Table of Contents

Overview
The Broadclub cuttlefish is a rather common cephalopod that ranges from the eastern fringes of the Indian Ocean to Fiji in the Pacific (1). It is usually found along coastal areas, at depths up to 30 m, hunting for shrimps, prawns and small fishes during the day. Like their close relatives, Octopuses and Squids, cuttlefishes are famous for their ability to alter skin colour patterns for camouflage and intra-species communications (2). The cuttlefish forms an important fishery as it is popularly consumed by humans.Description
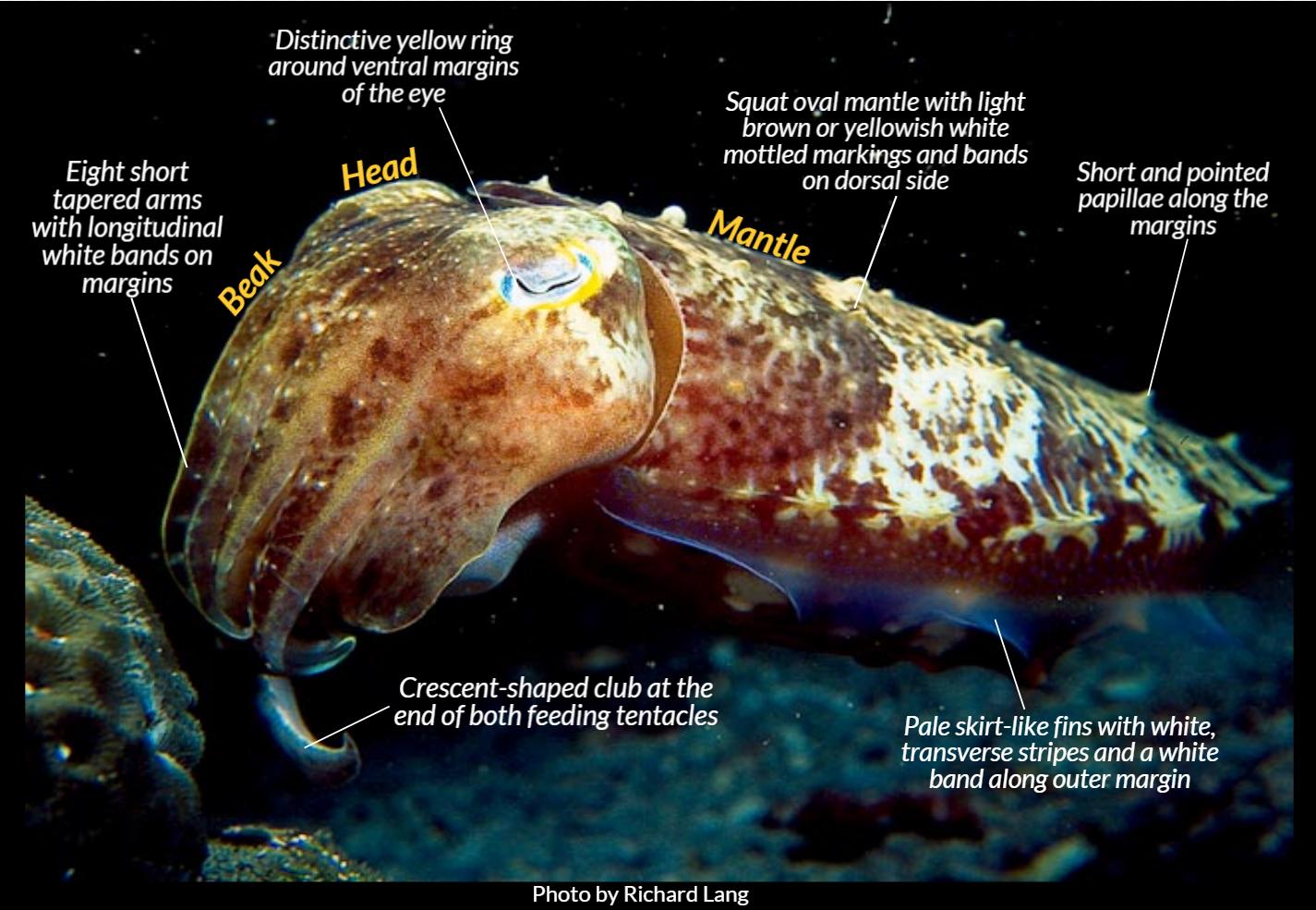
Its beak comprises 10 appendages (8 arms and 2 tentacles) surrounding its mouth (5). The short tapered arms have 2 to 4 suckers in transverse rows (5) and longitudinal white bands (7). These are used mainly for swimming and the tentacles for feeding, mating, intra-species communication and fighting (8). Tentacles have long stalks and a crescent-shaped club at the end with 5 to 6 suckers in transverse rows (3). Each tentacle has a large central nerve cord that runs along its entire length, allowing the cuttlefish to manoeuvre each of its suckers independently for maximum dexterity (3,8).
The head is robust, slightly narrower than the mantle with prominent eyes that have that are covered by a transparent membrane and conspicuous secondary fold on the eyelid (5). Unlike other cuttlefishes, the Broadclub cuttlefish has a distinctive yellow ring along the ventral margin of its eyes which have "W-shaped" pupils (3,6).
The mantle is oval and slightly flattened dorsoventrally (3). It is usually light brown, yellowish or dark brown, with white mottled markings and bands on dorsal side (2,3,4). There are narrow fins with white transverse stripe and white band, along outer margin of mantle (3,8). Dorsal mantle has numerous large papillae and with series of elongate papillae along each side (3). These create a three-dimensional skin texture and help with camouflage through expressions, such as against a coral background (9). The cuttlebone of the Sepia latimanus is bluntly rounded anteriorly and posteriorly with dorsal surface convex posteriorly and flat anteriorly (3). The spine is short, pointed and stout with keels absent (3).
The species appears to be sexually dimorphic (3,5,8). However, unlike most cuttlefishes, the hectocotylus (one of the arms of male cephalopods, specialized to store and transfer spermatophores to the female) is absent in the Broadclub cuttlefish (3). At maturity, male Broadclub cuttlefishes are usually darker (a dark-purple shade) and generally differ in size from the females (3,8,9). In Philippines, males are observed to be smaller while in Japan, males are observed to be larger (10).
For more detailed descriptions and an identification key of Sepiidae, more information can be found here.
Behaviour & Biology
Movement & Behaviour

Sepia latimanus are usually solitary except during aggregation for mating season (11). However, in a 2015 study in Okinawa, Japan, a group of Sepia latimanus was observed to form schools while exhibiting hunting behaviour (13). It is speculated that forming schools help increase success of foraging by increasing opportunities for finding food and compensate for the disadvantages arising from the lack of parental care in cephalopods (13).
Cuttlebone
Cuttlefishes are able to hover in midwater, with fins along mantle margins undulating as well as arms extended to aid stability (2,6,11). Buoyancy is achieved by regulating the relative amounts of gas and fluid in the chambers of the cuttlebone chambers, which are made of heavy calcium carbonate (7,8,11). These need to be strong enough to resist implosion from hydrostatic pressure, which increases with depth (11). Thus, cuttlebones of cuttlefishes that inhabit different depth differ in density and mass.Camouflage & Predation

Chromatophores
The most prominent feature of the Broadclub cuttlefish is its colour changing ability. Almost all Cephalopods, except the nautilus, have this ability as they possess chromatophores (2). Within the chromatophores are pigment granules (yellow, brown and red) in an intracellular sac known as cytoelastic sacculus, that has elastic walls (14). Connected to the cell membrane are radially-arranged muscle cells that when contracted, stretches the sacculus into a thin, flat disc (11,14). The diameter of the sacculus expands up to about 7 times its retracted state, increasing its area to about 50 times (12, 15).
In the Broadclub Cuttlefish, this colour-changing mechanism takes on a larger role. Underneath the layer of chromatophores are iridophores and leucophores which are cells that can generate iridescent and reflect light due to the unique pigment crystals within (12,15). By coordinating the multiple layers of pigmented cells, the cuttlefish can create bands of “flashing lights” (7,14,15). This enables the Broadclub cuttlefish to effectively subdue prey which have adapted to see through the cuttlefish’s camouflage (14). Rather than attempting to be inconspicuous, the Broadclub cuttlefish puts on a “Las Vegas style flashy light show” to visually captivate a wary and armed prey (such as crabs) into a trance, long enough for the Broadclub cuttlefish to grab hold of it with a swift strike of its feeding tentacles (12,15).
Papillae
Furthermore, muscles in the cuttlefish’s skin can flex and contort by expressions of its papillae (9) and changing its skin from smooth to very spiky. Although papillae morphology is unknown, it is probable that they depend on a muscular hydrostatic mechanism (6). Each papilla has a fixed maximum size but cuttlefish can control their form and degree of expression from not expressed (i.e., not observable, skin appears smooth) to completely expressed (papillae are extended maximally) (9,14). Papillae are an important aspect of camouflage as they allow cuttlefish to instantly change their textural appearance to capture the 3-dimensional aspect of its surroundings (9).
Excellent camouflage allows the cuttlefish to be exposed during the day to hunt.
Despite this chromatically-active behavior, studies on genetics and physiological discovered that (with one exception) cephalopods lack multiple photoreceptor types, meaning they are "colour-blind" (14). Cephalopods also fail certain behavioral trials designed to test for color vision by opponent spectral channels (14). Cuttlefish arms are innervated and have mechanoreceptors that allow the animal to gather perceptible information from its immediate surroundings (5) and it might seem intuitive that cuttlefish would use them to feel the substrate to gather cues for papillae expression. However, past work has suggested that papillae expression is driven by visual stimuli (14).
Growth & Reproduction

During mating season, which is usually between April to June, males establish a territory and defend a suitable coral head where the prospective females will visit and lay eggs (7). Courtship is highly ritualized and a conspicuous light display (2,8,12). Male cuttlefish will often compete fiercely for females, flashing bright warnings to their competitors and escalate into fights (2,8). While the larger males usually stand a better chance, there have been observations of smaller males “disguising” as females in an attempt to swim past the competitors and mate with the true females (8), but this has yet to be further studied.
This species mates head-to-head where the male places spermatophores (capsule containing spermatozoa) on the female's buccal membrane (7). Fertilisation takes place internally (2,12). As a female Broadclub cuttlefish can mate with multiple partners but will eventually choose only one spermatophore to fertilise her eggs, males often guard females to ward off rivals (2,12).
After mating, the female retreats to lays her eggs (2). She coats the eggs with a
protective sheath and carefully cements them deep within spaces among coral fingers (8). She leaves the eggs unattended to develop and to hatch on their own as she dies shortly after (7,8,12). It is not unusual for reproduction to make the end of cuttlefishes lifecycle. The eggs harden after they are laid, which makes them challenging for predators to reach and remove from the coral (12), although some coral fishes with long snouts are able to extract the eggs before they harden to consume the contents (16).After 38-40 days, embryos hatch into planktonic stage and live for some time before they grow larger and take up a benthic existence as adults. Juveniles often hide among the coral and coral rubble, mimicking mangrove leaves (3), as shown in the picture above.
Geographical Distribution
The Broadclub Cuttlefish is one of the most common cuttlefish species. It inhabits the Indian Ocean and Pacific Ocean (1), native to the FAO Marine Fishing Areas. It has a very wide geographic distribution, stretching from southern Mozambique on the East African coast northwards along the Indian Ocean coastline of Asia and the Middle East, throughout Eastern and Southeastern Asia and as far north as Southern Japan, and as far east as northern Australia, Papua and the islands of the Coral Sea (1,3). It is native to many countries including Bangladesh, Cambodia, China, Egypt, India, Indonesia, Korea, Malaysia, Philippines, Sri Lanka, Taiwan, Thailand, Vietnam and Yemen (1).
Common names of the Sepia latimanus in other countries include: Sotong-besar in Indonesia and Kobushime, Kubushime in Japan (3).
In Singapore
These cuttlefishes can also be found along Singapore's shores. They have been spotted along the Southern islands, such as at Pulau Hantu and Sisters' Island, and also along the coast of Tanah Merah. In 2011, divers at Pulau Hantu spotted a cool and calm Broadclub cuttlefish floating near the seabed, disguised as brown algae.
For more pictures of Broadclub Cuttlefishes along Singapore's shores, visit wildsingapore's webpage here.
Human Uses & Threats
Like most cuttlefishes, the Broadclub cuttlefish is popular for human consumption, but also as bait and are marketed fresh, frozen or dried. While it is common, Broadclub cuttlefish is regularly fished for its meat and cuttlebone, which is used as a calcium supplement for many pet animals (8). In the Philippines, the cuttlefish is split open, cuttlebone and viscera removed and dried in the sun without salt (1).
 |
| Photo by Jobazzard |
Many species of cuttlefish are important to fisheries over a great extent of its range (10,16). Fishing activity varies from local, or subsistence fisheries, to major export industries. It is caught in the local fisheries of western Japan and the Philippines using a variety of methods such as jigs, handlines, set nets and spears (10). Cuttlefishes are also an important component of finfish and prawn trawl bycatch (10,16).
Apart from overharvesting, the cuttlefish is also vulnerable to the rising acidity of ocean water (16). Studies have shown that under high pCO2 concentrations, cuttlefishes develop denser cuttlebone which is likely to negatively affect buoyancy regulation and reproduction (17). Other threats include habitat destruction, pollution by runoff and saltation which damages the marine environment (1,3,16). As cuttlefishes feed on shrimps, prawns and small/juvenile fishes, the destruction of coral reefs, such as through bottom trawling and land reclamation, indirectly affects Sepia latimanus.
However, the IUCN Red List of Threatened Species is unable to assess the Broadclub cuttlefish's and is hence listed as Data Deficient (DD) (1).
Taxonomy & Systematics
The species Sepia latimanus was first described by Quoy, J. R. and J. P. Gaimard in 1832 (18). It was described in Voyage de decouvertes de l'Astrolabe pendant les annees 1826-1827-1828-1829, Zoologie, under the chapter Mollusques (18).The type specimen was collected in New Guinea and is deposited at the Muséum National d'Histoire Naturelle in Paris (19).
Synonyms
- Ponderisepia eclogaria Iredale, 1926
- Sepia harmeri Robson, 1928
- Sepia rappiana Férussac, 1834 in Férussac and d’Orbigny (1834–1848)
- Sepia mozambica Rochebrune, 1884
- Sepia hercules Pilsbry, 1894
Sepia latimanus is likely to comprises a species complex (1,3). It has been suggested that Duc’s (1978) record of S. hercules Pilsbry, 1894 from Viet Nam is possibly a S. pharaonis instead (3). This requires investigation, particularly considering its fisheries significance. The impact of fishing cannot be evaluated when the distribution boundaries of species within the complex are not established (1). These distribution limits also need to be established before assessing the potential impacts of climate change on its coral reef habitats (1).
Taxonomic Hierarchy
The WoRMS taxon tree for Sepia latimanus can be found here.The hierarchy below is referenced from the Integrated Taxonomic Information System (18).
| Kingdom |
Animalia – Animal, animaux, animals |
| Subkingdom |
Bilateria |
| Infrakingdom |
Protostomia |
| Superphylum |
Lophozoa |
| Phylum |
Mollusca – mollusques, molusco, molluscs, mollusks |
| Class |
Cephalopoda Cuvier, 1797 – octopuses, squids, calmars, encornets, pieuvres, poulpes, náutilo, polvo, cefalopode, lula |
| Subclass |
Coleoidea Bather, 1888 |
| Superorder |
Decabrachia Boettger, 1952 |
| Order |
Sepiida Zittel, 1895 |
| Family |
Sepiidae Leach, 1817 |
| Genus |
Sepia Linnaeus, 1758 |
| Subgenus |
Sepia (Sepia) Linnaeus, 1758 |
| Species |
Sepia latimanus Quoy and Gaimard, 1832 |
About the Family Sepiidae
Three genera are currently included in the family Sepiidae: Sepia, Sepiella and Metasepia. The number of species in each genus is quite variable: over 100 species in the genus Sepia, 2 species in Metasepia, and 7 species in Sepiella. Sepiid geographical distribution is restricted to European, African and Indo-Western Pacific waters; only 3 are European (20). There are no species in American waters.However, based on studies conducted, the phylogeny of cuttlefish is still contentious and is continuously being reassessed and stabilized with the introduction of molecular methods and the discovery of new species. Currently, there are 2 main issues with the classification:
1) There is a lack of monophyly in the groupings when assessed with molecular data compared to morphological data (Refer to phylogeny tree below) - The molecular phylogeny agrees with some morphological studies established using shell characters, but the paraphyly of the genus Sepia emphasizes the necessity of reconsidering the weight of some characters (20).
2) Morphological differences, especially in the structure of cuttlebone, are traditionally used to deliminate species but is less than sufficient given the large range of individual varieties - In fact, the cuttlebone shows individual variations; in many species, a sexual dimorphism of the cuttlebone is observed (20). Moreover, specimens of the same species from distant geographical areas present differences in the shape of the shell, which has caused much confusion (20).
In the case of Sepia and Sepia latimanus, groups of ‘Sepia’ were identified, but with no clear phylogenetic link existing between them (20,21). There was no direct correlation between geographical distribution and subgenera or species complex established either on morphological characters or on molecular characters (20). There have been calls to reassess the Sepia, Metasepia and Sepiella groups to reflect molecular information.
Another problem in phylogenetic studies of Sepia latimanus is in scope limitations due to exportation restrictions. The Australian government restricted the international exportation of the Australian giant cuttlefish, Sepia apama (23). Thus, the species is rarely included in phylogenetic analysis. However, in a 2013 study by Kawashima et al., molecular analyses based on complete mt sequences strongly suggest a close relationship between Sepia apama and Sepia latimanus (23). This requires more studies for affirmation, but currently impeded by the exportation restrictions.
Gene Sequences
The following is a representative barcode sequence, the centroid of all available sequences for this species (22). |
| Image by Encyclopedia of Life. |
There are 5 barcode sequences available from BOLD and GenBank.
For genetic sequences, feel free to click on the links below:
External Links
- Encyclopedia of Life
- Index to Organism Names
- IUCN Red List of Threatened Species
- World Register of Marine Species
- Wikipedia
References
- Barratt, I. & L. Allcock, 2012.The IUCN Red List of Threatened Species 2012 - Sepia latimanus. http://dx.doi.org/10.2305/IUCN.UK.2012-1.RLTS.T162505A904969.en. Last updated 15 MAr. 2009. (Access 9 Nov. 2015).
- Hanlon, R. T. & J. B. Messenger, 1998. Cephalopod Behaviour. United Kingdom. Cambridge University Press. Pp. 232.
- Jereb, P. & C.F.E., Roper, 2010. Sepia latimanus Quoy and Gaimard, 1832. In: Cephalopods of the world. An annotated and illustrated catalogue of cephalopod species known to date. Volume 2. Myopsid and Oegopsid Squids. FAO Species Catalogue for Fishery Purposes. FAO, Rome. Pp. 91-92.
- Tan, R., 2013. Broadclub cuttlefishes, Sepia latimanus, Family Sepiidae. http://www.wildsingapore.com/wildfacts/mollusca/cephalopoda/cutbroadclub.htm. Last updated 1 Oct. 2013. (Accessed 7 Nov.2015).
- Jereb, P. & C.F.E., Roper, 2010. Family Sepiidae. In: Cephalopods of the world. An annotated and illustrated catalogue of cephalopod species known to date. Volume 2. Myopsid and Oegopsid Squids. FAO Species Catalogue for Fishery Purposes. FAO, Rome. Pp. 723-734.
- Mäthger L. M., R. T. Hanlon, J. Håkansson & D.E. Nilsson, 2013. The W-shaped pupil in cuttlefish (Sepia officinalis): functions for improving horizontal vision. Vision Research, 83: 19-24.
- MarineBio Conservation Society. Web, 2013. Broadclub Cuttlefishes, Sepia latimanus. http://marinebio.org/species.asp?id=1364. Last updated 14 Jan. 2013. (Accessed 7 Nov.2015).
- Blumenthal, P., 2013. The Bio Blog: Week of Feb. 18-24. https://pascalblumenthal.wordpress.com/2013/02/. Last updated 25 Feb. 2013. (Accessed 7 Nov.2015).
- Allen, J. J., L. M. Mäthger, A. Barbosa & R. T. Hanlon, 2009. Cuttlefish use visual cues to control three-dimensional skin papillae for camouflage. Journal of Comparative Physiology A, 195:547–555.
- Reid, A., P. Jereb, & C.F.E. Roper 2005. Family Sepiidae. In: P. Jereb & C.F.E. Roper, eds. Cephalopods of the world. An annotated and illustrated catalogue of species known to date. Volume 1. Chambered nautiluses and sepioids (Nautilidae, Sepiidae, Sepiolidae, Sepiadariidae, Idiosepiidae and Spirulidae). FAO Species Catalogue for Fishery Purposes. Rome, FAO. Pp. 57–152.
- Sherrard, K. M., 2000. Cuttlebone Morphology Limits Habitat Depth in Eleven Species of Sepia (Cephalopoda: Sepiidae). Biology Bulletin., 198: 404–414.
- Dan, S. , K. Hamasaki, T. Yamashita, M. Oka & S. Kitada, 2012. Age-based life cycle traits of the broadclub cuttlefish Sepia latimanus confirmed through release−recapture experiments. Aquatic Biology, 17:181-195.
- Yasumuro, H., S, Nakatsuru & Y. Iked, 2015. Cuttlefish can school in the field. Marine Biology, 162:763–771.
- Hanlon, R. T. & J. B. Messenger, 1988. Adaptive coloration in young cuttlefish (Sepia officinalis L.): the morphology and development of body patterns and their relation to behaviour. Philos Trans R Soc Lond B Biol Sci, 320:437–487.
- Kingston, A. C. N., A. M. Kuzirian, R. T. Hanlon & T. W. Cronin, 2015. Visual phototransduction components in cephalopod chromatophores suggest dermal photoreception. Journal of Experimental Biology, 218: 1596-1602.
- Norman, M.D., 2000. Cephalopods: A world guide. ConchBooks, Hackenheim. Pp. 320.
- Gutowska , M. A., F. Melzner, H. O. Pörtner & S. Meier, 2010. Cuttlebone calcification increases during exposure to elevated seawater pCO2 in the cephalopod Sepia officinalis. Marine Biology. 157: 1653-1663.
- Integrated Taxonomic Information System, 2003. Sepia latimanus Quoy and Gaimard, 1832. Taxonomic Serial No.: 556372. http://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=556372. Last updated 5 Jun. 2013. (Accessed 7 Nov. 2015).
- Global Biodiversity Information Facility, 2013. Sepia latimanus Quoy & Gaimard, 1832. http://www.gbif.org/species/2290748. Last updated 1 Jul. 2013. (Accessed 7 Nov. 2015).
- Bonnaud, L. & R. Boucher-Rodoni, 2006. Morphological character evolution and molecular trees in sepiids (Mollusca: Cephalopoda): Is the cuttlebone a robust phylogenetic marker?. Biological Journal of the Linnean Society, 89: 139 - 150.
- Tanabe, K., Y. Shigeta, T. Sasaki & H. Hirano, 2010. Molecular phylogeny among East-Asian cuttlefishes using three mitochondrial genes . Cephalopods - Present and Past. Tokai University Press, Tokyo. Pp. 15-21.
- Encyclopedia of Life, 2014. Barcode data: Sepia latimanus. http://www.eol.org/pages/491988/details#molecular_biology. Last updated 7 Sep. 2014. (Accessed 7 Nov 2015).
- Kawashima, Y., H. Nishiharab, T. Akasakia, M. Nikaidob, K. Tsuchiyac, S. Segawac, N. Okada, 2013. The complete mitochondrial genomes of deep-sea squid (Bathyteuthis abyssicola), bob-tail squid (Semirossia patagonica) and four giant cuttlefish (Sepia apama, S. latimanus, S. lycidas and S. pharaonis), and their application to the phylogenetic analysis of Decapodiformes. Molecular Phylogenetics and Evolution, 69: 980–993.
Contact
All graphics in this webpage are created by me, unless otherwise stated. The photos belong to their respective owners as stated. Permissions have been requested for usage of all photos.Lim Cheng Ling is contactable at lim.cheng.ling@nus.edu.sg.