Table of Contents

Creative Commons Attribution-Noncommercial 3.0 Unported License.
Overview
The spotted scat (Scatophagus argus) is a native fish of Singapore that could be found in both brackish and freshwater environments, including monsoon drains [1]. It has an average total length of 200 mm [2]. It is an important food fish, and brackish water aquarium fish in many countries [3], including Singapore [4]. It is also known by the name “kim kor” in Cantonese, and “Ikan Ketang” in Malay. Scatophagus argus is also known to have been caught by anglers in the coastal waters of Singapore*.
As this fish has venomous spines, it should be noted of and avoided when one enters its habitats especially the mangroves, or attempts to handle similar-looking fishes. This species page thus seeks to provide a comprehensive identification guide (see description and diagnosis) of the spotted scat, and information on the venomous spines possessed by the fish.
*Spotted scats are reported to have been caught by anglers at the Woodland Jetty. See http://woodland-jetty.blogspot.sg/2012/09/spotted-scat-caught-at-woodland-jetty.html for an example.
Name
Scientific name: Scatophagus argus (Linnaeus, 1766)
Common names: Spotted scat, Butterfish, Ikan kitang (Malay), Kim kor (Cantonese)
Etymology
Scatophagus: means feeding upon dung:Scat= ‘dung’, from Greek stem skat
-phagus= ‘eating, feeding on’, from Latin -phagus, from Greek –phagos
argus: The hundred-eyed giant of Greek mythology. It’s probably so named due to the many spots on its body, resembling many eyes. [5]
Synonyms
(Referenced from IUCN)Chaetodon argus Linnaeus, 1766
Scatophagus argus argus (Linnaeus, 1766)
Ephippus argus (Linnaeus, 1766)
Chaetodon pairatalis Hamilton, 1822
Chaetodon atromaculatus Bennett, 1830
Scatophagus purpurascens Cuvier, 1831
Scatophagus ornatus Cuvier, 1831
Scatophagus bougainvillii Cuvier, 1831
Sargus maculatus Gronow, 1854
Scatophagus argus ocellata Klunzinger, 1880
Scatophagus quadratus De Vis, 1882
Scatophagus aetatevarians De Vis, 1884
Description
Description
Body: greenish colouration; quadrangular body shape; many dark spots on body
Size: up to 350 mm total length
Sexual dimorphism: slight; differ in head profile and body colouration
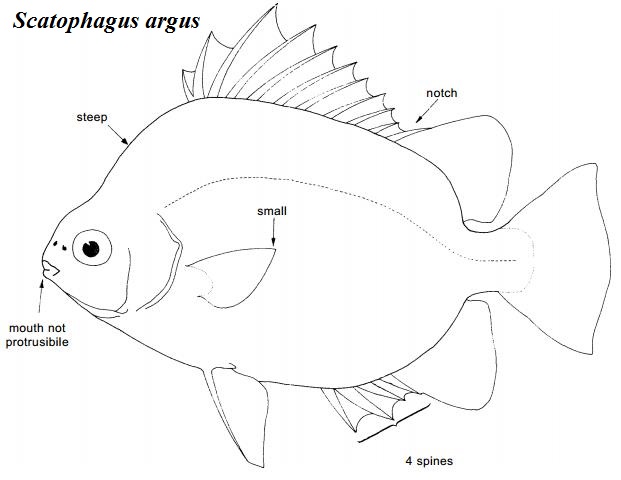
Generally, scats have a distinctly quadrangular and highly laterally compressed body which is usually greenish or silvery with dark spots or bars [6]. The figure below shows other descriptive characters of S. argus.
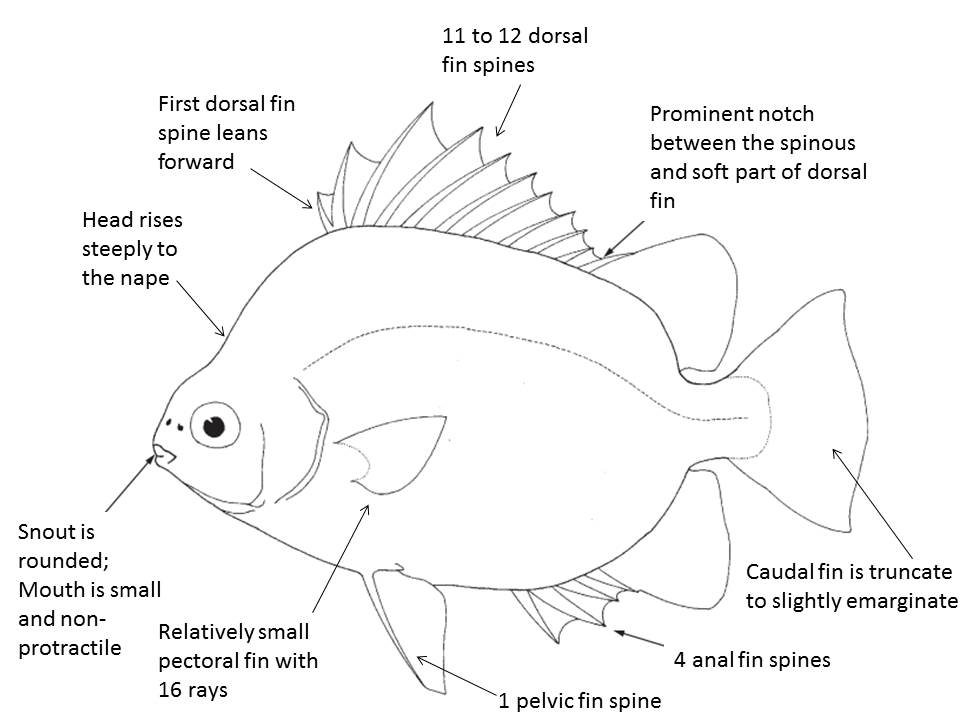
Figure 2. Diagnostic characteristics of the family Scatophagidae (adapted from Kottelat, 2001 [6] with additional labels added).
Size
The average total length of S. argus is about 200 mm [2], and they can grow up to a total length of 350 mm [6].Adults and Juveniles
In particular, adults S. argus have dark spots that could be faded, and more concentrated at the dorsal side of the body [6](figure 3). Juveniles resemble the adults but have dark spots about the same diameter as the eyes (figure 4, right) or have 5 – 6 dark and broad vertical bars along their body [6] (figure 4, center).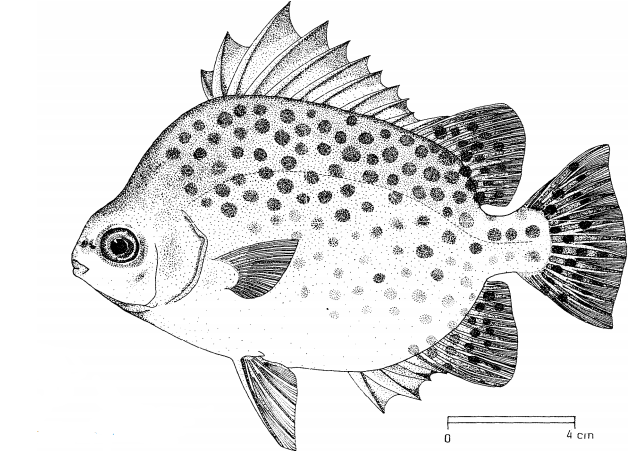
Figure 3. Illustration of S. argus in the adult stage (Image from FAO in De Bruin et al., 1995) [35].
Scatophagus argus go through a tholichthys postlarval stage [6]. The tholichthys larval stage is unique to fishes in the family Chaetodontidae (butterflyfishes) and Scatophagidae, and is characterized by having large body plates that enclose the body, particularly the head, forming a hard protective covering [7]. These larvae are small, ranging between a total length of 6mm to 12mm. These protective plates disappear as the larva develops into a juvenile [7].
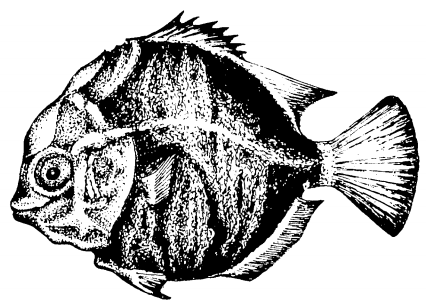

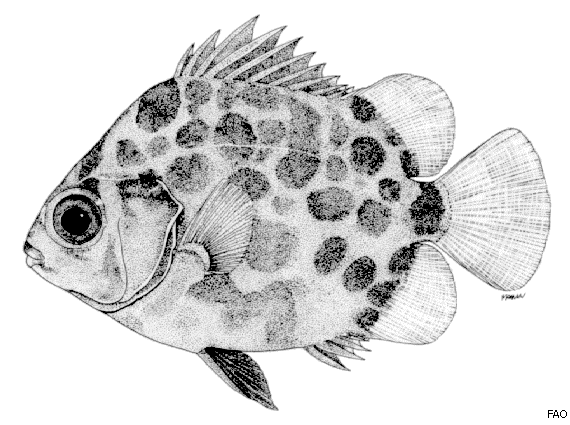
Figure 4. Left: An illustration of a tholichthys larva of S. argus of 12mm. Bony plates encloses the body and forms a protective cover around the head of the larva (Image by and used with permission from Barry & Fast, 1988[7]; Pending permission from journal). Center: Illustration of juvenile S. argus of 17.1 mm standard length (Image by FAO in Kottelat, 2001 [6]). Right: Illustration of juvenile S. argus of 30.2mm standard length (Image by FAO in Kottelat, 2001[6]).
Sex determination
Scatophagus argus exhibit slight sexual dimorphism. Males and females can be distinguished by their head profile [7]. In females, the head profile ascends in a gentler slope, whereas males have an abrupt steep sloping of the head above the eyes. In addition, females are of a lighter colour than males [8].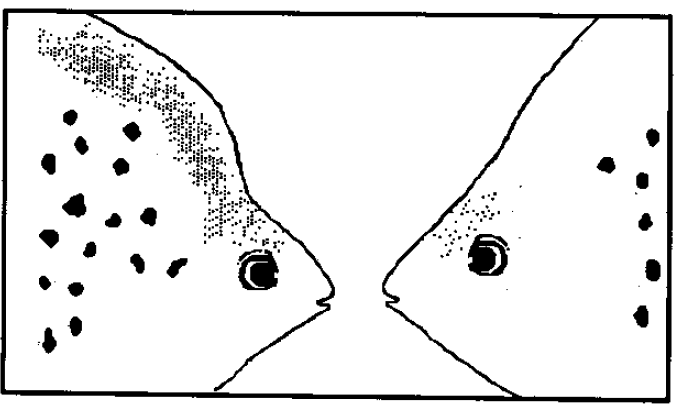
Figure 5. The head profile of male (left) and female (right) S. argus (Image by and used with permission from Barry & fast, 1992 [8]; Pending permission from journal).
Diagnosis
The family Scatophagidae consists of two genera, Scatophagus and Selenotoca [9].
Genus Scatophagus
Unlike Selenotoca, the gill membrane of Scatophagus forms a free fold that covers the isthmus (or throat area of fish) as depicted in figure 6a below [6].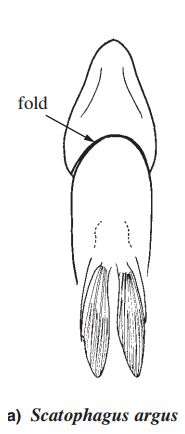
Figure 6. Ventral view of a) Scatophagus argus and b) and a Selenotoca species, Selenotoca multifasciata. In Scatophagus, the gill membrane forms a fold that covers the isthmus but not in Selenotoca (Image from FAO in Kottelat, 2001 [6]).
Species Scatophagus argus
The genus Scatophagus consists of only two species [9], the other being S. tetracanthus (Lacepède, 1802).Scatophagus argus can mainly be distinguished from S. tetracanthus by having a greenish silver coloured body with dark round spots on its upper part of its body [6] while S. tetracanthus has a yellow body with seven vertical dark bars across its body [10].

Figure 7. Left: Scatophagus argus has greenish silver body with dark spots on its body whereas, right: S. tetracanthus has a yellow body with seven dark vertical bars. (Image of S. argus by John E. Randall, obtained from Fishbase. Creative Commons Attribution-Noncommercial 3.0 Unported License; Image of S. tetracanthus by Liliane Moeremans, used with permission and obtained from Fishbase)
Similar-looking coastal fishes


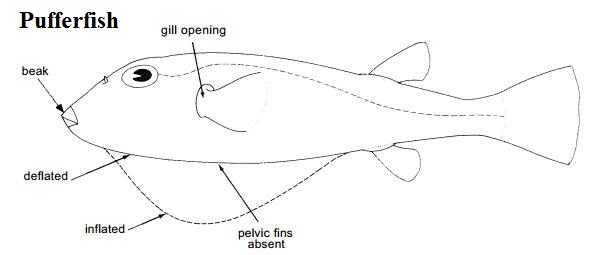
Figure 8. Both of these fishes can be found in the coastal areas of Singapore. On first look, both S. argus (left) and the spotted green pufferfish (right) may look alike (photos by and used with permission from Ria Tan).
Other native fishes, the spotted green pufferfish (Tetraodon nigroviridis) and Orange-spotted rabbitfish (Siganus guttatus) resemble S. argus superficially, and co-occur with S. argus in coastal waters of Singapore. Thus, these three fishes could be easily misidentified.
Nevertheless, it is important that handling of all three fishes with hands are avoided as they are all venomous- rabbitfishes and S. argus have venomous spines [11]while the pufferfish may excrete tetrodoxins through their skin which could be readily absorbed through our skin [33].
The spotted green pufferfish co-occurs with S. argus in the mangrove areas of Singapore [36] while the orange-spotted rabbitfish are more commonly found in the coastal areas further from the shore, where seagrass and corals are [11].
It seems that both the orange-spotted rabbitfish and S. argus could be fished from the same coastal area as accounted by an angler in a blog. Along with other species of rabbitfishes, the orange-spotted rabbitfish, like S. argus, are also sold in markets whole and eaten during the Chinese Lunar New Year period in Singapore (Kwik, 2014, pers comms).
The table below shows how the spotted green pufferfish and rabbitfish can be distinguished from S. argus by comparing their body shape, body colour, features of jaws, and dorsal fin.
Table 1. Table of comparison of features between S. argus, spotted green pufferfish [12; 13] and rabbitfish [14; 11).
Image Sahat Ratmuangkhwang from Fishbase |
 Image by Kuang Hsiang Liu. Creative Commons Attribution NonCommercial license. Obtained from Fishbase. |
Image by Sahat Ratmuangkhwang, obtained from Fishbase |
|
| Features |
S. argus |
Spotted green pufferfish |
Orange-spotted rabbitfish |
| Body shape |
Laterally flattened and quadrangular |
Blunt and round |
Laterally flattened, oval-shaped, deep-bodied |
| Body colour |
Greenish silver body with dark spots |
Bright green body and white belly; with dark spots |
Bluish colour at upper body, silvery below. Orange spots on body, large golden spot below the end of dorsal fin |
| Jaws (see figures below) |
Jaws bear narrow finger-shaped teeth and hidden in mouth |
Jaws modified to form beaks |
Jaws bear slender, close-set teeth |
| Dorsal fin (see figures below) |
Long and bears spines |
Short, bears no spine and located posteriorly on body |
14 dorsal fin spine, first dorsal fin spine pointed forward |
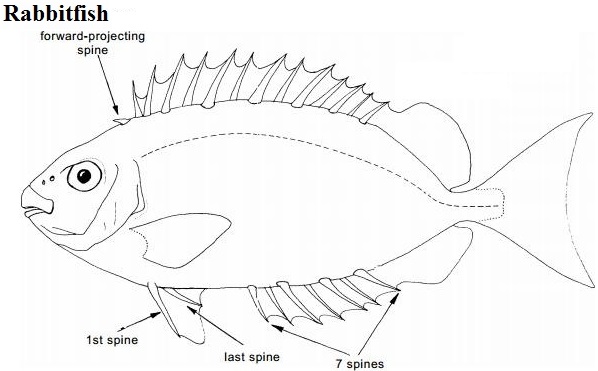
Native Distribution & Habitat
Distribution: From Indian ocean to Indo-West Pacific
Habitat: Freshwater, brackish
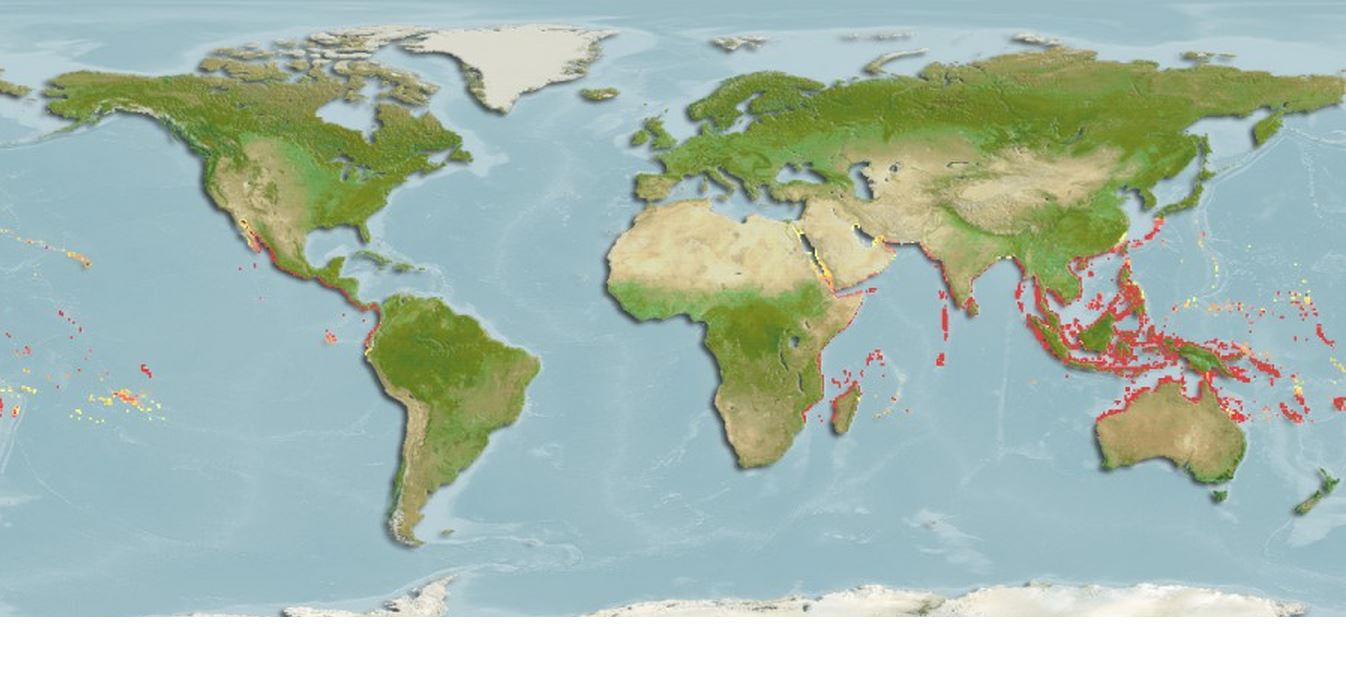
Scatophagus argus has a wide native range which spans across the whole coastal area of the Indian Ocean, and the tropical to warm temperate regions of Indo-West Pacific region, including Australia, Tahiti, and the Southern areas of China, and Taiwan [2, 6]. Scatophagus argus is the only Scatophagus species that occurs in the Southeast Asian region [9].
Scatophagus argus thrive in both freshwater and brackish waters, and are usually found between surface to a depth of 5m [15]. In Singapore, S. argus has been spotted along coastal areas, where mangroves and seagrass occur, as well as under jetties and near monsoon drains [4]. It has been caught or sighted along the northern (Johor Straits) and southern coast of Singapore.
Video 1. Scatophagus argus can be caught at the Woodlands Waterfront Jetty.
Listed below are examples of places in Singapore where S. argus was recorded.
At northern coast: Woodland Waterfront fishing jetty, Chek Jawa [4], Lim Chu Kang, Sungei Buloh Wetland Reserve, Pasir Ris, Pulau Seduku or Frog Island
At southern coast: Waters of Kallang, Changi, Tanah Merah
Inland: Lower Seletar Reservoir Yishun Dam fishing spots, Below Seletar North Link Bridge
Biology
Diet and feeding habits
Primary Diet: Omnivorous (mainly detritus and algae)
Scatophagus argus is usually gregarious, with adults forming smaller groups than juveniles (Kwik, 2014, pers. comms.). It is also known to feed actively during the day [2]. As mentioned in etymology, “Scatophagus argus” means dung-eater. It was so named as many were observed to feed on unwanted waste and offal discarded from ships at harbor [8]. However, its preference for dung matter remains unconfirmed [8]. Instead, its main diet appears to consists of detritus and algae [16]. Juvenile S. argus consumed a greater proportion of unicellular algae while adults consumed more multicellular algae, and a greater diversity of food items such as rotifers and sea anemones [16].
Scatophagus argus appear to be non-choosy feeders and would consume any food available though there might be a preference for filamentous algae in larger fishes [7,16]. Other animal matter such as copepods, fish scales and fish eggs have also been found in the guts of S. argus [16].
Barry & Fast [7] suggest that S. argus is primarily herbivorous and adapted to feed on plant material given its long gut length relative to body length and the presence of “small, sharp teeth used for scrapping and shredding plant material”.
Reproduction
Reproduction: Dioecious (having distinct male and female individuals); Oviparous; mature at one year of age; eggs can hatch within a day under certain conditions
Scatophagus argus is dioecious (having distinct male and female individuals) and is able to reproduce at 1 year of age [17]. It is estimated that males reach the age of sexual maturity at 115 mm standard length, weighing about 83.5g, while females, at 140 mm standard length and 150g [8].
The reproductive behavior of S. argus in the wild does not seem to have been documented thus far. Barry & Fast [7], however, observed the mating behavior of S. argus in captivity: Males, when placed in a tank with a female, appear to be aggressive towards each other and attempt to guard the female. The more aggressive male was observed to chase and attack the submissive male which held its dorsal fin erect most of the time. Nudging of the female’s abdomen by the domineering male was seen as well. Both male and female were observed to undulate their bodies in rhythm while holding each other by the lip. This behaviour led to wounded upper lips in both partners. Unfortunately, the spawning behaviour was not observed during the study.
In the life cycle of S. argus, it appears that both adults and their offprings have different salinity requirements- the offsprings live in brackish water [7] while adults have to spawn in waters of higher salinity [18].
The spawning season of S. argus likely correspond to that of the rainy season [7]. A female has an average absolute fecundity (number of eggs laid in a spawning season) of 456,320 eggs [17]. It is vital that spawning occurs in high salinity, such as 25‰ [17], with fertilised eggs that stay afloat in seawater [18]. The eggs have an average diameter of 0.75mm [17] and are round and transparent [7]. In fertilised eggs, oil droplets merge together, conferring buoyancy to the eggs [18] while this does not occur in unfertilised eggs which turn opaque and sinks [7].
With a temperature of 27—29 ℃ and salinity 25‰, fertilized eggs require about 20 to 24 hours to hatch and larvae measures between 1.75 to 1.88 mm in length [17]. First feeding begins after 3 to 4 days when the larvae’s yolk sac disappears [7,17]. The larvae then undergo a tholichthys larval stage [7] (see figure 4).
Tolerance to salinity changes
Scatophagus argus being euryhaline (able to thrive in both salt and freshwater) has been documented to be able to withstand sudden salinity changes between 0‰ to 30‰ [19].Venomous fin spine
Symptoms: pain, redness, swelling, throbbing sensation, dizziness
Treatment: treat wound with hot water, medical treatment is recommended
Nature of fin spine
Like all other Scatophagids, S. argus possess venomous fin spines for defence purposes [7] but the pain caused by S. argus is alleged to be more painful [20]. These spines are not used as offense but inflict painful wounds when the fish is handled carelessly- some fishermen who are oblivious of the venomous spines have been wounded by them [21]. Fortunately, the protein-based venom present in the spines is seldom life-threatening [3].The venomous spines of S. argus is well-described by Cameron & Endean [22]. All fin spines of S. argus contain venom glands- the 11 dorsal fin spines, 2 pelvic fin spines, and 4 anal fin spines. Each spine is sharp and pointed, and consists of two lateral grooves that contain venom gland cells. Smaller fishes were found to have relatively longer glands compared to larger fishes. However, the longest gland found in any of the specimens studied did not correspond to a particular fin spine, that is, the longest venom gland can occur in any of the fin spines in S.argus.
In the event that the spine punctures the skin, it erects and pressure exerted on the spine causes the venom to be released into the wound [21].
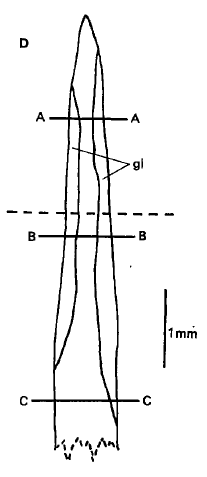
Venom's biochemical nature
In a study done by Sivan et al. [21] on the biochemical nature of the venom of S. argus, it was found that the venom causes haemolysis (lysis of red blood cells) in humans. In addition, when tested on mice, the venom appears to be “cytolytic, oedematic, nociceptive, proteolytic and myotoxic”, leading to tissue damage and pain.Symptoms
The effects of the venom could be felt within 5 to 10 minutes. In addition to unbearable localized pain at the wound that can lasts for an hour (Ibister, 2004), other symptoms include “redness, swelling and a throbbing sensation that extends to the limbs, followed by dizziness” [21]. The severity of the venom varies depending on the amount of venom injected, and the size of the fish [21].Treatment
The wound should be cleaned, and is typically treated by submerging it into hot water [21]. This suggests that wounds inflicted by S. argus are treated similarly as like any other venomous fish stings [23]. It is important to sought medical treatment in case there is any likely infection of the wound and if the pain does not subside [23].Uses
Uses: Popular aquarium fish; Food fish
Aquarium fish
Scatophagus argus is a popular aquarium fish sold in many countries including Singapore [24]. Juveniles are usually caught from the wild for aquariums fish trade [6].Video 2. Scatophagus argus in (left) freshwater aquarium and (right) brackish aquarium with the spotted green pufferfish and archer fish as tankmates (Video obtained from Youtube under fair use).
Food fish
Scatophagus argus is also sold fresh or salted in markets as food in the Indo-Pacific islands and Southeast Asian region in countries such as Cambodia [25]. In certain countries, S. argus is of minor commercial importance and is not frequently sold in markets [6] while in countries, such as the Philippines, it is highly priced and considered a delicacy [7].In Singapore, S. argus is sold in markets, such as those in Chinatown, particularly during the Chinese Lunar New Year. This is because the spawning period of S. argus happens to fall in the early months of the year, and when these fishes aggregate to spawn and females carry riped eggs, they are highly sought-after as food fish (Kwik, 2014, pers comms).
Taxonavigation
(referenced from Integrated Taxonomic Information System (ITIS))
Note: Each subsequent taxon is a subset of the one before it.
Animalia
Bilateria
Deuterostomia
Chordata
Vertebrata
Gnathostoma
Osteichthyes
Actinopterygii
Neopterygii
Teleostei
Acanthopterygii
Perciformes
Acanthuroidei
Scatophagidae*
Scatophagus Cuvier in Cuvier and Valenciennes, 1831
Scatophagus argus (Linnaeus, 1766)
* It must be noted that Scatophagidae in Pisces is not a junior homonym of Scatophagidae in Diptera. The spelling of “Scatophagidae” in Diptera is a misspelling of the correct term, “Scathophagidae”, which is based on the genus Scathophaga Meigen, 1803: 277 [30].
Phylogeny
Phylogeny: Yet to be resolved within Scatophagidae; Scatophagidae is likely to be closely related to Chaetodontidae and Pomacanthidae
Currently, it seems that the phylogenetic relationships within the suborder Acanthuroidei have yet to be resolved, with different families proposed to be the sister family of Scatophagidae [26, 27, 28].
Thus far, however, it seems that the Chaetodontidae (butterflyfishes) is closely related to Scatophagidae (27, 28).
According to the study done by Fessler & Westneat [28], Scatophagidae appears to be the sister group to Pomacanthidae (marine angelfish), and that this sister-family pair (Scatophagidae and Pomacanthidae) is the clade most closely related to Chaetodontidae (butterflyfishes) (see figure 9 below).
Within Scatophagidae, however, the phylogenetics has yet to be studied [29].
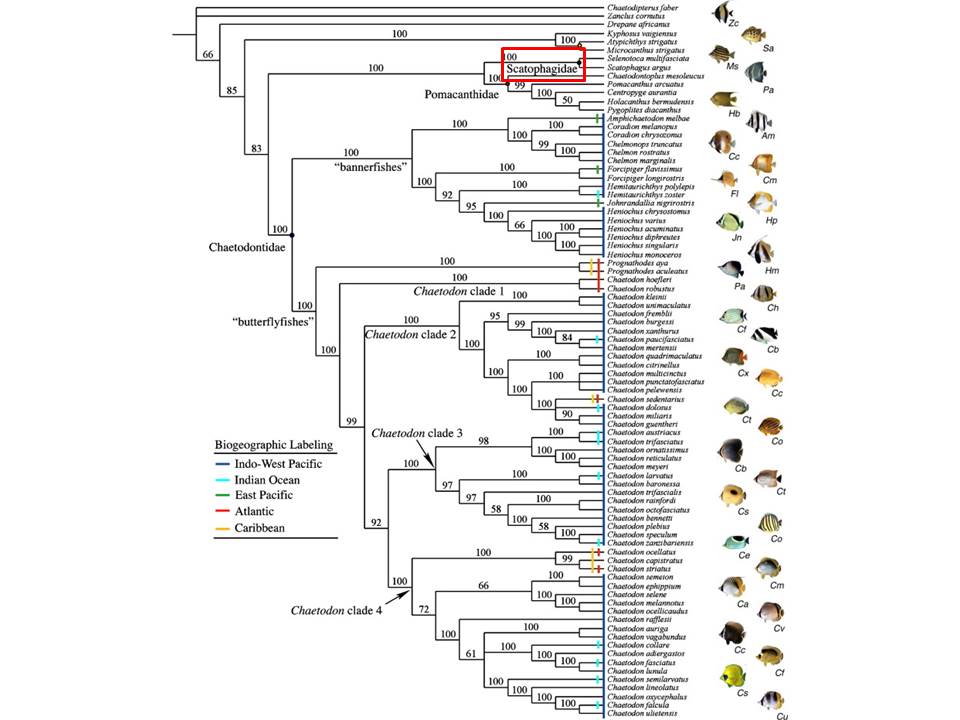
Figure 13. Phylogenetic tree derived for Chaetodontidae by Fessler & Westneat [28] through Bayesian analysis of mtDNA (12S, 16S, and ND3) and nuclear DNA (RAG2, and Tmo-4c4). It could be seen that the sister-family clade of Scatophagidae and Pomacanthidae is sister to the Chaetodontidae. Scatophagidae is boxed in red. (Figure adapted and used with permission from authors Fessler & Westneat, 2007; Permission Pending from journal).
Original description and Type information
The type locality of S. argus is India [30].
Scatophagus argus was first named and first described as Chaetodon argus by Linnaeus in 1766.
The original description of S. argus by Linnaeus in 1766 as Chaetodon argus was made based on a information and/or drawing given to him. According to Kottelat [30], the description was done based on “information and/or drawing of a specimen in Schlosser's collection communicated by Brünnich (Boddaert, 1770: 16–17) [31]. This specimen is described in detail and figured by Boddaerts (1770)”. It is likely that the holotype does exist however, its exact location is unknown [30].
The original description (figure below) of S. argus as Chaetodon argus by Linnaeus can be found on page 464 of his book,
Linnaeus, C. (1766). Systema naturae sive regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Laurentii Salvii, Holmiae. 12th ed. v. 1 (pt 1): 1-532.

Conservation status and threats
Conservation status: Least concern (IUCN Red List of Threatened Species)
Scatophagus argus does not seem to face any risk of extinction. It is not threatened nor endangered in Singapore [4] and is also listed as “Least Concern” on IUCN Red List of Threatened Species [32].
However, potential localized threats might exist given that S. argus occur in coastal areas, and is thus subjected to threats such as pollution in estuarine areas, and coastal habitat loss through development activities such as land reclamation (32, 4). In Singapore, coastal developments and over-fishing may affect local populations [4].
Acknowledgement
I would like to express my deepest appreciation to all those who provided me the possibility to complete this species page:
Professor Rudolph Meier for his guidance on how a proper species page should be done, and for the opportunity to create a species page for a Singaporean species that would contribute to the growing knowledge of wildlife in Singapore.
The many kind professors who granted me permission to re-use the images of their published papers.
Ms Lee Bee Yan for her guidance on taxonomy and phylogenetics, and Mr Low Bi Wei and Dr Jeffrey Kwik for sharing information on the spotted scat.
Personal Communications
Kwik, Jeffrey Teik Being, Post-doctoral Research Fellow, Freshwater and Invasion Biology Lab, Department of Biological Sciences, National University of Singapore
References
1) Tan, L. W. H. & P. K. L. Ng, 1988. A Guide to Seashore Life. Singapore Science Centre, 160pp.2) Allen, G.R., 1984. Scatophagidae. In: Fischer, W. & G. Bianchi (eds.), FAO species identification sheets for fishery purposes. Western Indian Ocean (Fishing Area 51). volume 4. Food and Agriculture Organisation, Rome. 4 pp.
3) Sivan, G & C. Radhakrishnan, 2007. Studies on Venom of the fish Scatophagus argus from Cochin estuary- a biochemical approach, PhD Dissertation, Cochin University of Science and Technology.
4) Tan, R., 2013a. Spotted scat, Scatophagus argus. Family Scatophagidae. Wild Factsheet, Wild Singapore. URL: http://www.wildsingapore.com/wildfacts/vertebrates/fish/scatophagidae/argus.htm (accessed on 1 November 2014).
5) Romero, P., 2002. An etymological dictionary of taxonomy. Madrid, unpublished. Accessible at: http://www.etymonline.com/index.php?allowed_in_frame=0&search=argus&searchmode=none (Accessed on September 2014)
6) Kottelat, M., 2001. Scatophagidae. Scats. In: K.E. Carpenter & V. Niem (eds.), FAO species identification guide for fishery purposes. The living marine resources of the Western Central Pacific. Vol. 6. Bony fishes part 4 (Labridae to Latimeriidae), estuarine crocodiles. Food and Agriculture Organisation, Rome. Pp. 3623-3626. [Link]
7) Barry, T. P. & Fast, A. W, 1988. Introductions. In: Fast, A. W (ed.), Spawning induction and pond culture of the spotted scat (Scatophagus argus Linnaeus) in the Philippines. Mariculture Research and Training Center, Hawaii Institute of Marine Biology, University of Hawaii at Manoa. Pp. 1-3.
8) Barry, T. P. & A. W. Fast., 1992. Biology of the spotted scat (Scatophagus argus) in the Philippines. Asian Fisheries Science, 5 (2): 163-179.
9) Parenti, P., 2004. Family Scatophagidae Bleeker 1876 — scats. California Academy of Science, Annotated Checklists of Fishes No. 36, 5 pp. URL: http://www.calacademy.org/sites/default/files/assets/docs/scatophagidae.pdf (Accessed on 8 November 2014).
10) Mostarda, E. & R. Anam, 2012. Field identification guide to the living marine resources of Kenya. Food and Agriculture Organization, Rome. 357 pp.
11) Tan, R., 2013b. Rabbitfishes, Siganidae. Wild Factsheet, Wild Singapore. URL: http://www.wildsingapore.com/wildfacts/vertebrates/fish/siganidae/siganidae.htm (accessed on 12 November 2014).
12) Shipp, R. L., 1978. Tetraodontidae. In: Fischer, W. (ed.), FAO species identification sheets for fishery purposes. West Atlantic (Fishing Area 31). volume 5. Food and Agriculture Organisation, Rome, Rome. Pp 1988- 1998.
13) Dekkers, W. J, 1975. Review of the Asiatic freshwater puffers of the genus Tetraodon Linnaeus, 1758 (Pisces, Tetraodontiformes, Tetraodontidae). Bijdragen tot de Dierkunde. 45 (1): 87-142.
14) Fischer, W. (ed.), 1978. FAO species identification sheets for fishery purposes. West Atlantic (Fishing Area 31). volume 5. Food and Agriculture Organisation, Rome, Rome. Pp 1988- 1998.
15) Allen, G.R. & M.V. Erdmann, 2012. Reef fishes of the East Indies. Tropical Reef Research, Perth, Australia. Volumes I-III. University of Hawai’i Press, Honolulu. 1292 pp.
16) Sivan, G. & C. Radhakrishnan, 2011. Food, feeding habits and biochemical composition of Scatophagus argus. Turkish Journal of Fisheries and Aquatic Sciences, 11: 603-608.
17) Cai, Z. P., Y. Wang, J. W. Hu, J. B. Zhang & Y. G. Lin, 2010. Reproductive biology of Scatophagus argus and artificial induction of Spawning. Journal of Tropical Oceanography, 29 (5): 180- 185.
18) Winfree, R. A., 1986. Progress in the population of valuable aquarium fishes: First recorded breeding of Scatophagus argus Presented at the 17th Annual meeting of World Mariculture Society, 17-23 January, Reno, NV, USA.
19) Ghaziloua, A., Chenarya, F., Morovvatib, H., Zolgarneinea, H., 2011. Time course of saltwater adaptation in spotted scat (Scatophagus argus) (Pisces): a histomorphometric approach. Italian Journal of Zoology. 78 (1): 82–89.
20) Marshall, T.C., 1964. Fish of the Great Barrier Reef and Coastal Waters of Queensland. Livingston publishing Co., Sydney. 566 pp.
21) Sivan, G, K.Venketesvaran & C. K. Radhakrishnan, 2007. Biological and biochemical properties of Scatophagus argus venom. Toxicon, 50 (4): 563–571.
22) Cameron, A. M. & R. Endean., 1977. Venom glands in Scatophagid fish. Toxicon, 8 (2): 171–178.
23) Isbister, G. K., 2004 Marine envenomation and poisoning. In: Dart, R. C. (ed.), Medical toxicology. 3rd ed. Williams & Wilkins, Lippincott. Pp. 1621- 1644.
24) Lim, K. K. & Low J. K., 1998. A guide to common marine fishes of Singapore. Singapore Science Centre, Singapore. 163 pp.
25) Rainboth, W.J., 1996. Fishes of the Cambodian Mekong. FAO Species Identification Field Guide for Fishery Purposes. Food and Agriculture Organisation, Rome. 265 pp.
26) Tyler, J.C., Johnson, G.D., Nakamura, I., Collette, B.B., 1989. Morphology of Luvarus imperialis (Luvaridae), with a phylogenetic analysis of the Acanthuroidei (Pisces). Smithsonian Contributions to Zoology, 485: 1– 78.
27) Bellwood, D.R. & L. van Herwerden & N. Konow, 2004. Evolution and biogeography of marine angelfishes (Pisces: Pomacanthidae). Molecular Phylogenetics and Evolution, 33 (1): 140–155.
28) Fessler, J. L. & M. W. Westneat, 2007. Molecular phylogenetics of the butterflyfishes (Chaetodontidae): Taxonomy and biogeography of a global coral reef fish family. Molecular Phylogenetics and Evolution, 45 (1): 50-68.
29) Chen, Jianhua, J. Chen, Y. Li, M. He, B. Yan, & X. Meng, 2013. Complete mitochondrial genome of the spotted scat Scatophagus argus (Teleostei, Scatophagidae). Mitochondrial DNA, Early Online: 1-2.
30) Kottelat, M., 2013. The fishes of the inland waters of Southeast Asia: a catalogue and core bibliography of the fishes known to occur in freshwaters, mangroves and estuaries. Raffles Bulletin of Zoology, Supplement 27: 1–663.
31) Boddaert, P., 1770. Epistola ad virum Johannem Burmannum […] de chaetodonte argo descripto atque accuratissima icone illustrato ex museo viri celeberrimi Johannis Alberti Schlosseri. Brief […] aan den weledelen hooggeleerden Heere Johannes Burmannus […] behelzende eene nauuwkeurige beschrijving, en naar het leven vervaardigde afbeelding, van den gevlakten klipvisch, uit de verzameling van wylen den weledelen zeer geleerden Heere Johannes Albertus Schlosser in zijn weled. Van Tongerlo, Amsterdam, 43 pp., pl. 2.
32) Collen, B., N. Richman, A. Beresford, A. Chenery & M. Ram (Sampled Red List Index Coordinating Team), 2010. Scatophagus argus. The IUCN Red List of Threatened Species. Version 2014.2. URL: http://www.iucnredlist.org/details/155268/0 . (Accessed on 8 November 2014).
33) Mahmud, Y., O. Arakawa, A. Ichinose, M. B. Tanu, T. Takatani, K. Tsuruda, K. Kawatsu, Y. Hamano & T. Noguchi, 2003. Intracellular visualization of tetrodotoxin (TTX) in the skin of a puffer Tetraodon nigroviridis by immunoenzymatic technique. Toxicon, 41(5): 605-611.
34) Computer Generated Native Distribution Map for Scatophagus argus (Spotted scat) (modelled future range map based on IPCC A2 emissions scenario). URL: www.aquamaps.org , version of Aug. 2013. Web. (Accessed 9 November 2014).
35) De Bruin, G.H.P., B.C. Russell & A. Bogusch, 1995. FAO species identification field guide for fishery purposes. The marine fishery resources of Sri Lanka. Food and Agriculture Organisation, Rome. 400 p.
36) Ng, P.K.L. & N. Sivasothi (eds.), 1999. A guide to the mangroves of Singapore I: The Ecosystem and plant diversity. Singapore Science Centre, Singapore. 160pp.
Meigen, J. W., 1803. Versuch einer neuen GattungsEintheilung der europäischen Zweiflügligen Insekten. Magazin für Insektenkunde (Illiger), 2: 259–286.