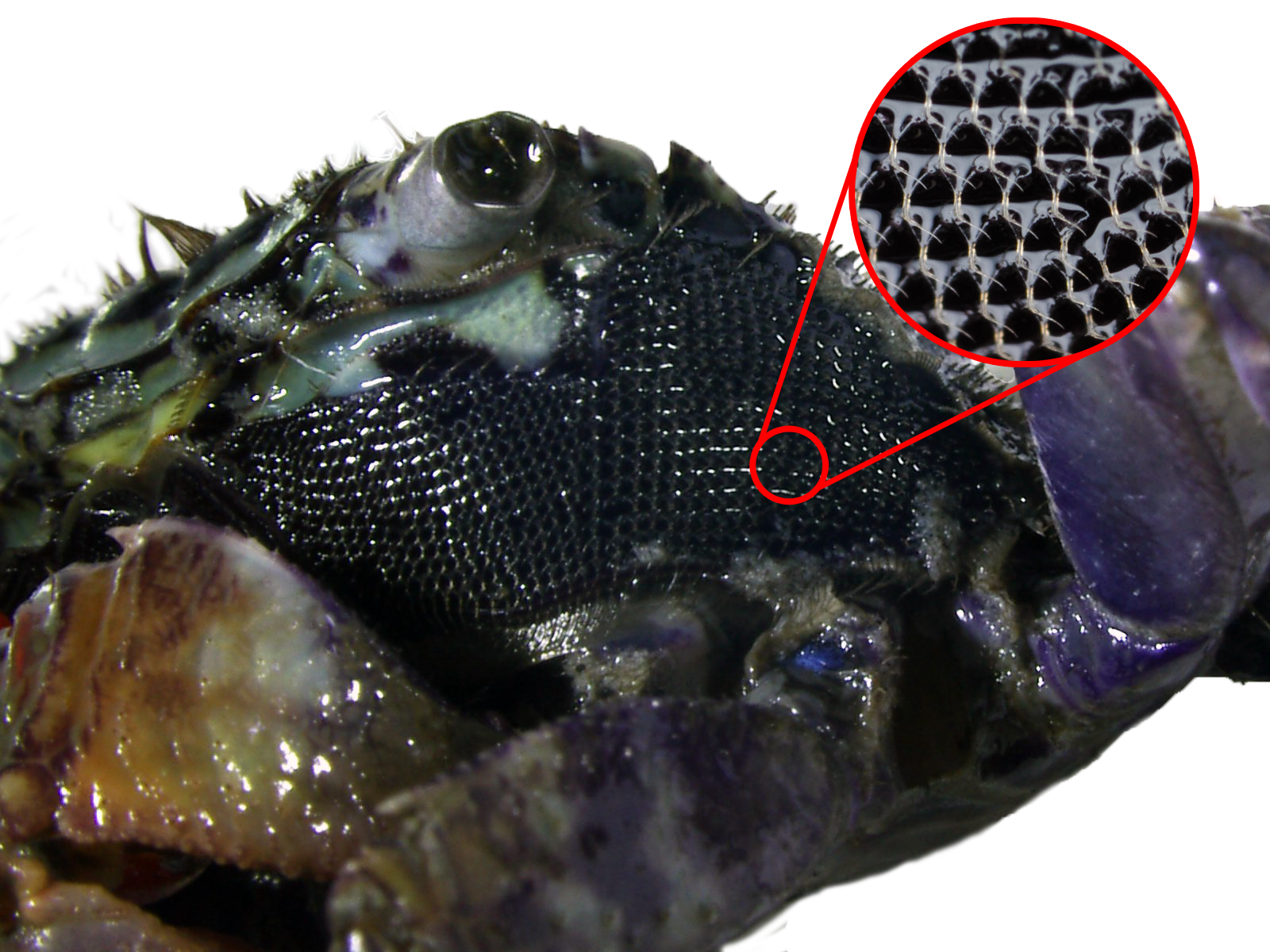 Fig. 22b. Pterygostomial region of P. eumolpe has retiuclated setae. (Photos: Jinfa©) |
|
Chelipeds (Pincers)
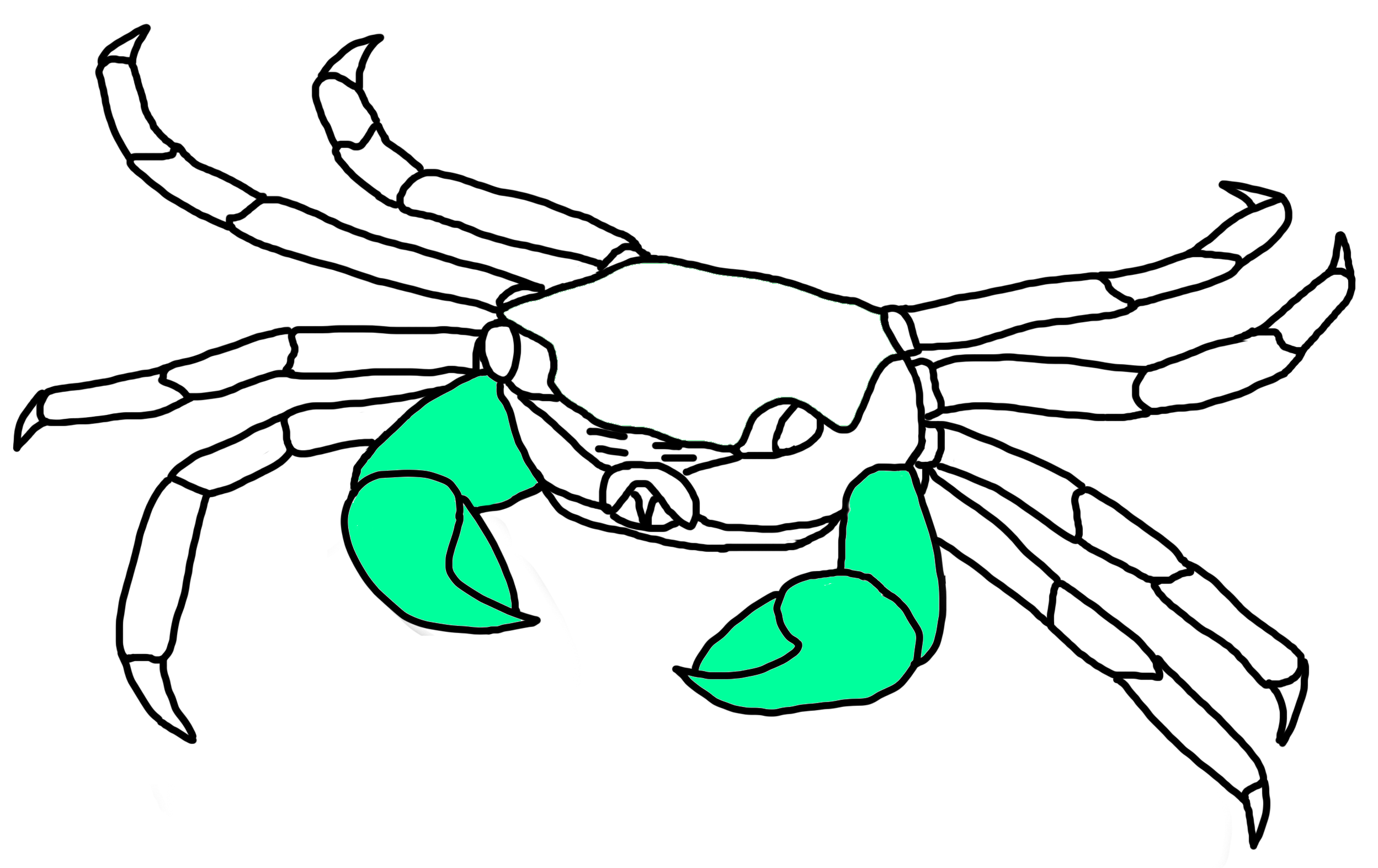 Fig. 23a. Cheliped locations of P. eumolpe (Illustration: Jinfa©) |
  Fig. 23b. Red chelipeds of P. eumolpe. (Photo: Jinfa & Christopher©) |
|
Walking legs
Fig. 24a. Walking leg locations of P. eumolpe (Illustration: Jinfa©) |
 Fig. 24b. Dactylus of P. eumolpe. (Photo: Jinfa©) |
To find out how to tell the difference between P. eumolpe & another similar looking crab, P. indiarum, refer to the Diagnosis section
Biology
Facial BandsThe facial bands of Perisesarma eumolpe are used to communicate their gender, maturity level, nutritional status and other qualities with one another. Firstly, the facial bands could be used by the crabs to recognize each other as conspecifics (members of the same species). When the facial bands of some of the crabs were blackened out in a study, other crabs seemed to ignore them. This could be because they were unable to recognize each other. [6][8] But, can these crabs really see colour? This was tested by Huang with heat associated learning. Crabs were observed to go in the direction of a Y-junction that had a different colour from the starting chamber that was heated up which suggested that they are able to see colour. [6] Majority of male crabs also have a blue band while majority of the females have green bands. This are used by crabs to discriminate gender. [5][6][7] Females were also found to have a positive correlation between the intensity of blue and their size, functioning as indicators of their maturity level. [5][6] The contrasting blue bands could also function in conspecific signalling during contestation for territory and matings rights. [5][6] Males were observed to display mate-guarding behaviours, choosing to move towards the male in a Y-chamber displaying male and female andromorphs.[7] This indicates that males gain more by fighting off other male competitors than by trying to attract females. Furthermore, when the facial bands of a male crab was blackened out, another male displayed less aggressiveness to it. These suggest the facial bands were important for male-male assessment. [5][6] Females similarly approached the female andromorph in the Y-chamber. This might be because they stand to gain more by keeping other females away from their territory compared to finding a mate, especially if it is resource-rich and are home to males. [7] In addition, both sexes choose to approach more brightly coloured conspecifics. [16] These evidence suggest that facial bands are honest signals and are used for assessing an individual's resource holding potential. [7]8][16] Resource holding potential is the capability of an animal to win if it engages in a fight. If a crab is not fit, it would not be able to expend resources to produce costly colours. Thus, dull coloured crabs can mean the crab is less fit and so do not pose a threat. This would help the crabs to avoid unnecessary fighting and injuries and thus save their energy for other activities such as foraging or burrowing. [28]  >>>>>>> >>>>>>>Fig. 25. P. eumople with green facial band. (Photo: Jinfa©)>>>>>>>>>>>>>>>>>Fig. 26. P. eumople with blue facial band. (Photo: Jinfa©) |
Facial Bands & CarotenoidsThe colours in the facial bands are most likely a result of their carotenoid-based diets. Differing from other mangrove sesarmids that rely heavily on mangrove sediments for assimilating carbon, Perisesarma crabs also feed on mangrove leaves, which contain carotenoids.[7][9][17] Wang tested if diet would affect facial colours by starving and then re-feeding some crabs. The results were a reduction in facial band brightness and colour saturation during starvation which increased again upon re-feeding.[7][8] This suggest that the colours could be cartenoid-based as carotenoids cannot be synthesised de novo and have to be obtained from their diet. [7][8] Further support was found from high performance liquid chromatography analysis of extracted facial band tissues that confirmed the presence of carotenoids.[7]17] Vid. 02. Facial band of P. eumolpe fading over time. (Animation: Jinfa©) For making this species page, I have also caught some crabs which I kept for about a month. In my glass tank, i had mangrove mud and leaves to ensure the crabs had enough food. However, I did observe that the one or two of the crabs with the yellow and green bands lost their colour completely within 2 weeks while crabs with the blue bands only became duller (personal observation).   Fig. 28a. P. eumolpe with healthy facial bands. (Photo: Jinfa©)>>>>>>>>>>>>>>>>>>>>>>>>>>Fig. 28b. P. eumolpe with faded facial bands. (Photo: Jinfa©) |
Feeding EcologyPerisesarma comprises one of the highest biomass of crabs in the mangrove, performing many ecological roles in the mangrove. [29] As Perisesarma feed on mangrove leaves, some of the energy stored in the leaves stay in the mangrove system, before they are lost when the tides wash them away. [10] Leaves that are broken into tiny pieces that not eaten and their faeces also enters the coprophagous food chain, providing food for other tiny organisms. [29] During foraging and burrowing, the crabs also perform bioturbation (stirring and mixing of the sediments), which can affect the growth of and reproduction of mangrove plants. [10][19] P. eumolpe are primarily sediment feeders that also feed on mangrove roots and leaves. [18] In additional, they are highly opportunistic and were noticed to feed on animal remains as well. [18][19] They were also found to prefer Avicennia alba leaves to other Singapore mangrove plant species and had no preference for fresh or old leaves. [18] Vid. 03. P. eumolpe feeding. (Video: Jinfa©) >>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> Vid. 04. P. eumolpe coming out of a mud pool and grooming itself before feeding. (Video: Jinfa©) |
StridulationOther than visual methods, P. eumolpe is also known to communicate with sound by stridulation, especially during the night when their facial bands are not visible. Sound is produced by rubbing the tubercles (Fig. 4 & 5) on the dorsal side of their chelae together (Fig. 29). [12] Boon et al. grouped the sound made by the crabs into rasp train and rasp series. [12] A rasp train is produced when one claw (rasping claw) is moved up and down repeatedly against the other stationary claw, between positions A1 and A2 (Fig. 27). [12] The rasp series is made up of a pure stridulation followed by the integral tap element. [12] Pure stridulation is a result of the crab moving its rasping claw upwards to reach position C from B, while the integral tap element is produced by striking the tip of the rasping claw on the propodus of the stationary contralateral claw (Fig. 27). [12] 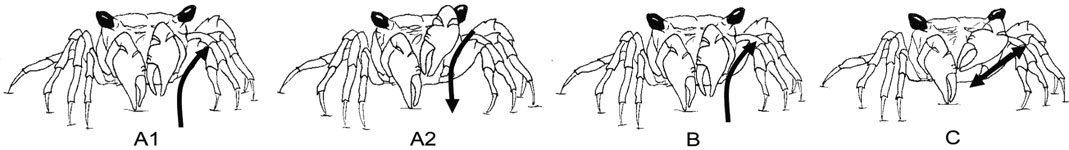 Fig. 29. Movement of chelae to produce sound (Illustration reproduced with permission from Boon et al, 2009) Sound is detected by the Barth's organ. This is found at the walking legs, on the underside of the meral-ischial joint, where the centre is bulging outwards from the surrounding depressed region (Fig. 30). [12]  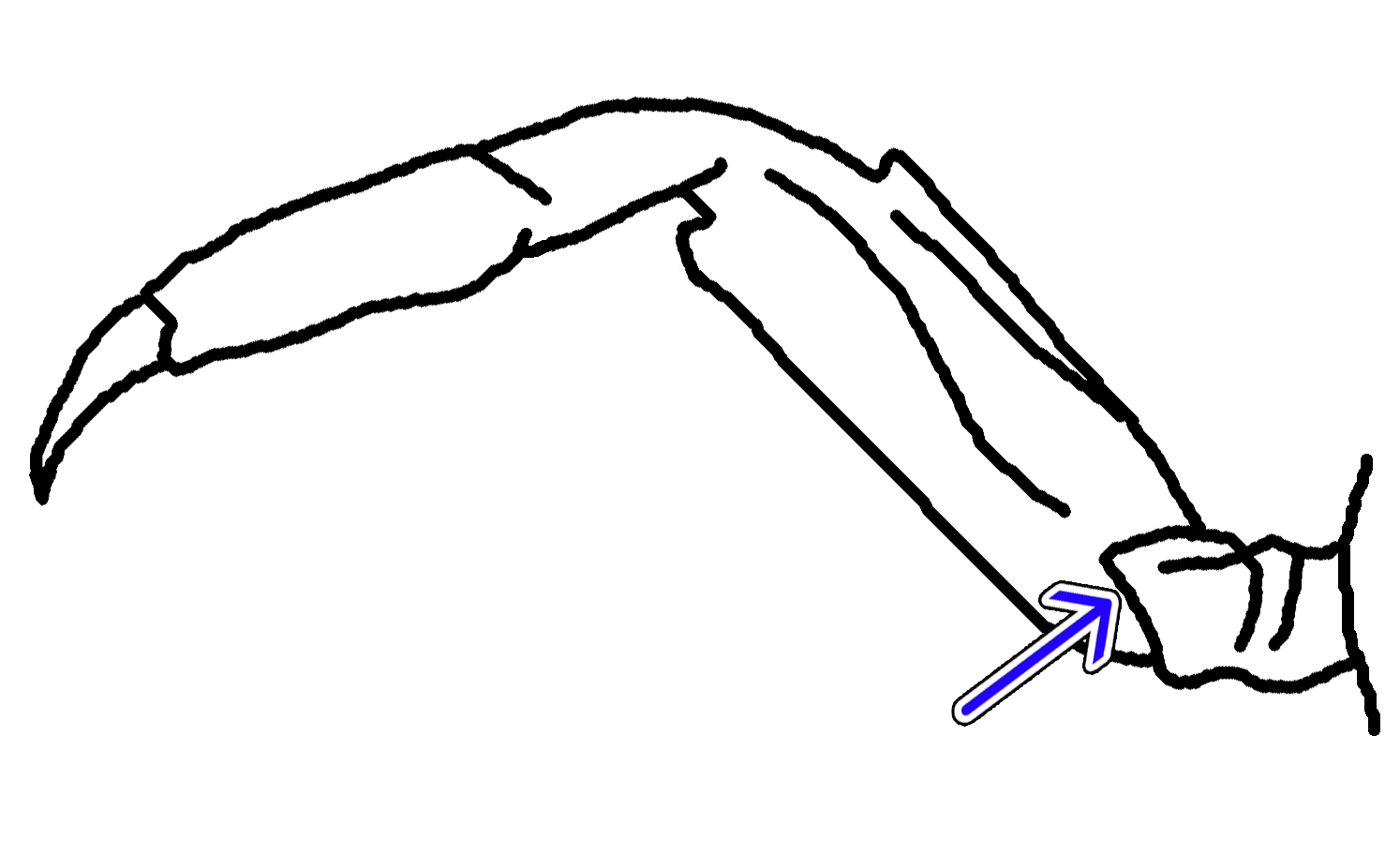 Fig. 30. Barth's organ on the meral-ischial joint (Photo & Illustration: Jinfa©) Unlike other animals where displays are usually performed before contact (to minimize wastage of energy), P. eumolpe was observed to display this stridulatory behaviour after contact, especially after intense fights. This is very unusual as displays are usually done beforehand. Chen (2014) also found that stridulations were displayed by the winners after the fights. Thus, this behaviour is most likely a victory display, exclusively used for asserting victory.[40] |
Branchial Water Movement & Reticulated SetaeHow do the crabs breathe when they are out of water? Water enters the branchial chamber by capillary action of the numnerous setae on the coxal joints of the walking legs. It then flows to the gills where gaseous exchange occurs before being pumped out. Most of the water that flows out would move across the pterygostomial region, which is covered with reticulated setae that traps it. [30] When on land, deoxygnated water that has passed through the gills can also be transported to the reticulated setae where it is aerated with oxygen before it is moved back to the branchial chamber. This gives the crab the ability to stay out of water for long periods. [11]  Fig. 31. Water movement of P. eumolpe. (Photo: Jinfa©)>>>>>>>>>>>>>>>>>>>>>>>>>> Vid. 05. P. eumolpe circulating water around its reticulated setae. (Video: Jinfa©)> |
ReproductionP. eumolpe males have been observed to initiate coutship by waving their claws to attract females. [31] During copulation, both crabs face their ventral sides to each other and have their abdomens extended so that the male is able to insert his gonopods into the female's paired vulvae to deliver his sperm (Fig. 14 & 16; Vid. 06). [31] Vid. 06. Sesarma rectum mating. (Video: wishikawa1) As a result of having to extend their abdomens to mate, it has been generalized that crabs can only mate after a female has recently moulted because the abdomen of the females are inflexible during inter-moults. [31] This might not necessarily be true for Sesarmidae as studies on some of the crabs in this taxon such as Amrases ricord have been been shown to mate during inter-moults. [31][32] This has has yet to be confirmed for P. eumolpe. However, the evidence of mate guarding by males might indicate that females only mate at certain stages in the moult cycle. [16] After fertilisation, females keep the eggs in her abdomen (Fig. 15) before spawning during a high tide on full or new moon. [13] The eggs hatch out into planktonic larvae known as zoea (Fig. 31). These would then grow and moult into megalopa (Fig. 32), the final stage as a zooplankton, before they become visible miniature crabs. [27] 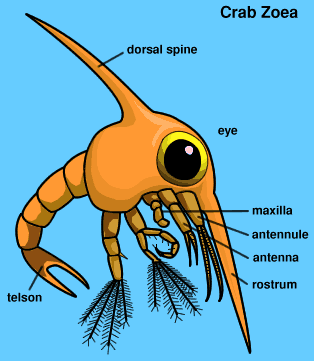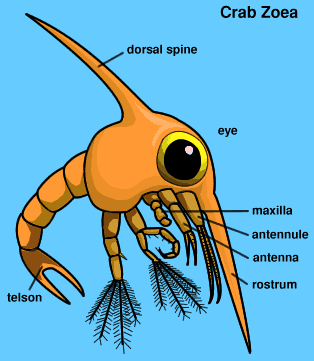 >>>>> >>>>>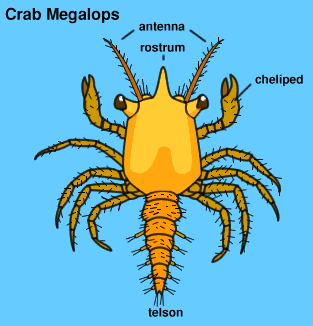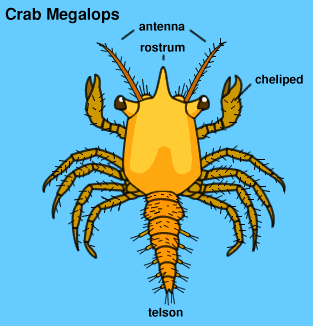 Fig. 32. Crab zoea illustration. (Permission pending from Davey©)>>>>Fig. 33. Crab megalopa illustration. (Permission pending from Davey©)> |
Taxonomy
Taxonavigation
Animalia
|
> Fig. 34. P. eumolpe hiding in a crevice. (Photo: Jinfa©) |
Taxonomic History
| It is important to know the different names (synonyms) used for P. eumolpe as research on any organism is published under its species name. Thus, one could miss out information by searching with just the accepted name, as the organism might have been filed under a different name. The following paragraphs describe how the name of P. eumolpe has changed over the years. P. eumolpe was first described by De Man and placed in the taxon Searma (Perisesarma). [2] Subsequently in 1909, Rathbun substituted the name Chiromantes Gistel, 1848 for de Man's Perisesarma. [33] This resulted in Tesch revising Sesarma (Perisesarma) eumolpe as Sesarma (Chiromantes) eumolpe in 1917. [3] Thereafter, in 1967, Campbell revised Sesarma (Chiromantes) and designated Sesarma dussumieri H. Milne Edwards, 1853 as the type specimen for this group. [34] Following in 1973, Holthuis proposed that Chiromantes Gistel, 1848 should be the replacement name for Pachysoma De Haan (1833). [35] This was because Rathbun designated Grapsus (Pachysoma) bidens De Haan, 1833, as the type species of Pachysoma incorrectly as De Haan did not originally include that species in Pachysoma. [35][36][37] Thus, Holthius designated Grapsus (Pachysoma) haematocheir De Haan, 1833 as the type specimen for Pachysoma De Haan, 1833. [35] On the other hand, (Pachysoma) haematocheir De Haan, 1833 was also the designated type specimen of the subgenus Holometopus H. Milne Edwards,1853 at that time. Therefore Chiromantes Gistel, 1848 became a senior objective synonym of Holometopus H. Milne Edwards,1853. [35] Thus, in 1973, Holthuis revised that Chiromantes Gistel, 1848, should be used for the taxon usually indicated as Holometopus, while the taxon usually indicated as Chiromantes should be known as Perisesarma De Man, 1895. He then proceeded to designate P. eumolpe as the type specimen for Perisesarma, not knowing that Sesarma dussumieri H. Milne Edwards, 1853 was already designated as the type specimen by Campbell in 1967. [34][35][38] Thus, the correct type specimen for Perisesarma is Sesarma dussumieri H. Milne Edwards, 1853. [38] In 2001, Sesarmidae was elevated to the family level from a sub-family. Consequently, Perisesarma was elevated to the genus level. [39] The current taxa nested in Sesarmidae is still very messy and are being revised. [27] For more information on how to identify P. eumolpe, refer to Description section. |
 Fig. 35. P. eumolpe hiding in a crevice (Photo: Jinfa & Christopher©)  . Fig. 36. P. eumolpe tucked in a hole in a wood (Photo: J©) |
Phylogenetics
| A phylogenetic tree construction by Gillkin and Schubart gave different tree toplogies with different selection criteria, both of which had high bootstrap support values (Fig. 37). When the selection criteria was parsimony, P. eumolpe formed an outgroup to the other 3 species of Perisesarma. This would suggest that P. samawati, P. guttatum and P. bidens were more closely related to one another than P. eumolpe is to them. On the other hand, when maximum likelihood was chosen as the selection criteria, P. eumolpe formed sister groups with the similarly coloured P. samawati. [4] This would suggest that P. eumolpe and P. samawati was more closely related to one another than to the other crabs. |
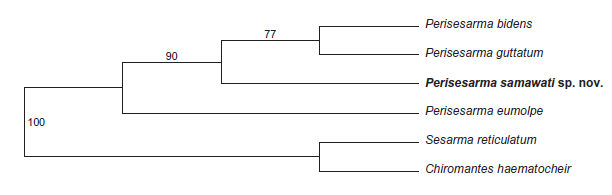 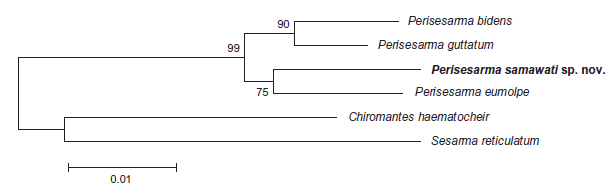 Fig. 37. Phylogenetic relationships of four Indo-Pacific species of Perisesarma spp. based on 575 base pairs of mtDNA coding for the 16S rRNA. Maximum parsimony tree (left) and maximum likelihood (neighbour joining, Kimura 2-parameter distances) tree (right) with confidence values established after 2000 bootstrap replicates. (Figure reproduced with permission from Gillikin & Schubart, 2004) |
| A more interesting phylogenetic tree would be to consider the sympatric similiar looking P. indiarium in the analysis. However, GenBank does not have the sequences for me to construct the tree. |
Acknowledgements
I would like to give my thanks to Dr. Todd for introducing this crab to me.
Ze Lin for going out to Mandai to catch some crabs with me.
Paul and Wei Kit for helping me get food for the crabs.
Benjamin for helping me get food for the crabs and releasing them.
Maosheng and Christopher for helping me in taking photographs and videos.
Dr. Naruse, Dr. Gillikin, Peiya, Simon and Ria for their photographs and figures.
Sufyan for helping me troubleshoot my flash animations when they don't work as planned.
Dr. Ng for helping me with Fig. 11.
All researchers who has generated the information for me to put on this page.
Fig. 38. P. eumolpe hiding under a plank (Photo: Jinfa & Maosheng©)
Comments
comments powered by Disqus
Links
|
Literature Cited
|