Haddon's Carpet AnemoneStichodactyla haddoni (Saville-Kent, 1893)
 |
| Figure 1: Haddon's carpet anemone out on a sand bar at Pulau Merambong (Image by: Rochelle Chan) |
Anemones are animals that can opportunistically prey on invertebrates, vertebrates and zooplankton that are usually smaller than them (Figure 1 and 2). They also have microscopic photosynthetic partners that provide them with their main source of energy and give them their colour!
Table of Contents
 |
| Figure 2: Stichodactyla haddoni individual in the midst of consuming an unknown crab species in Changi beach (Image by: Rochelle Chan) |
1. Diagnosis
Haddon's carpet anemone is a species of anemone that can be found in intertidal areas along the coasts of Singapore. The anemones are closely related to jellyfish and corals. They are animals with stinging cells that line their tentacles and aid in their capture of prey. After they root themselves into the substrate, they usually do not move around but have the capability to.1.1 Description
Stichodactyla haddoni can be easily differentiated from other anemones as it resembles a flat carpet. Its oral disc can be very large, up to 500 mm [4]. Haddon’s carpet anemone has short, bulbous tentacles that are sticky to the touch and densely covers its undulating oral disk (Figure 1) [5]. The best way to identify this species is through its exocoelic tentacles that fringe the edge of its oral disk (Figure 3-4) and smooth, tan-coloured body column without visible verrucae (Figure 5-6). These exocoelic tentacles are longer than, and alternate with its endocoelic tentacles (the rest of the tentacles that cover the oral disk) and are usually white. |
 |
| Figure 3: Stichodactyla haddoni with white, exocoelic tentacles lining the edge of the oral disk at Changi Beach (Image by: Rochelle Chan) |
Figure 4: Haddon's carpet anemone in-situ at Chek Jawa, with obvious white exocoelic tentacles and variegated tentacle colouration. (Image by: Dr Tay Ywee Chieh) |
 |
|
| Figure 5: Stichodactyla haddoni with smooth body column revealed. No verrucae observed. (Image by: Rochelle Chan) |
Figure 6: Stichodactyla mertensii with visible magenta verrucae in-situ at Pulau Jong (Image by: Ip Yin Cheong) |
1.2 Carpet anemones
In Singapore, there are four species of Stichodactyla that have been observed before: Stichodactyla haddoni, Stichodactyla tapetum (Mini carpet anemone), Stichodactyla gigantea (Giant carpet anemone) and Stichodactyla mertensii (Merten’s carpet anemone). The carpet anemones can be distinguished from the location that they are found in. Stichodactyla mertensii is subtidal, while the other three can be found in the intertidal. Stichodactyla haddoni and S. tapetum are usually found among seagrass beds or sandy substrates while S. gigantea is found among coral rubble. In addition, while the carpet anemones might look similar, there are a few distinct morphological observations that one can distinguish the four species. The dichotomous key below attempts to illustrate how to do a basic identification of S. haddoni when compared to the other three carpet anemones. For better comparison, Dunn et al. [6] provides a clearer and more precise dichotomous key that includes other anemone species also found in Singapore. However, S. mertensii was not included as it was not discovered in Singapore yet, therefore the dichotomous key below might be better in categorising the Stichodactyla species in Singapore.1. Tentacles not sticky, can be very large (up to 1 m in diameter), body column with large visible verrucae of magenta or orange coloration (Figure 6)… Stichodactyla mertensii
Tentacles sticky, less than 1 m in diameter, body column with visible small verrucae or no visible verrucae (Figure 5)…….….……….…................…...… 2.
2. Tentacles sparse, resemble spokes on a wheel, diameter generally less than 150 mm, smooth body column (Figure 7)…..…..............…….….…..…. Stichodactyla tapetum
Tentacles densely covers oral disk, generally larger, diameter can be more than 500 mm, body column smooth or small verrucae.................................. 3.
3. Tentacles slender with pointed tips, no exocoelic tentacles present, body column colourful with small colourful verrucae……...................................... Stichodactyla gigantea
Tentacles short with bulbous tips, outer edge of oral disk lined with (usually white) exocoelic tentacles, body column pale and smooth (Figure 4)…....... Stichodactyla haddoni
Stichodactyla tapetum might resemble the juvenile of S. haddoni closely (Figure 7-8). However, S. haddoni has consistent, densely covered oral disk with exocoelic tentacles while the tentacles of S. tapetum resemble spokes on a wheel and do not have exocoelic tentacles (Figure 7). For more information about how to differentiate between the different carpet anemones in Singapore, Wild Singapore provides a decent comparison.
 |
 |
| Figure 7: Stichodactyla tapetum with short bulbous tentacles that do not cover the entire oral disk, tentacles arranged in spokes-on-a-wheel formation and small oral disk (around 50mm). (Image by: Rochelle Chan) |
Figure 8: Juvenile Haddon's carpet anemone, oral disc small but tentacles densely cover oral disc. Exocoelic tentacles not shown. (Image by: Rochelle Chan) |
1.3 Coloration
Like corals, the anemones, including Stichodactyla haddoni derive their coloration from their symbiotic zooxanthellae [7], Symbiodinium sp.. The coloration of Haddon's carpet anemone usually varies from green, to reddish-brown to brown in Singapore [8]. When conditions become unfavourable, for example temperature increases and salinity changes, S. haddoni can bleach and eventually die if bleaching is prolonged [9] (Figure 9). The expelled zooxanthellae are unable to provide the anemones with products of photosynthesis. This expulsion of zooxanthellae is a common stress-response associated with high water temperature [26]. |
| Figure 9: Bleaching Haddon's carpet anemone. (Image by Ria Tan [48]) |
 |
|
| Figure 10: Stichodactyla haddoni on the sand bar at low tide on Cyrene Reef (Image by: Dr. Tay Ywee Chieh) |
Figure 11: Purple-coloured, bright and duller green-coloured S. haddoni on the sand bar. (Image by Ria Tan [44]) |
Back to top
2. Biology
2.1 Feeding habits
Through its autotrophic zooxanthellae endosymbionts, the anemone gains a constant supply of fixed carbon. However, the Haddon's carpet anemone is primarily a heterotrophic animal that is unable to synthesise its own food. Anemones are also able to obtain food by preying on zooplankton, invertebrates and vertebrates, especially when light exposure is limited or during bleaching [10] (Figure 2). They usually prey on organisms that are smaller than them, using their stinging cells called nematocysts [46]. These nematocysts line the tentacles of the anemone. A recent paper [11] found four novel peptide toxins in Stichodactyla haddoni that are lethal to crabs, indicating that these crustaceans might be a common prey item for the carpet anemone.2.2 Biological interactions
The first Marine symbiotic relationship that most would think of would commonly refer to the anemone and its anemonefish, popularised by Disney's Finding Nemo. Haddon's carpet anemone is one such anemone that forms mutualisic relationships with the Clownfishes (Amphiprion sp.). However, other symbionts also form relationships with the anemone, like the Tiny Carpet Anemone Shrimps (Pereclimenes sp.) and more importantly the ectosymbiont Symbiodinium sp.. It should be noted that for the anemonefishes, this symbiotic relationship is obligate, that the fishes are unable to live without a host anemone in the wild. The anemonefishes do not have a physiological reliance on the host anemones and are able to survive in captivity without predators [12]. However, the reliance on its ectosymbionts varies intra- and interspecifcally and can therefore be considered facultative mutualism for the anemones. However, without its endosymbionts, the anemone would not be able to survive long, concluding that it experiences obligate mutualism with Symbiodinium sp.. All in all, the anemone, ectosymbiont and endosymbiont form a unique trinity of symbiotic relationship in the oceans.2.2.1 Endosymbionts
As much as the anemonefishes help to protect the anemone from predators, there is a more important player in the survival of the anemone. The anemones harbour intracellular Symbiodinium sp. dinoflagellates inside their gastrovascular tissues [25]. The Symbiodinium sp. photosynthesise and provides the main source of fixed carbon and energy to the anemones. The anemone host provide a steady supply of nutrients (like Nitrogen) for the photosynthesis by the Symbiodinium sp. [26]. This supply of nutrients can be enhanced by the ectosymbionts like the anemonefish and the anemone shrimps [27]. On the other hand, bleaching of these endosymbionts have also been observed to correlate with the absence of anemonefish, indicating the importance and influence of this three-way symbiotic relationship [28].2.2.2 Ectosymbionts
Hosting the anemonefishes, Stichodactyla haddoni gain protection from their predators like chaetodontid fishes as the anemonefishes are known to display territorial aggression [13]. Some anemone individuals without resident fishes only survived if they could retract completely into crevices amongst the reef or if they were in partial-territory of other anemone fishes [14]. Actinians are known for their usual wide oral discs [12] that warrants greater vulnerability to predators. The reliance of host anemones on partners for anti-predator defence permitted these evolutionarily wide oral discs, as they expand to capture light for photosynthesis (via their obligate endosymbiotic zooxanthellae partners), which is their main source of energy [15]. Amphiprion had been observed by many researchers to carry food that was either too large or scrap material for its anemone [13, 16, 17] and thus was thought to be one of the benefits that anemones would receive. However, Fautin [12] rightfully argued that this behaviour, however intentional, benefits the anemones to a much smaller extent than the rich nitrogen, sulfur and phosphorus nutritional benefits of anemone fish wastes [18, 19].Stichodactyla haddoni hosts six species of anemonefish, namely Amphiprion akindynos, A. chrysogaster, A. chrysopterus, A. clarkii, A. polymnus, A. sebae [20]. However, in Singapore, the only ectosymbionts observed on Haddon's carpet anemone are the tiny anemone shrimp (Pereclimenes sp.) and the larger five-spot anemone shrimp (Pereclimenes brevicarpalis).
The tiny carpet anemone shrimps (Pereclimenes sp.) do not display territorial aggression, however anemone shrimps are also known to provide the host with Nitrogen and increase the density of zooxanthellae in their host tissue [21]. Other anemone shrimps like the Snapping shrimp (Alpheus armatus) are known to defend their host [22], however the species of anemone shrimps found in Singapore on Haddon's carpet anemone are commensal symbionts to the anemones. These Pereclimenes sp. shrimps are noctournal and hard to spot as they grow to about 1 cm and are almost transparent [45] (Figure 12). On the other hand, Periclimenes brevicarpalis shrimp is known to protect its host aneomone when provoked [23]. In addition, these anemone shrimps walks cautiously across the tips of the anemone tentacles to avoid being stung [24] .
 |
| Figure 12: Pereclimenes sp. shrimp on Stichodactyla haddoni. Photo by: Ria Tan [45]. |
2.2.3 Predators
Haddon's carpet anemone are prey items for Chaetodontidae fishes that live among coral rubble, sea grasses and rocks [13]. Anemonefishes and anemone shrimps can actively protect their host from these predators.2.3 Reproduction
The Haddon's carpet anemone, like other anemones, can undergo sexual reproduction [47] (Figure 13) via broadcast spawning or asexual reproduction via cloning [29]. The reproduction of the anemones are still relatively understudied as their spawning season can be unpredictable. Some congeneric species have been observed to release medusae at different, albeit overlapping timings [30] The anemones are sexual, ie. there are male and female individuals. Tropical anemones are long-lived and slow-growing [20], and ecological studies often do not extend for a sufficiently long time period, spanning such demographic events like asexual production [31].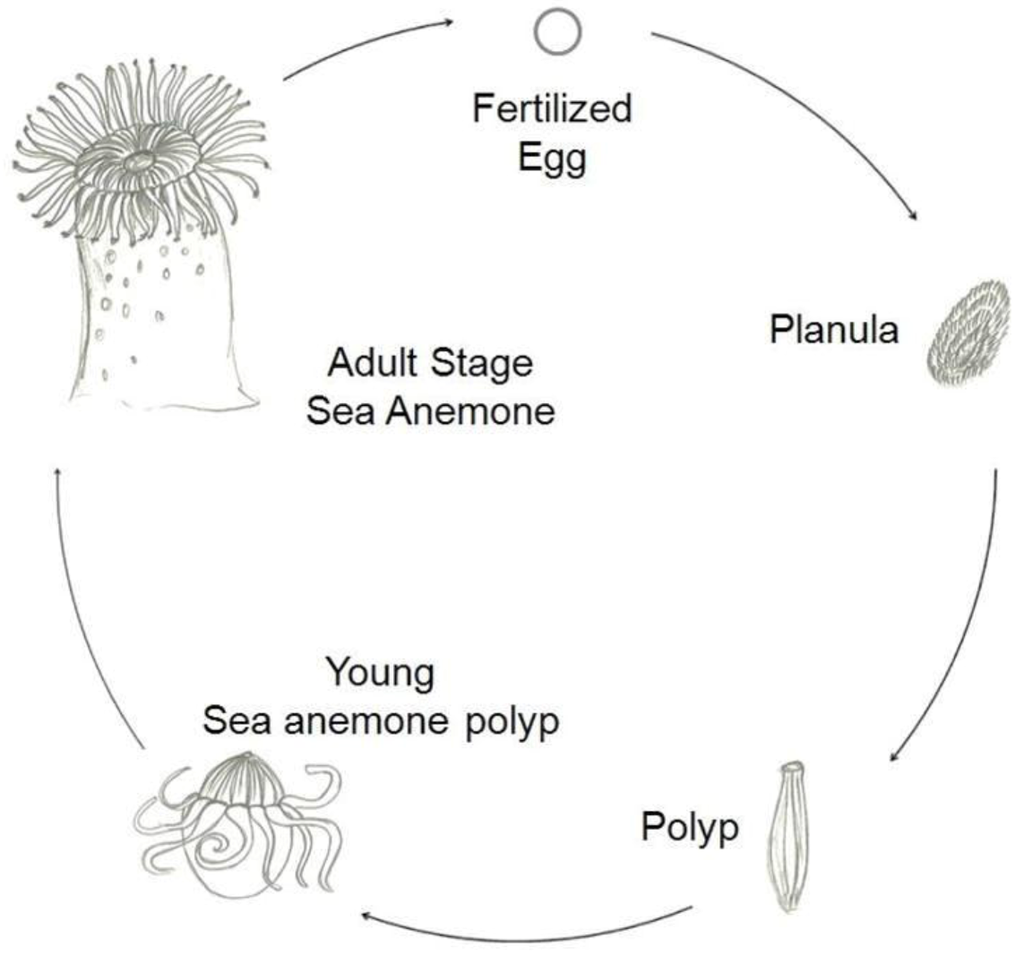 |
| Figure 13: Life cycle of anthozoans [47] |
2.3.1 Sexual
Adult S. haddoni polyps can undergo broadcast spawning that are synchronous and limited to annual breeding seasons [29]. These events are thought to be triggered by lunar cycles and environmental conditions. An observed study in East Timor once recorded spawning 15 nights after a full moon where numerous gametes were released into the water column in short bursts [32]. While spawning, the anemone's mouth protrudes and takes on a cone shape (Figure 14a). Interestingly, resident anemonefish can also opportunistically feed on the gametes of its host (Figure 14b), suggesting that this relationship is not always beneficial. |
| Figure 14: (a) Stichodactyla haddoni releasing gametes into the water column (b) Opportunistic feeding by ectosymbionts Amphiprion clarkii (Image by: Scott & Francisco, 2006) |
2.3.2 Asexual
Stichodacyla haddoni like other anemones and anthozoans can undergo asexual reproduction [12]. Asexual reproduction can occur via cloning by splitting, budding (video) and pedal laceration and unlike broadcast spawning, limits the dispersal of the offspring and local genetic diversity [33]. Splitting occurs when the anemone divides into two identical individuals while budding occurs when a smaller section of the parent anemone breaks off to become a separate individual [43]. Pedal laceration can occur when segments of the pedal disk divides and each develops into individual polyps [34] (Figure 15).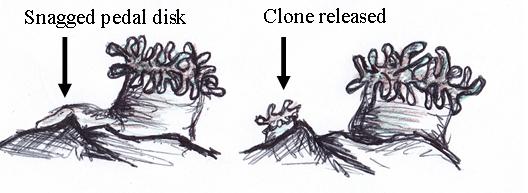 |
| Figure 15: Pedal laceration (Image by: Wood 2013) |
Back to top
3. Distribution
The distribution of S. haddoni in the world can be represented by Figure 16. However, Figure 16 was obtained from Global Biodiversity Information Facility (GBIF), which are biased towards scientific studies. In reality, S. haddoni likely occurs across tropical and subtropical seas from the Red Sea, to the Indian Ocean, New Caledonia, Japan and Australia [6, 27, 39]. In Singapore, this species of carpet anemone have been observed to occur in various areas but mostly around the Southern Islands (Figure 17).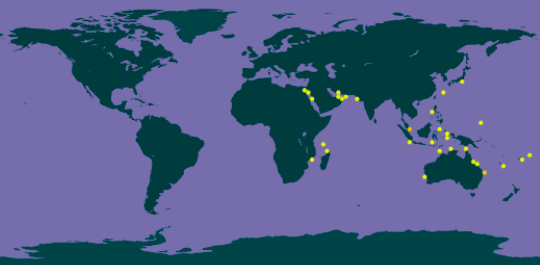 |
| Figure 16: Distribution of S. haddoni in the world, generally in the Indo-Pacific Ocean (Image adapted from: Global Biodiversity Information Facility, 2017 [38]) |
Figure 17: Local Singaporean distribution of Haddon's carpet anemone [6].
Back to top
4. Status and conservation
Stichodactyla haddoni has not been assessed by the IUCN Red List of Threatened Species [40], nor listed on CITES [41]. Its conservation status in the world is therefore unknown. In Singapore, they are not on listed among the threatened animals. However, considering the ever-changing landscape and modification of coast lines (land reclamation, removal of seagrass meadows) in Singapore, there is a need to conserve this species and its habitats (seagrass patches, rocky shores) which contain a diverse range of marine species. In addition, conserving this anemone also preserves its ectosymbionts and endosymbionts and maintains this three-way symbiotic relationship that is one-of-a-kind.4.1 Economic importance
Haddon's carpet anemone is highly sought after in the aquaria trade, together with its ectosymbiont, the anemonefish [37]. These host anemones can fetch for exorbitantly high prices, even up to 13 times the amount for anemonefishes, indicating that they might be preferentially harvested in the trade [38]. A single Stichodactyla haddoni individual can even cost up to US$650 on eBay (Figure 18) [49]. This figure is astounding and shows the high price (and possibly demand) for this animal in the aquaria trade.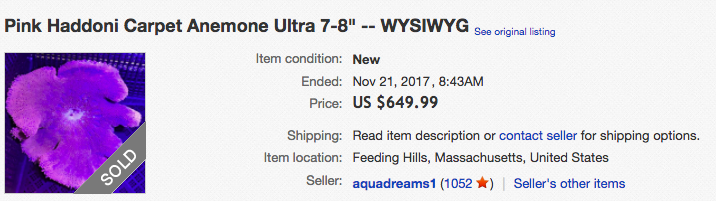 |
| Figure 18: Haddon's carpet anemone selling for close to US$650 (Image obtained by Rochelle Chan from eBay). |
!! However before going into the field to harvest these animals: bear in mind that these are precious, fragile, wild animals that belong in their natural habitat. Harvesting and maintaining them in aquaria is not easy and they tend to die sooner in captivity. In addition, harvesting them without permits could result in legal consequences.
In the study of Haddon's carpet anemone, Fautin et al. [6] notes that it is rare for this species to be seen without anemonefish ectosymbionts and hypothesises that this absence is likely due to removal by collectors. The absence of anemonefish on S. haddoni in Singapore might therefore correlate with a high demand in the aquaria trade for these anemonefishes. Without proper care, most anemone and anemonefish die off quickly, and even before reaching any aquarium.
Back to top
5. Taxonomy & Systematics
5.1 Nomenclature
Haddon's carpet anemone or Saddle Anemone are common names for Stichodactyla haddoni (Saville-Kent, 1893) (Figure 19). Stichodactyla haddoni was first described as Disocoma haddoni by Saville-Kent in his book on the Great Barrier Reef [1] (Figure 19) . Throughout the years, D. haddoni had been changed to Stoichactis haddoni [2] and then finally Stichodactyla haddoni [3] . |
 |
| Figure 19: First mention of Stichodactyla haddoni (Discosoma haddoni) by Saville-kent, and crediting the name to Alfred C. Haddon. |
5.2 Etymology
Saville-Kent (1893) was honoured as the person who first described D. haddoni because he had the first most accurate description. Haddon first provided simple data about this species but was vague and inaccurate. However, in order to honour Alfred C. Haddon, Saville-Kent named this species after Haddon [4] (Figure 19). Saville-Kent is a 19th century all-rounded naturalist who did important anthropological research in Australia and was pivotal in the discovery of this anemone.5.3 Taxonomic classification
| Kingdom |
Animalia |
| Phylum |
Cnidaria |
| Class |
Anthozoa |
| Order |
Actiniaria |
| Superfamily |
Actinioidea |
| Family |
Stichodactylidae |
| Genus |
Stichodactyla |
| Species epithet |
haddoni |
5.4 Type information
According to Dunn [5], no type specimen was located for S. haddoni. Even though Saville-Kent [1] mentioned that his coral collection was submitted to the British Museum (Natural History) (BMNH), there were no such actinians collected by him in BMNH [5].5.5 Phylogeny
There are seven Stichodactyla species in the world but only four are found in Singapore. The Stichodactylidae family also consist of genus Heteractis sp. that can be morphologically confused with Stichodactyla in Singapore. In addition, the COI genes of anthozoa are slow evolving and inter-specific differences vary [35]. As such, the COI barcoding might not be accurate in delimitating species of Stichodactylidae or Stichodactyla. Coral and anemone phylogeny studies have therefore used a combination of detailed morphological analysis and using specific combinations of genes for the delimitation of different species and clades. For Actinaria, it is common to use nuclear genes in phylogenetic studies as the nuclear gene evolution rate is known to be higher than the rate of evolution of mitochondrial genes [50]. Studies conducted have therefore used 12S, 16S, 18S, 28S rDNA to analyse phylogenetic relationships among Actinaria [51].
The phylogeny tree of Actinians have undergone many revisions. The latest revision attempts to categorise Anthopleura and Actinoidea. As with most of other phylogenetic studies, Stichodactyla haddoni, Heteractis sp., Macrodactyla sp., Anthopleura dorensis are likely to be in the family Stichodactylidae [42], taking note that the genus Anthopleura is not monophyletic as other species are found in other clusters. The authors used both nuclear and mitochondria DNA sequences and also cladistic analyses of verrucae and acrorhagi. However, acrohagi appears in genera other than Anthopleura, verrucae are not well defined in anatomy terminology. The evolutionary relationship between acrorhagi and pseudoacrohagi, verrucae and vesicles, are unknown.
Nonetheless, the authors sequenced 12S, 16S, 28S, COIII for several Anthopleura species from different locations, included Stichodactyla gigantea and Heteractis magnifica sequences from GENBANK, and used MUSCLE to align the sequences. ParitionFinder ver. 1.1.1 was used to separate each marker in each matrix before twenty maximum likelihood runs were performed on each data matrix RAxML ver. 8.1.16, with 1000 bootstrap replicates performed on the best-scoring tree (Figure 20). Stichodactyla gigantea and Heteractis magnifica clustered together as expected. As S. haddoni was not particularly studied in any phylogeny studies, its closest relative, S. gigantea can be used to identify the position of S. haddoni in the phylogeny tree [42]. Mostly, Heteractis and Stichodactyla are sister to each other.
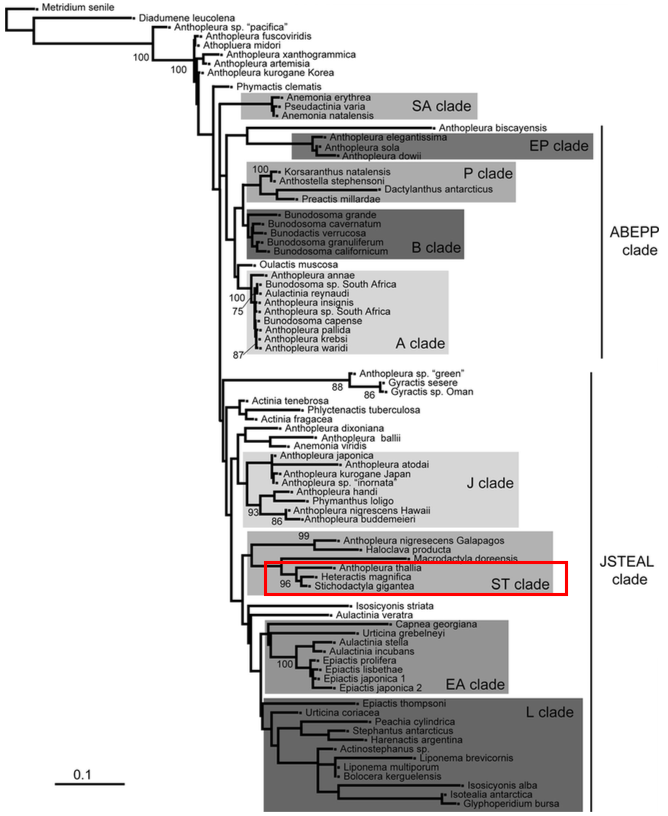 |
| Figure 20: Maximum likelihood tree derived from genetic analysis, with Stichodactyla and Heteractis boxed out in red [42]. |
In the morphological study of acrorhagi and verrucae, the authors placed the features into matrixes of two multistate characters marginal and column structures. To evaluate the pattern of character change, the characters were optimised on the tree of highest likelihood, optimised via simple likelihood in Mesquite. The results show that acrorhagi and verrucae are shared primitive features. The most parsimonious conclusion from the combined tree (Figure 21) is that acrorhagi was ancestral and verrucae to be "primitively present", while verrucae evolved into vesicles a few times. For Anthopleura, acorhagi and verrucae are pleisiomorphic. In the tree, Heteractis and Stichodactyla cluster together once again, showing support for the Stichodactylidae clade.
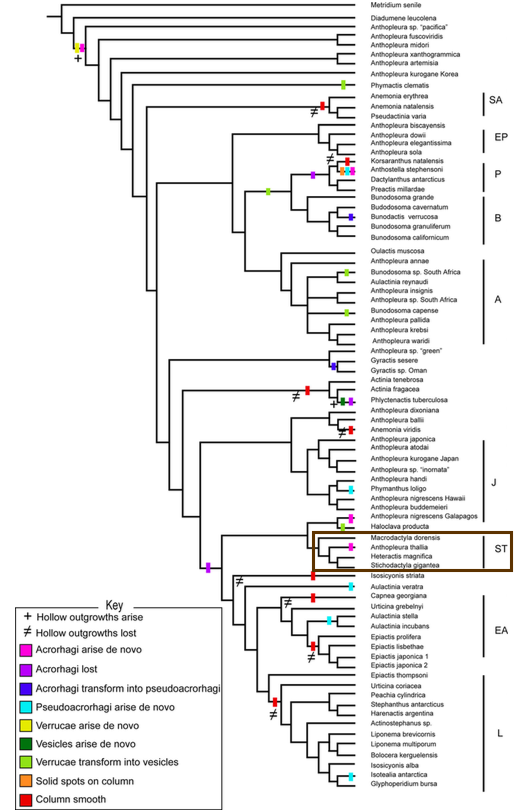 |
| Figure 21: Maximum likelihood tree of Actinaria derived using morphological characters, with Stichodactylidae clade boxed out in brown [42] |
Overall, this suggests that there is a great need for further studies in the phylogeny of Actinians and Stichodactyla genus, in particular, to delimit species. Stichodactyla haddoni as a species are not well represented in phylogenic studies and are mostly delimited through distinct morphological traits, like its exocoelic tentacles, or the presence of the type of ectosymbiont. It is also important to note that phylogenetic relationships among hexacorallians are unclear as they can be independent of anatomy, life cycle and genetic sequence analysis [51].
Back to top
6. Glossary
Acrorhagi - bulbous marginal structures that are tightly packed with nematocysts, characteristic of Anthopleura and several other genera in ActiniidaeAutotroph - an organism that can make its own food via photosynthesis (phototrophs) or oxidation (chemotrophs)
Broadcast spawning - a method of sexual reproduction that involves the release of sperm and eggs into the water column for external fertilisation to occur
Commensal symbiont - an organism that contributes zero net harm to its host but itself benefits from the relationship
Congeneric - of an animal or plant species that belong to the same genus
Endocoelic - a tentacle whose cavity communicates with the endocoel
Exocoelic - a tentacle whose cavity communicates with the exocoel
Heterotroph - an organism that consumes organic carbon in order to produce energy for metabolism
Nematocyst/ cnidocyte - a cell that contains a coiled, barbed thread that is triggered upon contact, releasing toxin
Pedal disk - the basal plate of an anemone that attaches to substrates
Pleisiomorphic - a characteristic of an organism that is derived from its ancestor
Polyp - a (cnidarian) individual that has a cylindrical body with vase-shaped body
Variegated - exhibiting different colours, especially as irregular patches or streaks.
Verrucae - small bumps in rows on the body column that act as “suckers” and allow the anemone to grasp substrate.
Back to top
7. References
[1] Saville-Kent (1893). URL: http://www.biodiversitylibrary.org/item/40631#page/218/mode/1up (Accessed on: 10 Nov 2017).[2] Uchida, H., K. Okamoto, and T. Fukuda. 1975. Some observations on the symbiosis between anemonefishes and sea-anemones in Japan. Bulletin of the Marine Park Research Stations 1(1): 31-46.
[3] Stoichactis haddoni (Fautin, D. (2015). Stoichactis haddoni. In: Fautin, Daphne G. (2013). Hexacorallians of the World. Accessed through: World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id=367767 on 2017-09-08)
[4] Fautin, D. G., Allen, G. R., Allen, G. R., Naturalist, A., Allen, G. R., & Naturaliste, A. (1992). Field guide to anemonefishes and their host sea anemones.
[5] Dunn, D. F. (1981). The clownfish sea anemones: Stichodactylidae (Coelenterata: Actiniaria) and other sea anemones symbiotic with pomacentrid fishes. Transactions of the American Philosophical Society, 71(1), 3-115.
[6] Fautin, D. G., Tan, S. H., & Tan, R. (2009). Sea anemones (Cnidaria: Actiniaria) of Singapore: abundant and well-known shallow-water species. Raffles Bulletin of Zoology, 121-143.
[7] Cesar, S. A., De Dios, H. H. Y., Amoin, N. B., & Dy, D. T. (2014). Thermal stress affects zooxanthellae density and chlorophyll-a concentration of the solitary mushroom coral, Heliofungia actiniformis. Philipp J Sci, 143, 35-42.
[8] Chan, W. W. R. (2017) Pers. obs.
[9] Hobbs, J. P. A., Frisch, A. J., Ford, B. M., Thums, M., Saenz-Agudelo, P., Furby, K. A., & Berumen, M. L. 2013. Taxonomic, spatial and temporal patterns of bleaching in anemones inhabited by anemonefishes. PloS one,8(8), e70966.
[10] Leal, M. C., Nejstgaard, J. C., Calado, R., Thompson, M. E., & Frischer, M. E. (2014). Molecular assessment of heterotrophy and prey digestion in zooxanthellate cnidarians. Molecular ecology, 23(15), 3838-3848.
[11] Honma, T., Kawahata, S., Ishida, M., Nagai, H., Nagashima, Y., & Shiomi, K. (2008). Novel peptide toxins from the sea anemone Stichodactyla haddoni. Peptides, 29(4), 536-544.
[12] Fautin, D. D. (1991). Developmental pathways of anthozoans. Hydrobiologia, 216(1), 143-149.
[13] Mariscal RN (1970) The nature of the symbiosis between Indo-Pacific anemone fishes and sea anemones. Marine Biology, 6(1): 58–65.
[14] Ross, R. M. (1978). Territorial behavior and ecology of the anemonefish Amphiprion melanopus on Guam. Ethology, 46(1), 71-83.
[15] Godwin J & Fautin DG (1992) Defense of host actinians by anemonefishes. Copeia, 1992(3): 902-908.
[16] Sluiter CP (1888) Ein merkwiirdiger Fall yon Mutualismus. Zool. Amz. 11: 240–243.
[17] Allen GR (1972) The anemonefishes: their classification and biology. T. F. H. Publications, Ltd., Neptune City, New Jersey
[18] Roopin M, Henry RP & Chadwick NE (2008) Nutrient transfer in a marine mutualism: patterns of ammonia excretion by anemonefish and uptake by giant sea anemones. Marine Biology, 154(3): 547–556.
[19] Cleveland A, Verde EA & Lee RW (2011) Nutritional exchange in a tropical tripartite symbiosis: direct evidence for the transfer of nutrients from anemonefish to host anemone and zooxanthellae. Marine Biology, 158(3): 589–602.
[20] Fautin, Daphne G.; Allen, Gerald R. (1997). //Field Guide to Anemone Fishes and Their Host Sea Anemones//. Western Australian Museum. ISBN 9780730983651.
[21] Spotte, S. (1996). Supply of regenerated nitrogen to sea anemones by their symbiotic shrimp. Journal of Experimental Marine Biology and Ecology, 198(1), 27-36.
[22] Smith, W. L. (1977). Beneficial behavior of a symbiotic shrimp to its host anemone. Bulletin of Marine Science, 27(2), 343-346.
[23] Ng, P. K. L., Lim, S. S. L., & Tan, L. W. H. (Eds.). (2007). Private Lives: an Exposé of Singapore's Shores. National University of Singapore, Department of Biological Sciences.
[24] Leong, K. P., Chua, S. C., Low, J. K., Gouw-Iwata, L., & Wong, D. (2003). Singapore waters: unveiling our seas. Nature Society Singapore, Marine Conservation Group.
[25] Ollerton J, McCollin D, Fautin DG & Allen GR (2007). Finding NEMO: nestedness engendered by mutualistic organization in anemonefish and their hosts. Proceedings of the Royal Society of London B: Biological Sciences, 274(1609): 591–598.
[26] Finn, M. H., Lönnstedt, O. M., Rizzari, J. R., Jones, G. P., & Frisch, A. J. (2016). Experimental bleaching of a tropical sea anemone in situ. Marine Ecology, 37(3), 691-696.
[27] Thornhill, D. J. (2012). Ecological impacts and practices of the coral reef wildlife trade. Defenders of Wildlife, 187.
[28] Jones, A. M., Gardner, S., & Sinclair, W. (2008). Losing ‘Nemo’: bleaching and collection appear to reduce inshore populations of anemonefishes. Journal of Fish Biology, 73(3), 753-761.
[29] Scott, A., & Harrison, P. (2007). Broadcast spawning of two species of sea anemone, Entacmaea quadricolor and Heteractis crispa, that host anemonefish. Invertebrate Reproduction & Development, 50(3), 163-171.
[30] Fautin, D. G. (2002). Reproduction of cnidaria. Canadian Journal of Zoology, 80(10), 1735-1754.
[31] Holbrook, S. J., & Schmitt, R. J. (2005). Growth, reproduction and survival of a tropical sea anemone (Actiniaria): benefits of hosting anemonefish. Coral Reefs, 24(1), 67-73.
[32] Scott, A., & Francisco, B. (2006). Observations on the feeding behaviour of resident anemonefish during host sea anemone spawning. Coral Reefs, 25(3), 451-451.
[33] Waller, R. G. (2005). Deep-water Scleractinia (Cnidaria: Anthozoa): current knowledge of reproductive processes. In Cold-water Corals and Ecosystems (pp. 691-700). Springer Berlin Heidelberg.
[34] Wood, N. (2013). Entacmaea quadricolor. URL: http://www.gbri.org.au/SpeciesList/Entacmaeaquadricolor%7CNicolaWood.aspx?aid=1&PageContentID=4465 (Accessed on: 12 Nov 2017).
[35] Huang, D., Meier, R., Todd, P. A., & Chou, L. M. (2008). Slow mitochondrial COI sequence evolution at the base of the metazoan tree and its implications for DNA barcoding. Journal of Molecular Evolution, 66(2), 167-174.
[36] Fautin D.G. (2017). WoRMS Hexacoral: Hexacorallians (Actiniaria) of the World (version 2017-10-08). In: Roskov Y., Abucay L., Orrell T., Nicolson D., Bailly N., Kirk P.M., Bourgoin T., DeWalt R.E., Decock W., De Wever A., Nieukerken E. van, Zarucchi J., Penev L., eds. (2017). Species 2000 & ITIS Catalogue of Life, 30th October 2017. Digital resource at www.catalogueoflife.org/col. Species 2000: Naturalis, Leiden, the Netherlands. ISSN 2405-8858.
[37] Scott, A., Hardefeldt, J. M., & Hall, K. C. (2014). Asexual propagation of sea anemones that host anemonefishes: implications for the marine ornamental aquarium trade and restocking programs. PloS one, 9(10), e109566.
[38] Shuman CS, Hodgson G, Ambrose RF (2005) Population impacts of collecting sea anemones and anemonefish for the marine aquarium trade in the Philippines. Coral Reefs 24: 564–573.
[39] “Georeferenced Data: Map of global distribution of Stichodactyla haddoni” by Global Biodiversity Information Facility. Stichodactyla haddoni, 2017. URL: https://www.gbif.org/species/7812410 (Accessed on 15 Nov 2015)
[40] IUCN. (2017). The IUCN Red List of Threatened Species. Version 2017-1. Downloaded on 18 May 2017.
[41] CITES (2017). The Checklist of CITES Species Website. Appendices I, II and III valid from 4 October 2017. https://www.cites.org/eng/app/appendices.php (accessed on 15 Nov 2015).
[42] Daly, M., Crowley, L. M., Larson, P., Rodríguez, E., Saucier, E. H., & Fautin, D. G. (2017). Anthopleura and the phylogeny of Actinioidea (Cnidaria: Anthozoa: Actiniaria). Organisms Diversity & Evolution, 1-20.
The Anemone FAQ
[44] Tan, R. (2017). Stichodactyla haddoni. URL: http://www.wildsingapore.com/wildfacts/cnidaria/actiniaria/haddoni.htm (Accessed on 12 Nov 2017)
[45] Tan, R. (2017). Pereclimenes sp. URL: http://www.wildsingapore.com/wildfacts/crustacea/othercrust/shrimp/carpet.htm (Accessed on 12 Nov 2017)
[46] Cnidocyte. (2017). URL: https://en.wikipedia.org/wiki/Cnidocyte (Accessed on 11 Nov 2017)
[47] Frazão, B., Vasconcelos, V., & Antunes, A. (2012). Sea anemone (Cnidaria, Anthozoa, Actiniaria) toxins: an overview. Marine drugs, 10(8), 1812-1851
[48] Tan, R. (2017). Bleached Haddon's carpet anemone (Stichodactyla haddoni). URL: https://www.flickr.com/photos/wildsingapore/537045847/in/photostream/ (Accessed on 3 Dec 2017).
[49] eBay (2017). Pink Haddoni carpet anemone. URL: https://www.ebay.com/itm/Pink-Haddoni-Carpet-Anemone-Ultra-7-8-WYSIWYG/222710966132?hash=item33da9ba774:g:-vUAAOSwPAxaAk31 (Accessed on 3 Dec 2017).
[50] Daly M, Chaudhuri A, Gusmao LC, Rodriguez E (2008). Phylogenetic relationships among sea anemones (Cnidaria: Anthozoa: Actiniaria). Mol Phylogenet Evol 48: 292–301.
[51] Fariman, G. A., Javid, P., & Shakouri, A. (2015). Morphology and phylogeny of the sea anemone Stichodactyla haddoni (Cnidaria: Anthozoa: Actiniaria) from Chabahar Bay, Iran. Turkish Journal of Zoology, 39(6), 998-1003.
Back to top