|
| Frontal picture of the face of Blyth's Horseshoe bat Picture credits: Nick Baker, EcologyAsia.com |
Table of Contents
Introduction
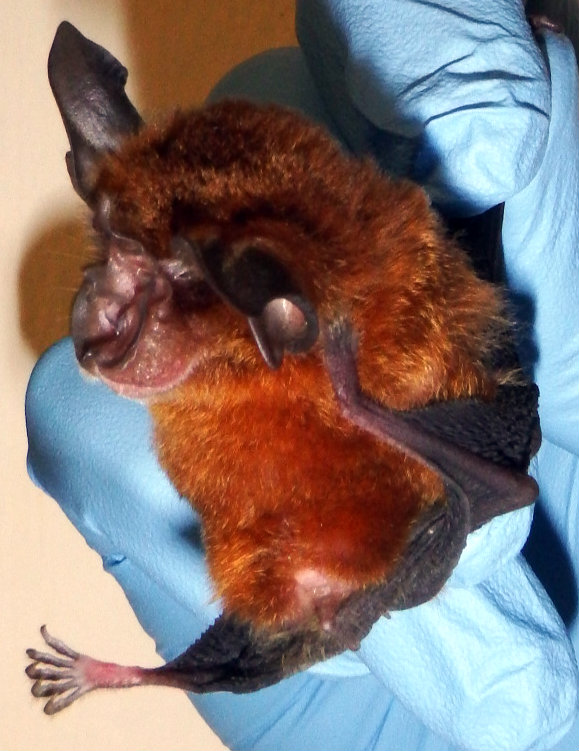 |
| Image of a male Blyth's Horseshoe bat in Orange morph. Notice the small eyes and complex nose structure. Picture used with permission and credit to Benjamin Lee. |
Blyth's Horseshoe bat (Rhinolophus lepidus) is one of the many species of horseshoe bat belonging to the genus Rhinolophus. They acquired their name from their unique spear or leaf shaped nose structure, as well as their fur which gives off a glossy look when view from an angle. They generate their echolocation from the nasal structure (nostril) and the echos received with the nose, which differs from other insectivorous bats which utilize laryngeal echolocation. As such it is possible for the bat to feed and echolocate concurrently! Like most insectivorous bats, they have small eyes and their field of vision often limited by their noseleaf[1] . Here is a link to an introductory video of the Blyth's Horseshoe bat by Dr Tigga Kingston from Texas Tech University.
| Distribution map of the Blyth's Horsehoe bat. Image taken from IUCN |
They are insectivorous and their wings are adapted to give them extremely flexible flight control to hunt for insects among a clustered environment[1]. With their complex nose structure, they are able to use echolocation to help them navigate and detect objects in the environment. They do so by emitting high frequency sounds and analyzing the returning sound echo to orientate and "see" their environment[3] . Their highly acrobatic flight along with their echolocation capabilities allow them to hunt down flying insects in mid-air with great efficiency, also known as aerial hawking. Here is a video showing them in action.
| Conservation status of Blyth's Horseshoe bat. Image taken from IUCN website |
According to the IUCN, R. lepidus is considered least concern in terms of vulnerability to extinction. This is likely due to their widespread distribution as well as abundance in the areas. However, they also face threats from human such as bush meat hunting and tourism souvenir trade. In recent years, more and more attention have been directed to R. lepidus and other bats due to emerging diseases in recent decades and in general, bats potential role as disease reservoirs.
Behavior and Biology
Flight morphology and locomotion
| Wing of a bat showing the different sections of flight membrane. Image credit to J. Gebhard, 1982. Image taken from The Biology of Bats, by G. Neuweiler, 2000. |
The wings of the bat is a modified mammalian forearm. The bones are elongated and form a supporting structure for the membrane resembling the tough and rigid frame of an umbrella[4] [5] . The membrane can be divided into 4 general regions: the plagiopatagium (fifth finger to rump); the propatagium (shoulder to wrist); the chiropatagium (fifth finger to wing tip) and the uropatagium (legs to tail). The first two regions support the main bulk of the body during flight, while the third helps the bat to propel forward during flight and the last helps the bat to catch prey during mid flight.
Bats are capable of both hovering and horizontal flight. The flight style of the bat is related to the shape of the wing (aspect ratio) and the wing loading (for technical details, do refer to this useful web page). Bats that forage in open space are characterized by long and pointed wings for fast, energetically efficient flight but at the cost of reduced maneuverability; whereas bats that forage in more cluttered habitats have short and broad wings, increasing their maneuverability at the cost of slower flight and energetically expensive long distance flight[6] .
Rhinolophus lepidus have short, broad wings (low aspect ratio) and a low wing loading. This allow them to perform flights with great maneuverability and perform acrobatic turns to pursue flying insects in the foliage and hover while hunting.
Echolocation
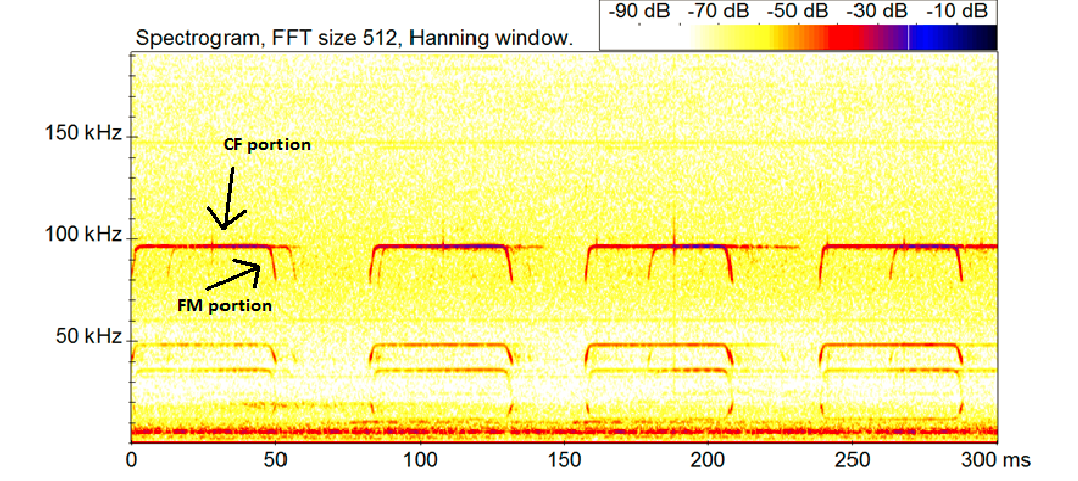 |
| A sonogram of the echolocation call of the Glossy Horeshoe bat. CF stands for constant frequency and FM stands for frequency modulated. The call peaks around 97khz and the bands below 97khz are harmonics. Image used with permission and credit to Benjamin P. Y-H. Lee. Annotation by Lim Zong Xian |
Each call structure (CF, FM, CF-FM) is adapted from an acoustic environment niche and as such imposes ecological restriction for foraging bats [6][7] . In open uncluttered areas, bats often use long search calls with narrowband and in intervals of 2-3 wing beats. Whereas for bats foraging in highly cluttered areas, a larger bandwidth call is emitted at a higher frequency to help orientate the bat. The call structure of each bat species is shaped by their diet, habitat,flight morphology and they could vary depending on the environment[4] [6] .
Echolocation was also used for water body recognition aside from foraging. Study also suggest such habitat recognition is likely to be innate in bats[8] . The above video (taken from Youtube channel Nature) showcase the experiment using flight cages and a variety of surfaces to test their hypothesis. This innate behavior is found in a wide variety of bats, including a closely related species of R. lepidus.
Rhinolophus lepidus echolocates using Quasi-CF calls with a peak of 97.8 khz enclosed by an initial and terminal FM downsweep[3] [4] . Their echolocation structure is suited for a cluttered environment (tree foliage), aimed at hunting flying insects at edges and gaps in the foliage by aerial hawking [4] [5] [9] . The CF component aids in medium range detection of prey while the short FM downsweep improves the classification and localization of the targets along with a more defined "picture" of the background (vegetation, objects)[6] .
Diet
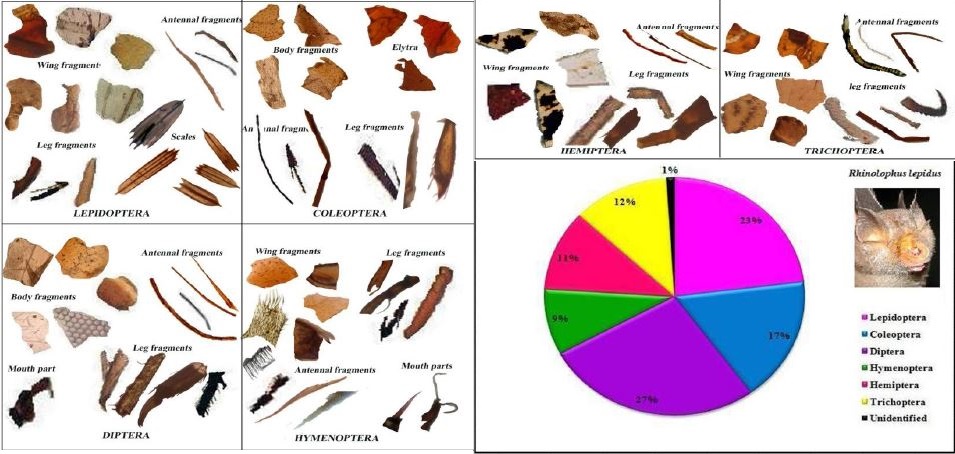 |
| Prey remains isolated from the feces of the Blyth's Horseshoe bat. Image credit to Ponmalar & Vanitharani, 2014. |
The diet for insectivorous bats is dependent on a variety of factors such as skull and dental structure, wing morphology and echolocation behavior[4] [10] . The size of the skull and dental structure determines the type (hard vs soft) and size of prey. The wing morphology and behavior helps to determine the manner of foraging and the environment best suited to the bat.
Generally, bats with longer signal at lower frequencies are able to search out a wider area due to lower atmospheric attenuation whereas bats with shorter calls at higher frequency have a smaller search area. However, bats with shorter calls are more suited to detect small insects[4] .
Rhinolophus lepidus mainly prey on soft flying insects such as butterflies, moths and wasp by aerial hawking . The peak frequency call (97.8khz) of the bat allows them to prey on eared moths effectively as the call are above the hearing range of the moths (20-70khz)[11] . However recent studies suggest that the hearing range of eared moths are wider and subjected to variation depending on geographical location[12] [13] . In this case, it is likely that high frequency calls are "softer" and thus the moths can only detect the bats at a much shorter distance, reducing their response time and providing the bat with a higher chance of success[11] .
Roosting and foraging
Rhinolophus lepidus is a cave roosting species with a wide foraging range up to 11km a night[14] . They roost in small groups of up to four bats to large colonies of around 400 bats in caves and their habit of roosting in clusters is rather unique among the Rhinolophids[15] . They will roost wherever they can hang freely and will wrap their wings fully around their body, unlike most other bats which fold their wings at the side.They typically hunt alone in the foliage of trees (cluttered environment) and may perch under a branch to enjoy their meal. They seem to display high fidelity to a hunting site and have been observed to hunt near man-made houses (under the porch and shelter), especially during rainy periods[15] .
Breeding
Individuals reach sexual maturity at about 2 years of age and females have a gestation period of around 7 weeks. Youngs are generally born in late spring and in a study conducted in India, clusters are found to be sexually segregated when the pups are born in early May[1] . Typical of most bats, R. lepidus is generally long-lived (6-7 years) and give birth to a single pup each time.Significance
Ecological role
|
|
Rhinolophus lepidus serves the role of both predator and prey in the ecosystem. Some of the common natural predators of bats include bat hawk and vipers. These predators usually hunt for the bats during their emergence period or when they are returning to their roost[10] . Specific predators of R. lepidus and bats in general are not well documented and literature are not informative on that matter in Singapore context. However it can be assume the common predators of other species of bats prey on R. lepidus as well, such as the sighting of Crested Goshawks (link to Singapore bird group) preying on fruits bats by nature photographers in Singapore.
Every night, insectivorus bats such as R. lepidus, can consume large amount of flying insects, keeping insect population in check. Bats play an important pest control role in agriculture. Their nightly feeding frenzy helps to control the population of pest insects on agriculture crops, saving the industry across the world billions of dollars[10] .
Emerging diseases and Disease reservoir
Many animals serve as natural reservoirs for various viruses, including wild ungulates (cows and horse)[16] , rodents[17] and birds[18] [19] . Bats, both Megachiropteran and Microchiropteran, play host to a wide variety of viruses and diseases (natural reservoir) as well. Due to their unique immune system, they rarely succumb to diseases and thus are able to carry and host the disease vectors for long periods of time [20]. Fortunately, such diseases rarely spread to humans because bats are rarely in contact with humans[21] .
However, with the rise of globalization, deforestation, and increased human-wildlife interaction, many previously dormant and highly pathogenic diseases were introduced into human society due to human encroachment into wildlife territory and the bridging of the natural barrier[22] [23] [24] . Both the Nipah virus outbreak in 1999 in Malaysia and Singapore[25] [26] and the SARS-Cov outbreak in 2003[27] [28] , involved bats as one of the potential reservoir hosts[20] in the transmission pathway of the disease, from wildlife to humans. The Chinese Horseshoe bat (Rhinolophus sinicus) were found to be natural reservoirs of SARS-CoV[29] [30] and Nipah virus was transmitted from infected pigs to humans with the flying foxes acting as reservoirs for the virus[24] .
A hypothesis on how transmission to humans occurs involves vectors (something akin to a middleman). Some of these vectors could be arthropods such as mosquitoes and midges which feed on both the blood of bats and humans; mammals such as pigs, dogs and horses[31] [32] that could possibly come into contact with the body fluids (urine, saliva, excretion) of bats with the viruses. Other hypothesis includes direct contact such as consumption of bush meat or accidental bite from handling and aerosols transmission in highly populated bat caves[21] .
Much research has been done and is ongoing with regards to the pathogenicity (ability to infect) of bat-borne virus to humans[21] . Aside from that, it is important to focus on other underlying causes of emerging diseases, such as the encroachment of human activities into previously uninhabited habitats (including bat roosts). All possible factors associated with the emergence of such infectious disease should be emphasised and evaluated so that bats do not become the sole target for persecution and extermination[33] .
Threats
Although bats in general have been hunted for bush meat in various parts of the world, the main threat to R. lepidus populations is likely to be impact from tourism. Clearing of forest and converting natural bat habitat into tourism attractions (caves, karst structures) reduces the number of available roost and foraging grounds. In certain areas, bats may be forced to form smaller colonies due to lack of available roost, which might limit and impede their reproduction . Many bat species, including R. lepidus were caught and mounted into a glass box to be sold as a tourism merchandise.Description and morphology
For further identification needs, here is a morphological key for the bats in Southeast Asia.| Illustration of the general anatomy of the insectivorous bat. Image taken from Boonsong and McNeely, 1988, Mammals of Thailand. |
|
General descriptions
|
||||||
|
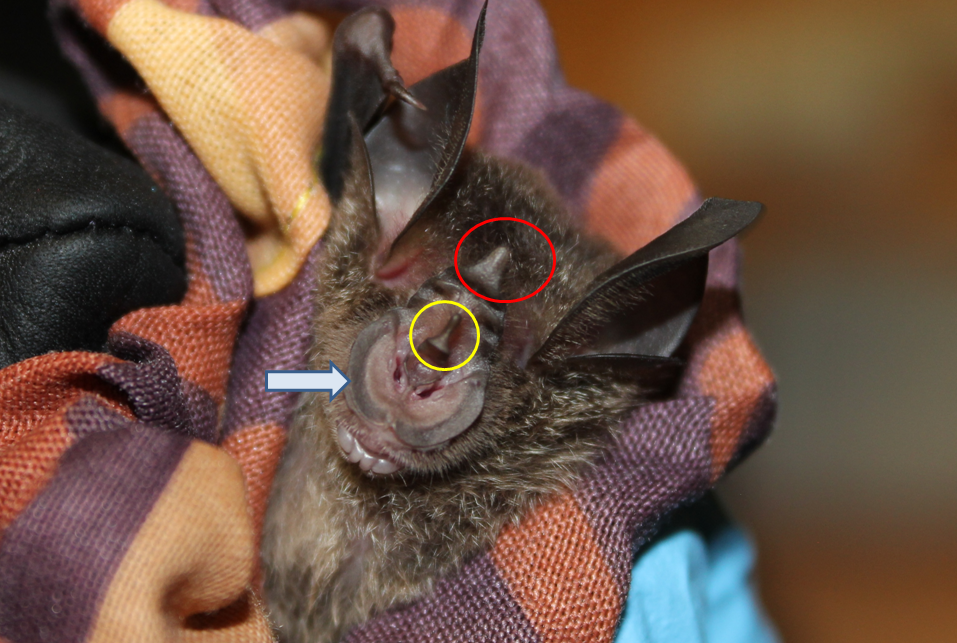
Nasal structure
|
||||||
|
Skull and Dental structure
|
Taxonomy and Phylogenetics
| Kingdom |
Animalia |
| Phylum |
Chordata |
| Class |
Mammalia |
| Order |
Chiroptera |
| Suborder |
Yinchiroptera |
| Family |
Rhinolophidae |
| Genus |
Rhinolophus |
| Species |
Rhinolophus lepidus |
Recent advances in molecular technology and techniques have allowed researchers to better understand the phylogenetics of the various bat taxa. This has resulted in better clarity of the relationships among the various taxa as well as resolving conflicts in past studies, in which higher classification of Chiroptera were complicated by the use of different data set across studies. Molecular techniques used in recent studies have also uncovered important evidences to suggest that many traditionally categorized groups are no longer monophyletic[35] .
The Family Rhinolophidae and genus Rhinolophus
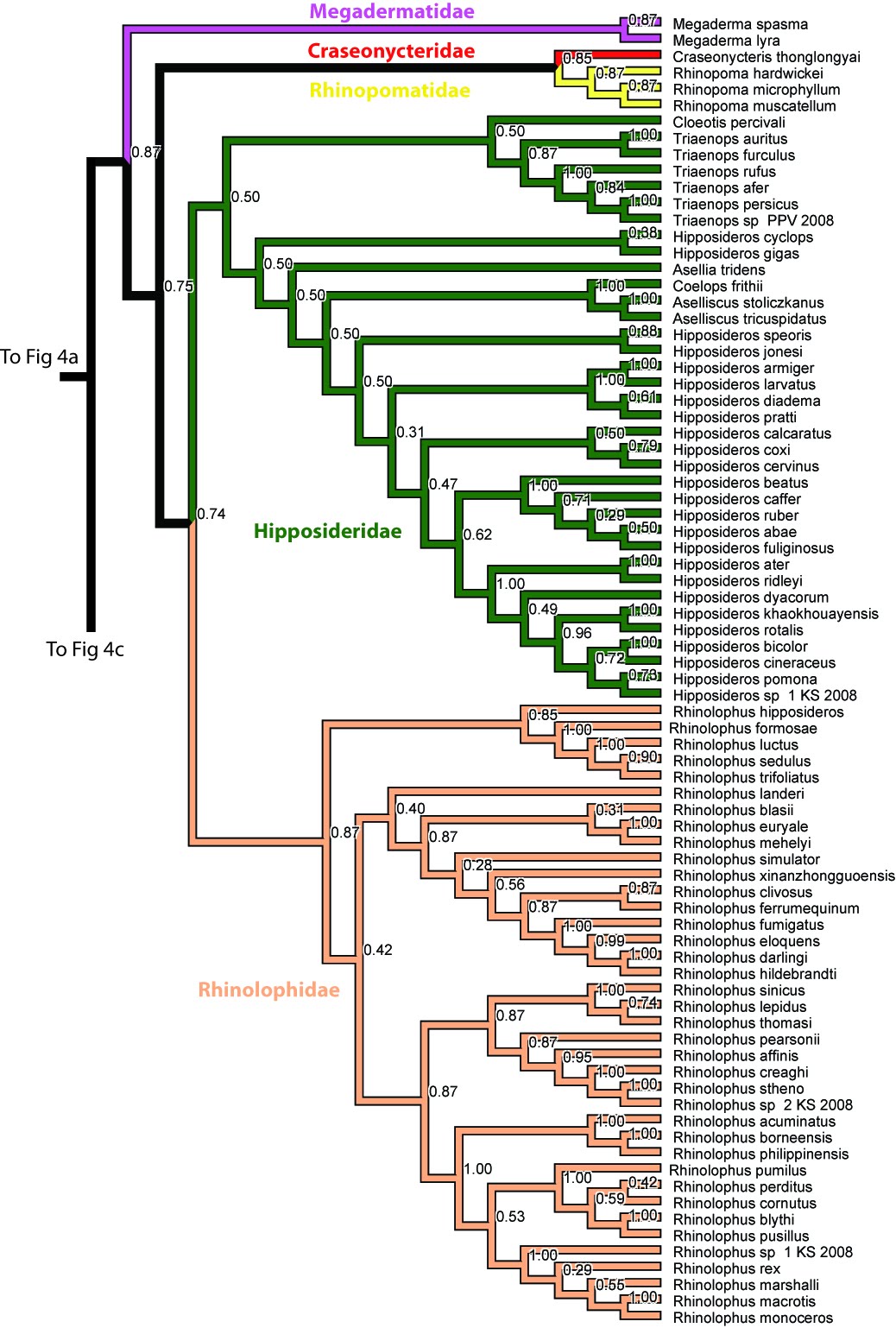
In a recent study conducted by Teeling et al. in 2000[36] , the authors challenged the concept of microbat monophyly. Their study conducted a phylogenetic analysis of bat relationships with the use of four nuclear genes and three mitochondrial genes. From the results, the authors proposed that the superfamily Rhinolophoidea (in which Rhinolophidae is part of) are closer in relationship to the megabats than to other microbats. This subsequently implied that complex echolocation in bats could have evolved twice, independently in the rhinolophids and other microbats or echolocation was lost during the evolution of megabats. A link to the article can be found here.
|
|
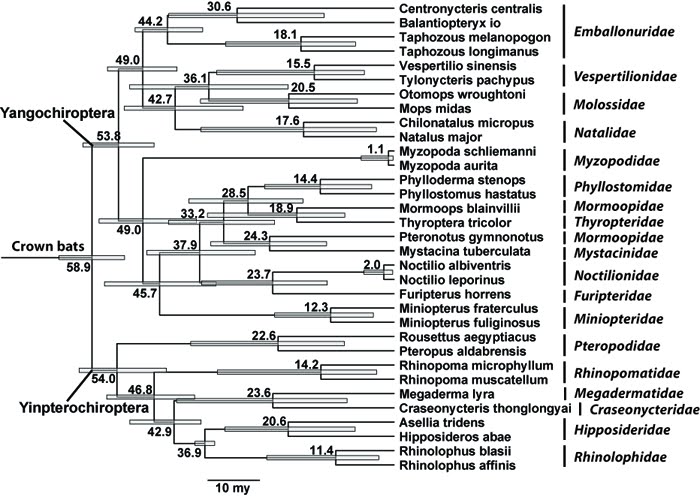
The divergence analysis shows that the Rhinolophidae diverged with the Hipposideridae 36.9 million years ago (m.y.a), which is supported and estimated from the age of their fossil[41] . The divergence time analysis shows the split of the Oriental clade (representative Rhinolophus affinis) and African clade (representative Rhinolophus blasii) to be roughly 11.4 m.y.a.
Rhinolophus Lacépéde, 1799, is a taxon exclusive to the Old world[42] . They are distinguished from other clades due to the fusion of various bones (manubrium, first 2 ribs, last cervical and 2 thoracic vertebrae) to form a solid pectoral ring which allow them to echolocate without moving. The phylogenetics are rather poorly resolved with many deviance in the trees with different techniques used.
|
|
The species Rhinolophus lepidus
Rhinolophus lepidus was first described by Blyth in 1844 (Blyth, 1844). The type specimen used by Blyth was obtained from India, Bengal, Calcutta. A holotype is currently stored at Natural History Museum London.Holotype for Rhinolophus lepidus
GBIF ID: 1056419048
Catalog no.: 1879.11.21.151
Other catalog no.: NHMUK (ecatalogue: 4284943)
Sex/stage: Male Adult
Properties: Both wet and dry (skull) samples
Locality: India
| Subspecies |
Rhinolophus lepdius cuneatus K. Andersen, 1918 Rhinolophus lepidus feae K. Andersen, 1907 Rhinolophus lepidus monticola K. Andersen, 1905 Rhinolophus lepidus refulgens K. Andersen, 1905 |
There have been many studies on the limit and recognition of the subspecies. Rhinolophus shortridgei was initially considered and published as a subspecies as Rhinolophus lepidus shortridgei based on traits such as skull, mandible and hind foot length. However upon investigation of the type skull along with specimens from the other subspecies, the naming was resolved and R. shortridgei is considered a full species[35] .
Etymology
Glossary
- Noseleaf - A form of complex nasal structure present in bats from Rhinolophidae, Phyllostomidae and Megadermatidae
- Echolocation - A method for animals to recognize objects in their environment by emitting calls and listening to the echos
- Pelage - Fur, hair or wool of a mammal
- Tragus - A small pointed protuberance on the external ear, usually partially covering the ear canal
- Aerial hawking - A form of hunting by aerial predators, prey pursued and caught in flight
- Paleotropical - A zoogeographical region comprising of Africa, tropical Asia, New Guinea and many Pacific island
- Sonogram - A graph representing a sound, showing the distribution of energy at different frequencies
- Megachiroptera - Suborder of Chiroptera, consist of the fruit bats in general
- Microchiroptera - Suborder of Chiroptera, consist of the echolocating bats
- Insectivorous - A diet consisting mainly of insects and invertebrates
- Frugivorous - A diet consisting mainly of fruits or preferred to fruits
- Fidelity - Adherence
- Gestation - Period of development inside the womb between conception and birth
- Emerging diseases - Infectious diseases that has appeared in a population for the first time or previously existed but is rapidly spreading in cases or geographical range.
- Disease reservoirs - Long term host of a pathogen of an infectious disease
- Karst structures - structures formed from the dissolution of soluble rocks such as limestone. Characterized by sink-holes and caves
- Bush meat - wildmeat, meat from non-domesticated animals
Resources
Morphological keys/ Identification guides for bats- A key to the bats of South Asia
- Field guide to Amazonian Bats
- The Biology of bats by Gerhard Neuweiler, 2000
- Bats of Krau Wildlife Reserve by Tigga et al., 2006
- Mammals of Thailand by Boonsong and McNeely, 1988
- Ecology, evolution, and behaviour of bats: The proceedings of a symposium held by the Zoological Society of London and the Mammal Society: London, 26th and 27th November 1993
Literature
- ^ Boonsong, L., & McNeely, J. A. (1988). Mammals of Thailand. Saha Karn Bhaet.
- ^ Wilson, D. E., & Reeder, D. M. (Eds.). (2005). Mammal species of the world: a taxonomic and geographic reference (Vol. 1). JHU Press.
- ^ Simmons, J. A., Fenton, M. B., & O'Farrell, M. J. (1979). Echolocation and pursuit of prey by bats. Science, 203(4375), 16-21.
- ^
Neuweiler, G. (2000). The biology of bats. Oxford University Press on Demand.
Denzinger, A., Kalko, E. K., & Jones, G. (2004). Ecological and evolutionary aspects of echolocation in bats. Echolocation in bats and dolphins, 311-326.
Schnitzler, H. U., & Kalko, E. K. (2001). Echolocation by Insect-Eating Bats We define four distinct functional groups of bats and find differences in signal structure that correlate with the typical echolocation tasks faced by each group. Bioscience, 51(7), 557-569.
Greif, S., & Siemers, B. M. (2010). Innate recognition of water bodies in echolocating bats. Nature communications, 1, 107.
Shi, L. M., Feng, J., Liu, Y., Ye, G. X., & Zhu, X. (2009). Is food resource partitioning responsible for deviation of echolocation call frequencies from allometry in Rhinolophus macrotis?. Acta Theriologica, 54(4), 371-382.
Rydell, J., Skals, N., Surlykke, A., & Svensson, M. (1997). Hearing and bat defence in geometrid winter moths. Proceedings of the Royal Society of London B: Biological Sciences, 264(1378), 83-88.
Struebig, M. J., Kingston, T., Zubaid, A., Le Comber, S. C., Mohd-Adnan, A., Turner, A., ... & Rossiter, S. J. (2009). Conservation importance of limestone karst outcrops for Palaeotropical bats in a fragmented landscape. Biological Conservation, 142(10), 2089-2096.
Gortázar, C., Torres, M. J., Vicente, J., Acevedo, P., Reglero, M., De la Fuente, J., ... & Aznar-Martín, J. (2008). Bovine tuberculosis in Donana Biosphere Reserve: the role of wild ungulates as disease reservoirs in the last Iberian lynx strongholds. PLoS One, 3(7), e2776.
Patz, J. A., Graczyk, T. K., Geller, N., & Vittor, A. Y. (2000). Effects of environmental change on emerging parasitic diseases. International journal for parasitology, 30(12), 1395-1405.
Guan, Y., Zheng, B. J., He, Y. Q., Liu, X. L., Zhuang, Z. X., Cheung, C. L., ... & Butt, K. M. (2003). Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science, 302(5643), 276-278.
Melaun, C., Werblow, A., Busch, M. W., Liston, A., & Klimpel, S. (2014). Bats as potential reservoir hosts for vector-borne diseases. In Bats (Chiroptera) as Vectors of Diseases and Parasites(pp. 25-61). Springer Berlin Heidelberg.
Wibbelt, G., Moore, M. S., Schountz, T., & Voigt, C. C. (2010). Emerging diseases in Chiroptera: why bats?. Biology letters, rsbl20100267.
Jones, K. E., Purvis, A., Maclarnon, A. N. N., BININDA‐EMONDS, O. R., & Simmons, N. B. (2002). A phylogenetic supertree of the bats (Mammalia: Chiroptera). Biological Reviews, 77(2), 223-259.
Stoffberg, S., Jacobs, D. S., Mackie, I. J., & Matthee, C. A. (2010). Molecular phylogenetics and historical biogeography of Rhinolophus bats. Molecular Phylogenetics and Evolution, 54(1), 1-9.