Blood Cockles
Blood Cockles Tegillarca granosa (Linnaeus, 1758)
Tegillarca granosa (Linnaeus, 1758)
Introduction
Welcome to the secret life of the blood cockle, seen from an animal perspective!This species page aims to help you to understand and know more about the beloved blood cockles that you often see in classic Singaporean dishes like Laksa and Char Kway Teow. Although there will be recipes and food-related information in the Cultural and Culinary section, we would be focusing more on the animal aspects of the Blood Cockle. In some of the sections, there are general information about the animal group of 'Bivalves' - includes many other edible shellfish like clams, cockles, scallops and mussels!
Table of Contents
- a form of an animal.
- part of a big and varied group of animals called 'Bivalves'
Guide to reading this webpage:
- the names Tegillarca granosa, Arca granosa, Anadara granosa, blood cockle and sea hum will be used interchangeably to refer to the same and main species in discussion here.
- since this page is rather lengthy, please navigate through the page using the table of contents on the right-side of the page.
- Webpage too long? Skip to these 5 interesting sections: What's with the blood?, Behaviour/ Movement section, Health and Safety section, Cultural and Culinary Section, How to pick cockles at the supermarket?
Name
Scientific name: Tegillarca granosa (Linnaeus, 1758)Vernacular English (Common) names: Blood Cockles, Blood Clam, Granular Ark, Mud cockle,'sea hum/ sea ham' (colloquial), (common names in other languages)
Synonyms
Anadara granosa (Linnaeus, 1758)Anadara bisenensis Schrenck & Reinhart, 1938
Anadara thackwayi Iredale, 1927
Anomalocardia pulchella Dunker, 1868
Arca aculeata Bruguière, 1789
Arca corbicula Gmelin, 1791
Arca corbula Dillwyn, 1817
Arca granosa Linnaeus, 1758
Arca granosa kamakuraensis Noda, 1966
Arca nodulosa Lightfoot, 1786 (invalid: junior homonym of Arca nodulosa O. F. Müller, 1776)
Arca obessa Kotaka, 1953
Tegillarca granosa bessalis Iredale, 1939
Etymology

Figure 2: Origin of the Ark Shells group that the sea hum species belongs to (Source: Google Results).
- original Latin translation for chest is reflected in the genus of the original species name, Arca (see above in synonyms section)
- English usage of the word 'ark' is referred to the a type of boat. This reference might be stemming from the boat-like inner surface of ark shells, as mentioned here.
Biology
What's with the blood? (Viewer's Discretion Advised)
Wow, that's some messy business. So the main question is: Is the red liquid really blood?
The red liquid that is the first thing that you notice when you see or smell the blood cockle. As its name suggests, the red liquid is actually similar to the human blood that we have! The liquid also carries haemoglobin and red blood cells that help it to survive in environments with low oxygen levels[1] . In addition, an added edge is that the oxygen-carrying capacity of the red blood cells increase as environmental temperatures increase[2] , hence it can better cope with these two stressors at the same time.
- Other interesting sections below: Behaviour/ Movement section, Health and Safety section, Cultural and Culinary Section, How to pick cockles at the supermarket?
Gender
There are rare instances where there are individuals that are hermaphrodites (<1.5%) for the blood cockle[3] , but otherwise, they are mostly unisexual - either male or female[4] .Behaviour/ Movement
As compared to many other animals, bivalves are way less active. However, there are instances when bivalves make surprisingly big movements!One such movement has to do with the use of the bivalve's feet to dig into the ground to seek cover, or even travel by leaping (Video 1)!
[[media type=youtube key=6TjNYOG9AB8 width=560 height=315 align=center width="560" height="315" align="center"]]
Video 1: Bivalve using foot to move and jump. (Source: YouTube, 10/8/2010, 'i Love Shelling', The Cockle Hop.mov, permission pending)
Another sort of movement that the blood cockle does is the closing and opening of its shell, in response to changing environmental conditions. When the salinity of the surrounding waters drops below 19 ‰ (parts per thousand), the blood cockle reacts by clamping down and closing its shell. This attempt to protect itself is only effective in the short-term. if low salinity conditions persist for more than three days, it could result in mortality. On the other end of the spectrum, the blood cockle would widely open its shell, when not submerged in water for long periods of time. In such low oxygen conditions, opening its valves has the purpose of increasing extraction of oxygen from atmospheric air [5] .
- 3 more interesting sections below:Health and Safety section,
Culture and Culinary Section, How to pick cockles at the supermarket?
Reproduction
The blood cockle reproduces sexually. When male and female Tegillarca granosa reach sexual maturity (usually when male lengths = 20mm, females = 24mm [6] ), the gametes (sperm and egg) will just be actively released into the water. The sperm is released in a steady stream, while the egg is released in spurts. Fertilisation occurs in the water and the eggs eventually develop into a free-swimming 'trochophore'[7] . A trochophore is a larvae that lives in the water column, tiny enough (cannot be seen by the naked eye) to be swayed and moved along by the currents.The blood cockle is a 'dribble spawner'[8] (FAO, 2017), where it spawns a little throughout the whole year, with some peak months that differ every year[9] . In Penang, this period is in July to October[10] , while it is July to August in Pattani Bay, Thailand[11] .
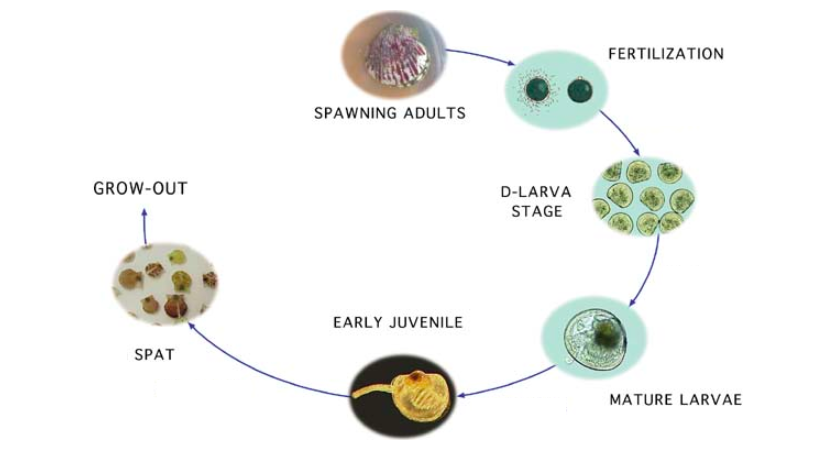
Life Cycle
Following the trochophore stage mentioned under the Reproduction section,- develop and move on to the veliger (something like infant) stage. The blood cockle is extremely vulnerable during this two stages, and mortality is high then[12]
- undergo metamorphosis and transition into a non-swimming, sessile stage, the spat (like a mini shell)
- finally grows out into an adult
Feeding Habits
Blood cockles are filter feeders[13] , a subset of suspension feeders. This means that it 'sucks in' water in the water column, sieves this water to obtain their 'food', and then expel out the excess water. Bivalves are able to selectively take in organic matter like phytoplankton that they eat as food, among other inorganic material in the water column[14] . Other such organic matter that serves as food include algae, detritus and nanozooplankton[15] . Even eating bacteria has been shown to biomass increase in bivalves. However, there is also a critical linking organism, nanozooplanktons, in this relationship. Nanozooplanktons also feed on bacteria. By eating the bigger nanozooplanktons, the bivalves can better retain the bacteria that the nanozooplankton ingests[16] .During feeding, the ctenidium, or the gills, act as the filter, as well as the organ creating the flow of water in and out of the bivalve[17] .
Habitats
Tegillarca granosa is generally found in muddy areas in the intertidal zone.Salinity
Bivalves can be found in areas of different kinds of salinities, from freshwater to hypersalinehabitats[18] . Since blood cockles thrive in the intertidal zone, the environment is largely estuarine, with low salinities. The blood cockles are also cultivated in the intertidal with salinities ranging from 10-32 ‰ ('parts per thousand')[19] . Also, in Malaysia, it has been known to persist in waters that have salinities of about 30 ‰[20] . In the tropics, changes in salinity at a certain area can be more volatile. When there is increased rainfall, the salinity of the water in that area could fall drastically. In turn, this can affect the presence of Tegillarca granosa as well as other marine animals that are used to a certain range of salinity[21] .
Water and substratum conditions
The Tegillarca granosa species prefers to reside in water that has low wave action such as shallow inlets and bays, although some water flow is still desirable[22] . These small movements ensure the constant suspension of organic material in the water[23] , potential food for the blood cockles. Increased turbidity would mean that the blood cockles have more opportunies to 'catch' the food in the water, since they are filter feeders. More information about their feeding behaviour can be found in the 'Biology' section below. Areas with 'soft flocculent' and silty mud seem to also be favoured by Tegillarca granosa[24] [25] . However, there have been instances in Phuket, Thailand where the species have been found on sediments that are more sandy in nature[26] .
Temperature
In the tropical regions that the blood cockles reside, the blood cockles thrive in areas with temperatures ranging from 25°C to 40°C[27] .
!Important! Health and Safety - So are blood cockles safe to eat?
Yet again, as filter feeders with high filtration rates, and as sessile organisms, blood cockles are more likely to accumulate pollutants[28] .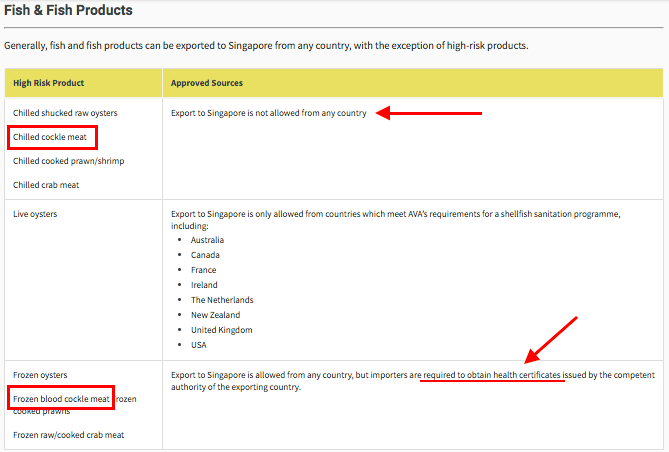
Blood cockle meat, either chilled or frozen is considered a high-risk seafood in Singapore, as classified by the AVA[29] . The import of chilled cockles are banned totally, while that of frozen cockles have to be accompanied by a health certificate[30] , because of 'food safety reasons'. What might these reasons be? Let's check it out!
In the paper by Mirsadeghi et al. in 2013, Tegillarca granosa has also shown to bioaccumulate polycyclic aromatic hydrocarbons (PAHs). Bioaccumulation is the transfer of in chemicals from the environment into the tissue of an aquatic organism (i.e. blood cockles), resulting in an increase in concentration of the chemical in the tissues[31] . In environments with higher amounts of tPAHs, Tegillarca granosa that grow there have greater levels of tPAHs in their tissue as well. Since PAHs are carcinogenic and toxic, it is important to know the source of the blood cockles that you consume.
Okay, so they might have increased concentraions of this PAH compound. What does this mean for us? Firstly, PAHs are carcinogenic. This means that PAHs have the potential to be cancer-causing. More carcinogenic foods can be found here. Increased exposure of PAHs can increase the likelihood of getting skin, liver, bladder, lung and gastrointestinal cancers[32] . Secondly, PAHs are also endocrine disrupters. In particular, increased exposure can affect the reproductive health of animals and humans[33] . Lastly, it has also been mentioned that consumption is linked to cholera, hepatitis A and dysenteric shellfish poisoning[34] . Apart from PAHs, levels of radioactive isotopes of Polonium that are higher than safe limits have been found in blood cockles[35] . The cockles sampled were near a coal power plant in Malaysia, which could have resulted in the high concentrations.
Blood cockles have also been found to contain the bacteria Vibrio parahaemolyticus that can cause gastroenteritis if eaten raw, or not well-cooked. In fact, in room temperature, infectious doses of Vibrio parahaemolyticus can be cultivated in the cockle in just a few hours[36] .
In Thailand, high popularity of the blood cockles eaten only half-cooked might have led to the high number of cases of people getting diarrhoea[37] .
- 2 more interesting sections below: Culture and Culinary Section, How to pick cockles at the supermarket?
Distribution
South-East Asia and Northern Australia houses the few densest areas of Blood Cockles in the world. Also widespread in the Indo-Pacific region[38] (Narasimham, 1988).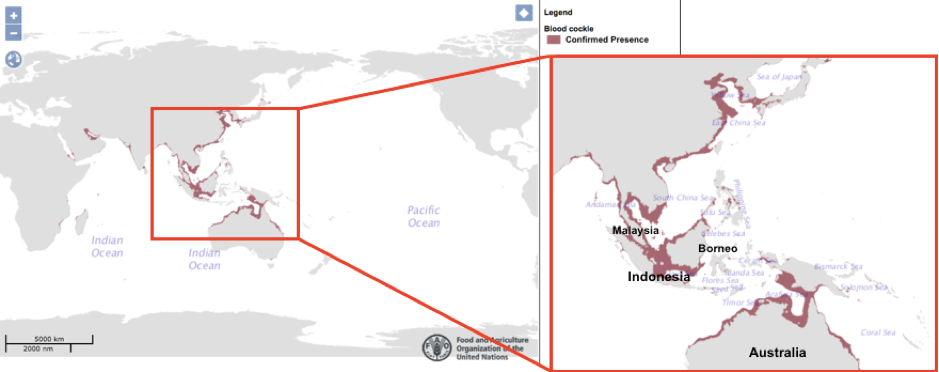
The Eastern banks of China also has a substantial area of blood cockles concentration. Being a shellfish that is eaten as food, they are being extensively farmed especially in South-East Asia. Here in Singapore, you would be surprised that the Blood Cockles are also being harvested off the coasts of Eastern Singapore, one prominent company being ‘Ah Hua Kelong’[39] .
Anatomy*
*this section is largely based on NUS’ (National University of Singapore’s) LSM4299 lecture by Dr Tan Koh Siang (TMSI/ NUS) on 17 August 2016.You would be surprised to find out not only do blood cockles have a circulatory system and lips, they have feet as well! Aren't they starting to sound like animals now? Figure 7 below would be used to describe the major parts (labelled a - h) of the blood cockles’ anatomy.
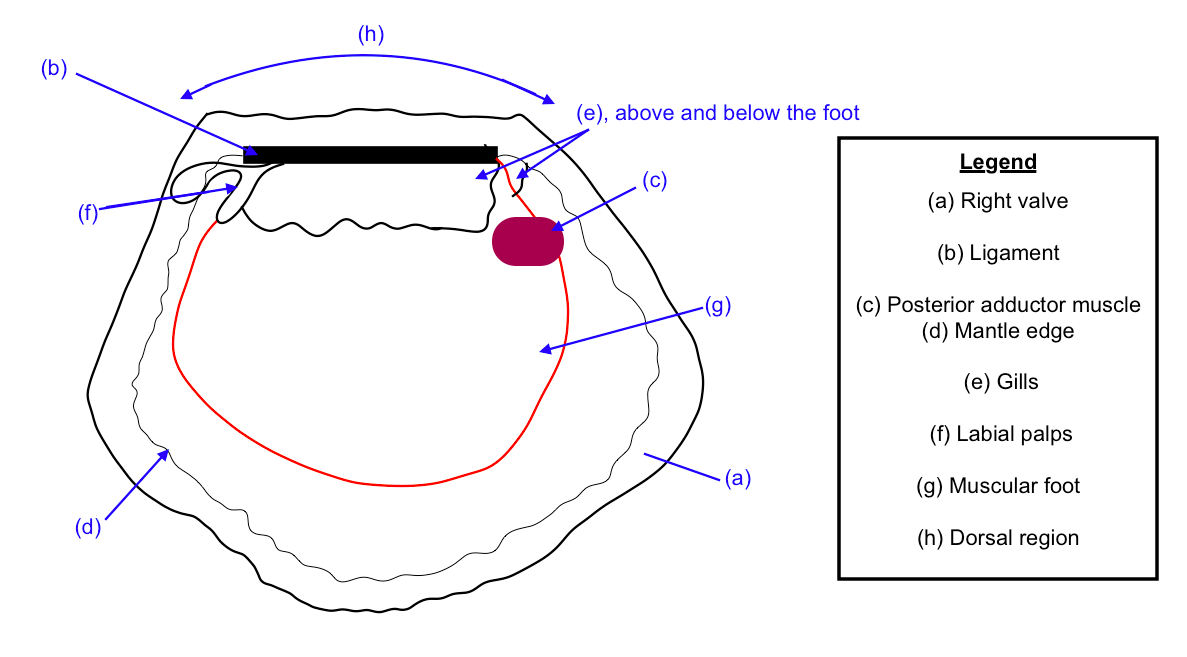
If you are not able to spend time reading this detailed section on the blood cockles' anatomy, Table 1 below is a summary of the parts (a) - (h)
Table 1: Summary of Anatomy sections (a) - (h)
| Part of animal |
Main points/ Function (s) |
| Right Valve |
positioning of the animal can be deduced from where relative positions of certain anatomical features are |
| Ligament |
Control the opening and closing of the shell. This can also help in movement (e.g. to swim or burrow) |
| Posterior Adductor Muscles |
Muscles that relax to open the shell, and contracts to shut the valves. |
| Mantle Edge |
The 'skirting' encompassing the soft body inside |
| Gills |
Used to breathe and to filter food that the sea hum 'sucks' in |
| Labial Palps |
'Lips' |
| Muscular Foot |
Used to move, e.g. by hopping or digging into the sand/ mud |
| Dorsal Region |
The region where the hinge and 'teeth' are present. |
All bivalves have a right and left shell (or valve), even though it is tough to see a difference between the two shells from the exterior. To align the animal appropriately, you would first need to ascertain the anterior and posterior end of the blood cockle. This is mainly done by examining the content of its body inside the shell. One good way is by identifying the labial palp (part f). The labial palp is actually the ‘lips’ of the animal. LIPS? Yes, the blood cockle, like many other bivalves, have lips surrounding the mouth of the animal. Hence, where the mouth is, is where the anterior end is. After identifying the anterior end, point the anterior end upwards. Then, imagine that the hinge (where the two shells are connected, and where is ligament is) is your spine, and your head would be the anterior end, while the bottom half of your body is the posterior. By using your body as a model, it would be easy to ascertain which shell (or valve) is the left or right one. Figure 8 below shows a demonstration of the correct alignment.
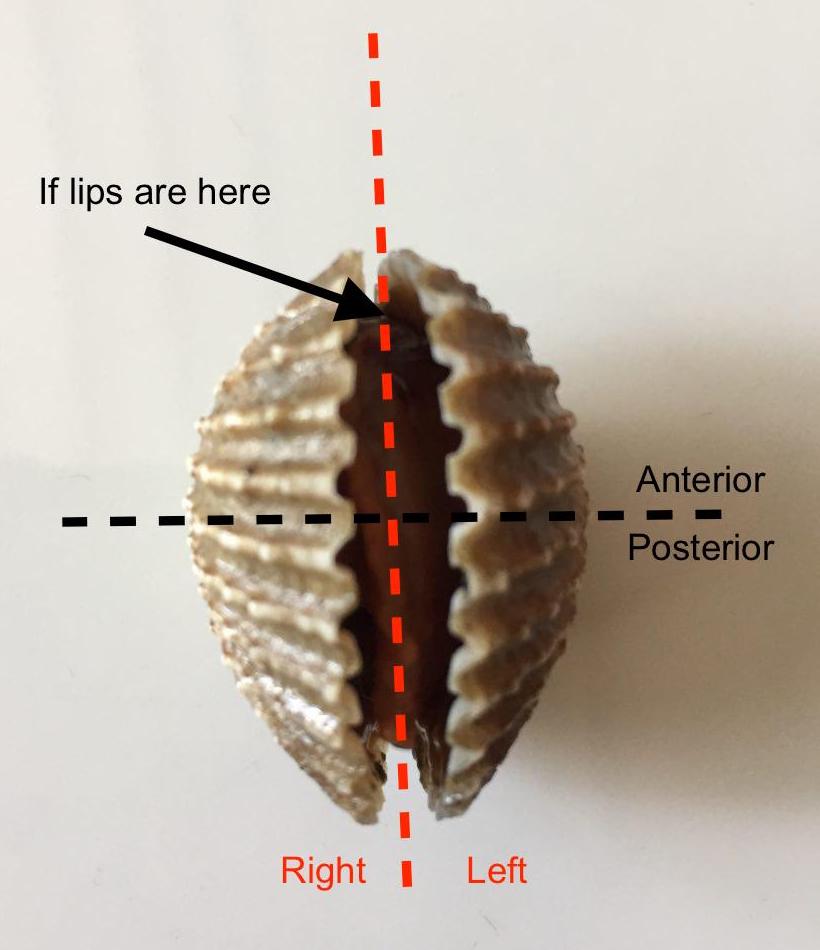
(Author’s personal collection)
b) Hinge Ligament
This ligament is a proteinaceous material that allows the cockles to control the opening and closing of the shell. The hinge ligament works like a muscle, in co-operation with the adductor muscles (c). To open the valves, the adductor muscles contract, while the ligament relaxes[40] . The reverse is true when the bivalve closes. Figure 9 shows how a shell closes from its form in A to B. In the figure, the ligament consists of the ‘fibrous’ and ‘lamellar’ layers.
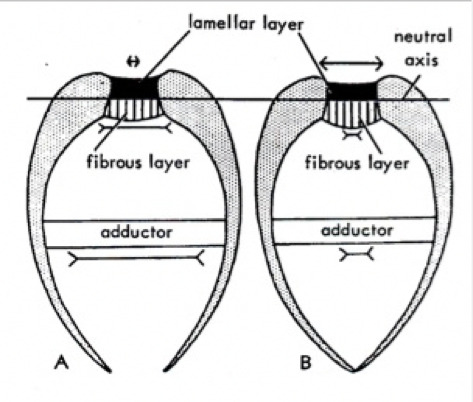
In addition, for some groups of bivalves, like the ‘swimmers’ and ‘burrowers’ use their ligaments to move. ‘Swimmers’ open and close their shells quickly to swim, while burrowers also use it to aid it in burrowing into the mud or ground[41] .
c) Adductor Muscles
The adductor muscles are muscles that are attached to both sides of the valves, making sea hums difficult to open before you toss the ‘meat’ into your mouth. When forcing the shells open, the adductor muscles usually break in the process. As seen in Figure 9, they are vital in helping the animal control the opening and closing of its shell.
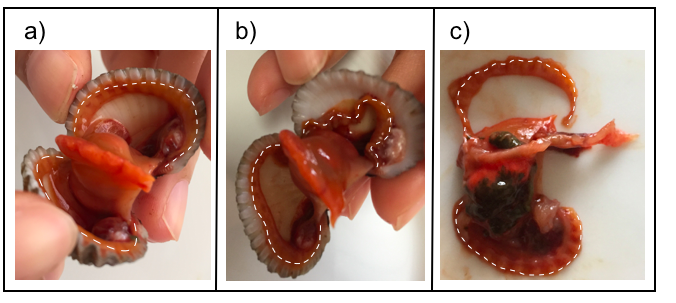
The sea hum has an anterior and posterior adductor muscle, just many other bivalves. The anterior posterior muscle is the one at the anterior of the sea hum, the same side as where the lips (part f) are. However, there are exceptions like the well-known green mussel (Perna viridis), that only has posterior adductor muscles. The positioning and appearance of these muscles can be seen in Figure 10. It appears white-ish, and is the muscle that you feel the resistance from when you are pulling the shells apart. When torn apart, usually there would be a ‘residue’ of the muscle on the inner surface of the shells as seen also in Figure 11.

d) Mantle Edge
The mantle edge is the ribbed ‘skirting’ that encompasses the main body inside. The mantle edges line the internal shell on both sides. Figure 12 shows how the mantle edges look like in a raw blood cockle.
e) Gills (Ctenidium)
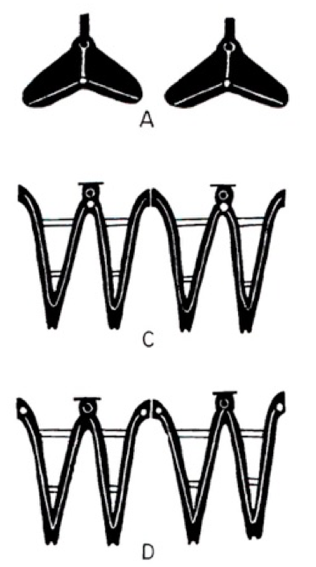
Just like fish have gills, as a marine or estuarine organism, bivalves have gills to breathe, although they might be somewhat structurally dissimilar. There are three different structures of gills, classifying bivalves into three groups: Protobranchs, Filibranchs and Eulamellibranchs. The blood cockle is a filibranch bivalve, as are all other species in the family Arcidae[42] . It might be rather difficult to identify the ctenidium of the blood cockle without any tools, but Figure 13 shows how the three different structures look differ, if you manage to.
The gills are also the organ that filters out their 'food' from the water column (See section on Feeding Habits).
f) Labial Palps (The ‘Lips’)
It is rather difficult to distinguish the labial palps of the blood cockle just by looking at the body inside. However, as compared the other parts of their body, they feel significantly more hardy, similar to how cartilage feels like. As mentioned above, the labial palps are an anatomical component of the body that helps us to discern the anterior and posterior end of the blood cockle, as well as most bivalves. Figures 14 and 15 gives us a closer look at how the lips look in reality.
(Author’s personal collection)
In Figure 15, the labial palps can be seen connected to the gastrointestinal tract, part of the digestive system of the blood cockle.
g) The muscular foot
Like us, bivalves have ‘feet’ as well! However, its anatomy and physiology is very different from the feet that we have. Their ‘feet’ is entirely made up of muscle, and is very versatile in its uses. For a burrowing bivalve such as the blood cockle[43] , one of the main functions of the foot is to dig into enable the the sea hum to dig and burrow into the mud. This could be the case when the bivalve is trying to seek shelter in the sand from the sweltering heat in the intertidal area. The foot is the most noticeable part of the cockle (Figure 16), probably the only part that you initially thought consisted of its ‘body’.
h) Dorsal region
This part of a bivalve is not really part of its anatomy, but more of a position. For the sea hum, it is an area of the bivalve where the hinge, teeth and ligament is. Table 1 below further clarifies these three parts of the sea hum.
Table 2: Descriptions and picture of the three components in the dorsal area of the blood cockle.
| Hinge |
The point where both valves are ‘stuck’ together. |
| Teeth |
Just inside of the hinge. Sea hums have ‘taxodont’ teeth that looks like a row of short, lined indentations (pictured below, author's personal collection). |
| Ligament |
Part b) above |
Ecological Interactions
PredatorsSnails Natica maculosa, Thais carinifer , Bedeva blosvillei
Birds Skates, Tringa nebularia (greenshank)
Fish Plotosus anguillaris
Starfish Asterias amurensis
Infestations
The pea crab (Pinnotheres alcocki, now Arcotheres alcocki) is a species of crustacean that uses the blood cockle as a host[44] [45] . Although there seems to be little information of how the pea crab interacts with the blood cockle, studies of similar species seem to show that the female pea crab functions as a parasite [46] . In other bivalves, the presence of similar crabs in the bivalves show a negative effect as compared to uninfected bivalves. Another example is how Pinnotheres maculatus reduces the ability of mussels to breathe, by reducing filtration rates of the gills [47] .
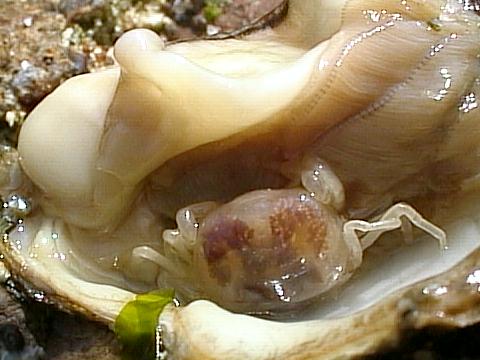
Also, the barnacle Balanus amphitrite can be found encrusted on the shells of aquacultured blood cockles[48] . Being strategically mounted on the posterior side of the bivalve, the side where the blood cockle 'eats' from, the barnacle is essentially 'stealing' the blood cockles' food[49] .
Red Tides & Algal Blooms
As filter feeders (more information in 'Feeding habits' under 'Biology' section below), bivalves like the blood cockle are more vulnerable to changes in water quality. Examples of instances where they might be adversely affected will be in the cases of 'red tides' and algal blooms[50] . Red tides occur when there a great amounts of dinoflagellates (microscopic one-celled algae) [51] (Figure 18) in the sea, tainting the sea red (Figure 19).
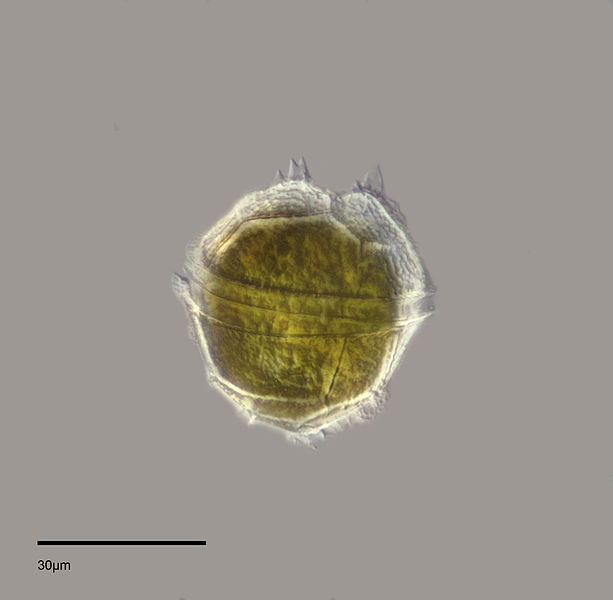

These dinoflagellates can be harmful to a variety of marine animals. There are a variety of effects, but for shellfish like Tegillarca granosa, high levels of toxins and low oxygen conditions that can result from these blooms can threaten their lives.
Ecosystem Significance
Since the blood cockles 'eat' the suspended organic material that is in the water, they also 'clean up' the waters they reside in, resulting in clearer waters. This special characteristic of many bivalves could be used by humans to filter out harmful bacteria in natural areas. One such example is of researchers thinking of 'cleaning up' Mountain Lake, a waterbody that is full of harmful bacteria by using mussels or clams[52] .Many marine bivalves are also used as bioindicators to measure the health of an environment. As group of organisms that is usually ever-present and one that filters huge volumes of water in the environment (at the same time accumulating contaminants), such bivalves serve as important study material to assess characteristics and the ability of an environment to support life[53] . In other words, bivalves are good bioindicators.
Economic Significance
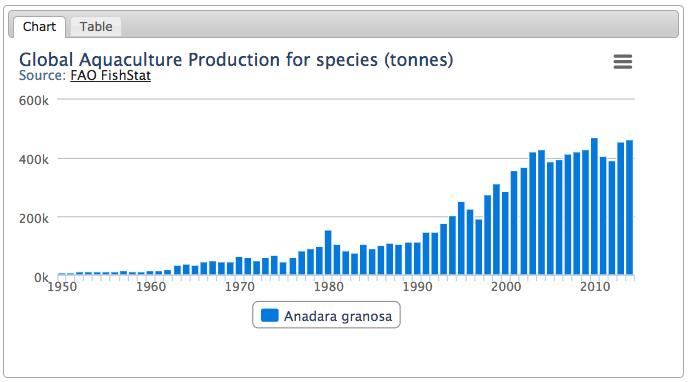
Like many other species in the Anadara genus, the blood cockle is an important seafood, and is cultivated on a large-scale basis for economic purposes in countries like Malaysia and Thailand[54] . This species is actually an ‘Anadarinid’, a ‘marine bivalve mollusc harvested commercially and on a subsistence basis in many warm temperate to tropical countries’[55] . Globally, the aquaculture of Tegillarca granosa has been rising since the start in the early 1950s until 2014 (Figure 20).Figure 20: Global trend of aquaculture production of Anadara granosa (Tegillarca granosa) Source: Food and Agriculture Organization of the United Nations, no date, no author, Species Fact Sheets - Tegillarca granosa, http://www.fao.org/fishery/species/3503/en. Reproduced with permission.
Singapore
Locally, Singapore relies heavily on the import of Blood Cockles from Malaysia[56] . Singapore imports an average of about two thousand tonnes of Blood Cockles from 2014 to 2016 from Malaysia. However, the yield of Blood Cockles in the three major areas (Selangor, Perak and Johor) of blood cockles farming in Malaysia have been dwindling[57] since as early as 2012[58] . Heightened levels of water pollution seem to be the main culprit in reducing these numbers. Since Blood Cockles is an irreplaceable ingredient in many local dishes such as Char Kway Teow and Laksa, it is getting increasingly difficult for the supply from Malaysia as well as one source in Singapore to meet the demand here. One of the rising hypermarkets in Singapore, Sheng Siong, has noted that the price of blood cockle imports has on average, risen by half of its original price half a decade ago[59] . Local sellers seem to be struggling to cover the extra costs. However, our local fishery, Ah Hua Kelong, situated in the East of Singapore seems to be alleviating the stress on supply since it seems that the waters in the East are unaffected by the water pollution[60] .
Asia
As a popular shellfish that is consumed widely in most of Asia, its cultivation forms a significant fishery industry in these Asian countries. China has practised cultivation of the blood cockle for at least 18 years (since 1999), especially on the coastal fringes from Zhejiang to Guangdong as a high-value seafood[61] . The Tegillarca granosa (‘mud cockle’) was the most prized and extensively cultivated among the three major cultivations of blood cockles in the late 20th century, the other two species being Scapharca subcrenata and Scarpharca broughtonii[62] . In Indonesia, cockle production occurs on coast of West Sumatra, Central and South Java, East and West Kalimantan and other muddy bottoms in Sulawesi, Maluku and Papua[63] . In 2009, the annual productions of the blood cockles was valued at US$23.72 million[64] .
Closer to home, Malaysia has been cultivating this species ever since 1948[65] . In fact, the biggest cockle industry is in Malaysia, specifically, the state of Perak (Kuala Gula, Kula Sangga-Matang, Kuala Trong, Sungai Jarum)[66] . In 2009, the annual production of blood cockles was valued at US$36.6million[67] . Other major areas of cockle cultivation are in Kedah (Merbok), Pulau Pinang (Juru), Perak (Kuala Gula, Kula Sangga-Matang, Kuala Trong, Sungai Jarum), Selangor (Kuala Selangor) and Johor (Muar)[68] . Then, it was popular as a cheaper source of protein in their diets[69] . Places in Malaysia where there are significant culturing activities include Penang, Perak and Selangor[70] . Other Asian countries that cultivate the blood cockles include South Korea, Taiwan and Thailand[71] .
Threats
The natural stocks of the blood cockle as an ecological species has been mentioned to be potentially threatened by a parasitic trematode. Such trematodes use bivalves as an intermediate host during its larval stages before moving on to carnivorous fishes that are their definitive hosts [72] . As a cultivated seafood species, there are local and regional concerns that the harvests of farmed blood cockles are dwindling fast. As mentioned in the Economic Significance section, there have been reduced blood cockle harvests in the country with the biggest blood cockle fishery industries in the world, Malaysia. Decreasing harvests of blood cockles is largely suspected to be because of water pollution that flow through the intertidal into the sea[73] [74] . Being in the intertidal zone, these filter-feeders might have accumulated high levels of toxic from the wastes that are being washed down. Other theories include the increasingly fluctuating weather patterns in recent years[75] , resulting in unstable temperatures. Increased greenhouse gases is another threat that affects shelled marine organisms in general. Bivalve shells are made largely of calcium carbonate[76] . When there are higher concentrations of acidic greenhouse gases like carbon dioxide in the atmosphere, these gases dissolve in the seawater, raising the pH of the water (Ocean acidification). The dissolved carbonic acid then react with the calcium carbonate of bivalves, effectively dissolving the shells of bivalves[77] . Figure 21 shows us how badly bivalves (not just Tegillarca granosa) can be affected by ocean acidification.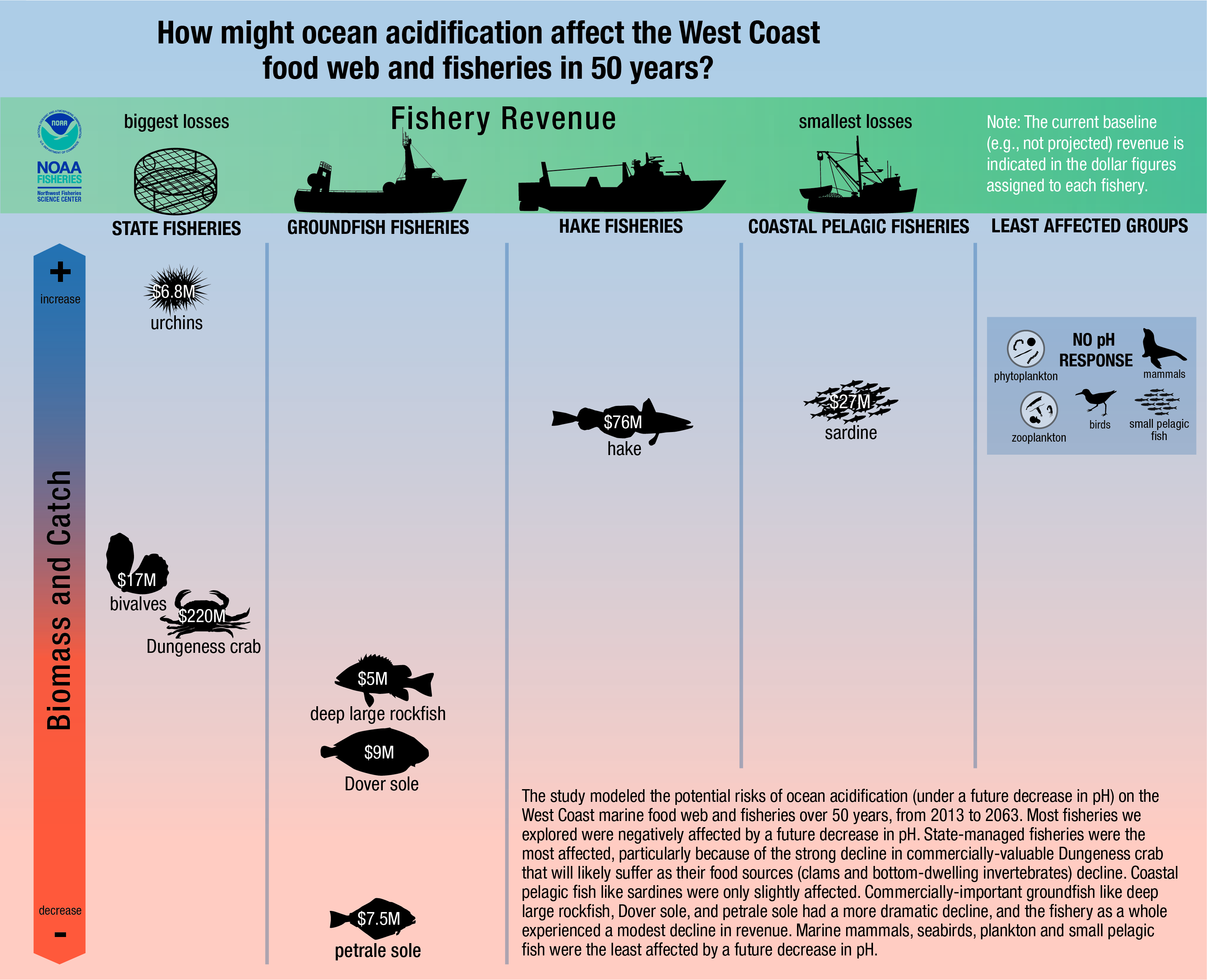
Conservation Status
The conservation status for Tegillarca granosa has not been assessed, and hence not listed in the IUCN (International Union for Conservation of Nature) Red List. It is also not in the CITES database either. On a national scale, it is not listed in the Singapore Red Data Book (2008 edition). Despite there being threats, the Tegillarca gransoa species might not be listed as a conservation priority because as of now because of the active aquaculture of this economically important species as a seafood. Also, there might still be a multitude of locations where they can still thrive.
Cultural and Culinary Significance
SingaporeThe sea hum is an irreplaceable ingredient in popular local dishes in Malaysia and Singapore. One such dish is the Singaporean Laksa (Figure 22).
Along with coconut milk and fishcake, the sea hum is usually a signature ingredient in this dish that is usually found in most hawker centres in Singapore. This dish has origins from Peranakans living in the Katong area[78] , hence also laden with heritage significance.
Another popular Singaporean dish which uses the sea hum is 'char kway teow' (Figure 23). The char kway teow is a Teochew dish that is widely available in hawker centres, and other casual dining establishments in Singapore. Although it has Teochew origins, its name 'char kway teow' means fried kway teow (a type of noodle) in Hokkien. This dish is representative of the mixing of cultures in Singapore, as well as other places like Selangor and Penang in Malaysia that also have their own versions of this dish[79] . This inexpensive dish has been eaten in Singapore ever since about 1950s.

Want to make your own Char Kway Teow? Links to the recipes of the various versions of Char Kway Teow can be found in the recipes section below! Otherwise, head over to the nearest Char Kway Teow stall in Singapore. One of the more famous Char Kway Teow stalls in Singapore is 'Hill street char kway teow' (Figure 24).

Asia
In Malaysia, Malays like to eat it boiled or cooked in curry, while the Chinese boil to eat it with chilli sauce, and also with vermicelli[80] .
In Thailand, blood cockles are very popular and often consumed. Blood cockles are also sold as street food in Thailand, where semi-boiled varieties, 'Hoy kraeng luak' are preferred for their juicier texture[81] . However, because bivalves usually have increased concentrations of harmful chemical and substances (mentioned in Health and Safety section), this practice has health implications.
In South Korea, the blood cockle is called 'ggomak', and is most frequently consumed in the winter, usually in January. A simple yet classic Korean blood cockle dish is the ' ggomak muchim' or blood cockles with soy sauce. A recipe for this Korean dish is linked under the 'Recipes' section. Actually, the Koreans use mainly three types of blood cockles, 'pijogae', 'sae-ggomak' and 'cham-ggomak'[82] . However, there is limited literature on the exact species of each kind. In general, the 'ggomak' is the Tegillarca granosa species (Oh, 2009). Also, one of the best places for tasty cockles in Korea would Beolgyo, in the Jeollanam province, South of South Korea. Cockle farming and cooking seem to be an important part of the economy in that area[83] . Lastly, another way to eat cockles would be to order 'ggomak jungsik' or cockle table, a slew of different varieties of cockles served in different ways, served together with other side dishes[84] .
There are so many dishes that uses the blood cockle as an ingredient. However, reducing stocks stemming from increased water pollution could make it increasingly harder to obtain it (mentioned in Economic Significance section). There have also been notices of stores no longer adding blood cockles into their laksas. One such notice was found in a halal hawker-like food establishment in Singapore, Encik Tan (Figure 25).

- Last interesting section below: How to pick cockles at the supermarket?
How to pick cockles at the supermarket?
Fresh cockles usually:- Do not smell bad
- are tightly closed
- close their shells when touched (Like in Video 2 below)
This information and more about the processes from harvesting to selling different kinds of bivalves at the supermarkets can be found at this link. Video 2 below shows how different individuals of blood cockles react to touch.
Video 2: Blood cockles in supermarket reacting to touch, 'Blood Cockles (//Tegillarca granosa//) in Singapore Supermarket' (Author's own video)
Recipes
Laksa: http://thehoneycombers.com/singapore/laksa-recipe-singapore-style/Laksa (Beginner's): http://rasamalaysia.com/laksa-recipe/2/
Char Kway Teow: http://www.noobcook.com/char-kway-teow/2/
Penang Char Kway Teow: http://www.geniuskitchen.com/recipe/penang-char-kway-teow-32468
Korean ggomak muchim: http://bburikitchen.com/recipe-ggomak-muchim-blood-cockles-with-soy-sauce-dressing
Taxonomy
Description
Informal description:Generally, this bivalve is relatively small and compact. Looking at the blood cockle from the top, the surface of its shell is deeply ridged with vertical lines that run down from its umbo to the head of the animal. From the lateral (side) view, the edges of the shell form an undulating (wave-like) pattern. Its umbo is also prominently protruded.
Formal Description:
The original description of this species was by Linnaeus in his book, Systema Naturæ, which was written in Latin. An English adaptation can be found in a journal article by Narasimham in 1998[85] . The main descriptions are summarised in Table 2. Size of type specimen is under section on Types.
Genus description: 'Shell equivalve or inequivalve with large radial ribs which are nodulated or smooth, ligamental area with chevron markings, taxodont teeth in single straight series, inner margin of shell crenulated and byssus present or absent'[86] .
In summary, this just means that species under the genus Anadara have shells that might or might not be symmetrical to one another, with a distinct ribbed pattern. They have teeth that are in a straight line (Table 1 above in Anatomy section). Some species in the genus can have byssus threads.
Table 3: Main descriptions of the species Anadara granosa, described by Narasimham, 1988[87]
| Shell |
‘of medium size, fairly thick, ovate, convm, inflated, equivalve and inequilateral ; dorsal margin straight, anterior end rounded, sloping ventrally, posterior end obliquely rounded and ventral margin concave’ |
| Size |
‘Height 75.4-90.0 percent in length (mean 81.3); width 66.0-82.0% in length (mean 73.6)’* |
| Teeth |
‘with taxodont dentition in one straight series of short fine teeth which become slightly larger at extremities’ |
| Adductor muscle scars |
‘Posterior adductor scar elongately squarish and anterior scar similar but smaller’ |
| Body |
'Soft body blood red in colour. ' |
Type Information
The 'type' of a genus is a species that is deemed representative of the genus, while the type for a species is a specimen or individual that is deemed representative of the species. A type serves the function of being a reference of the all the attributes of the genus or species. There are many kinds of types, but for the blood cockle, there exist only a syntype. To find out more about types, click here.The type locality is the Mediterranean Sea, according to Linnaeus (although disputed)[88] .
Syntypes exist in Sweden, while original Linnaean specimen are kept by the Linnaean Society in London. The tampering of Linnaean's shell collection after his death rendered the original specimen not useful as a type for the species.
Location of syntype: Zoological Museum, Institute of Zoology, University of Uppsala, Uppsala, Sweden
Description of syntype: Length: 45.7mm, Height: 37mm, Width: 31.8mm
The type species for the genus Anadara that was originally used for this species, is actually //Arca antiquata Linnaeus, 1758[89] .
Taxonavigation
Order: Arcida Family: Arcidae Genus: Tegillarca Species: Tegillarca granosa| Table 4: Hierachy and taxonomic rankings |
||
|---|---|---|
| Taxonomic Hierachy |
Common Name |
|
| Kingdom |
Animalia |
Animal |
| Phylum |
Mollusca |
Molluscs |
| Class |
Bivalvia Linnaeus, 1758 |
Bivalves |
| Subclass |
Pteriomorphia Beurlen, 1944 |
- |
| Order |
Arcoida Stoliczka, 1871 / Arcida |
- |
| Superfamily |
Arcoidea Lamarck, 1809 |
- |
| Family |
Arcidae Lamarck, 1809 |
Ark Clams |
| Subfamily |
Anadarinae Reinhart, 1935 (Combosch & Giribet, 2016)* |
- |
| Genus |
Tegillarca Iredale, 1939 |
- |
| Species |
Tegillarca granosa (Linnaeus, 1758) |
- |
*reference, not author of subfamily name
Phylogeny
Morphology-related phylogeny (Superfamily level)
How do we use morphology to compare organisms?Using morphology to determine how closely-related different species or groups of bivalves seem to be the more popular method, as opposed to using genetic methods (only utilised more recently). Comparing morphology is studying the form and anatomy of the organism in relation to another that you are comparing it with. For example, you can use the number of ridges that are on the shells of the bivalve to compare which are more similar to each other. However, it is important to choose a feature that has some evolutionary significance.
However, it is important to note that using morphology to distinguish species is essentially using the 'Morphological Species Concept' to group individuals to form separate species. There are many species concepts that are used by different taxonomists and scientists studying different organisms. For example, a concept uses the basis of interbreeding populations as a measure of being the same species (i.e. Biological Species Concept), while another, the Phylogenetic Species Concept sensu Mishler and Theriot uses the principle of monophyly to recognise a species. A brief explanation of the mentioned species concepts can be found here.
In the case of the blood cockle, there is a study that uses anatomical features like type of gills and type of 'teeth' to compare the superfamily (Arcoidea) of the blood cockle to other bivalve superfamilies. In the case of these two traits, gill type gives relevant information about the age of when the organism evolved, while 'teeth' type is a very distinguishable structure that can be clearly defined and fall into a set number of categories for the bivalve Class. Such characteristics are important in choosing what features you would use to compare in a morphological analysis.
The mentioned study by Piazzi et al. in 2011[90] , made morphological analyses based on a matrix of A, gill grade, B, hinge, C, gill cilia, D, stomach type, E, labial palps, F, shell microstructure. From comparing all the features from A-F simultaneously, and then using maximum likelihood analyses and maximum parsimony to select the best-fit tree, the study ultimately came up with one phylogenetic tree, marked in differing colours in Figure 26 below.
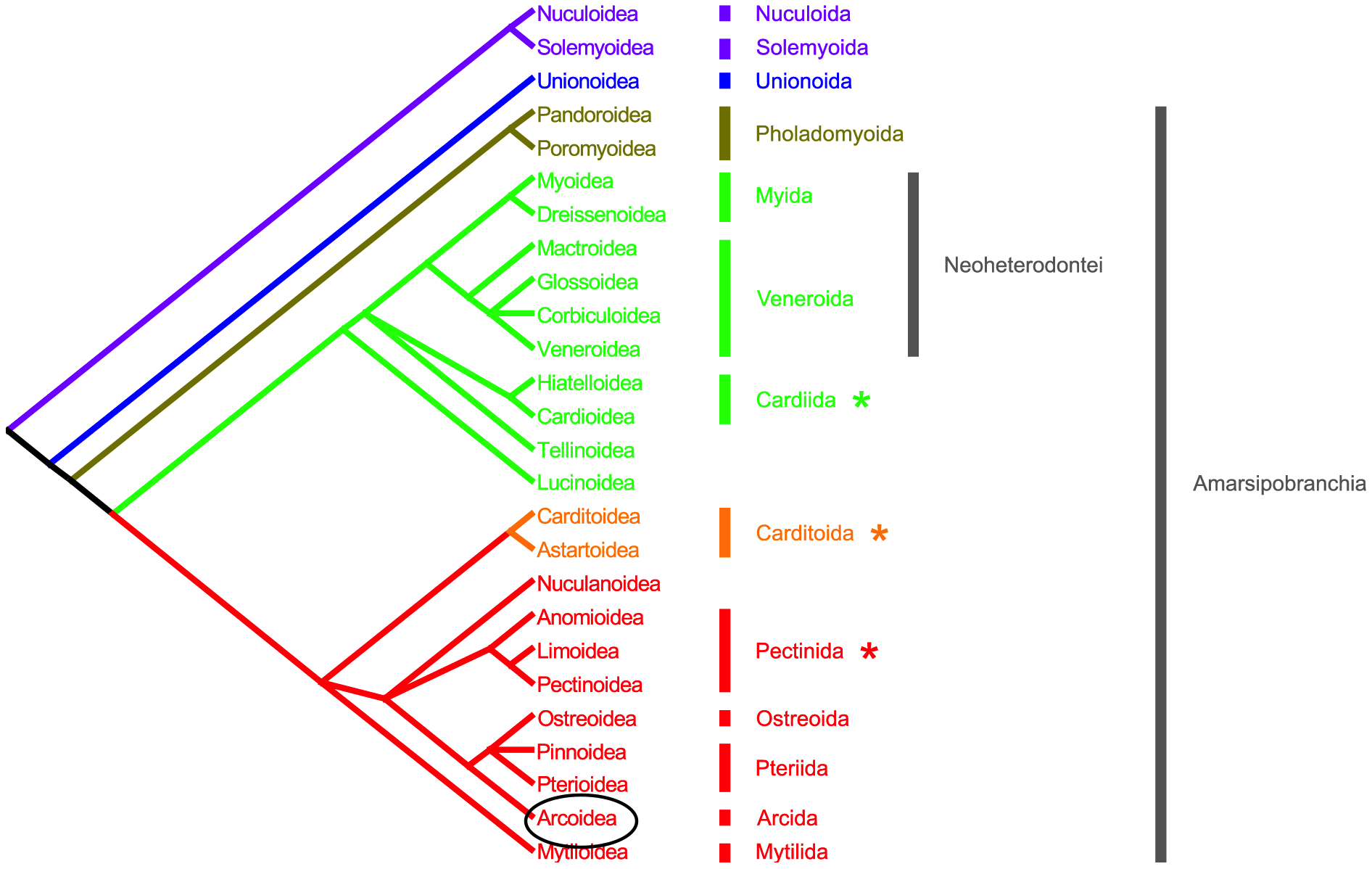
From this phylogenetic tree, we can say that the superfamily Arcoidea is most closely related to the superfamily Pterioidea, the superfamily consisting of species like the pearl oyster. However, sometimes, some scientists prefer to use genetic studies instead of morphological because there might be less subjective discrepancies, and also because there are cases where two species could be undistinguishable. Now, we can look at a family-level genomic analysis for Arcidae, the family of Tegillarca granosa.
Genomic-related phylogeny
Family levelUsing 18S rDNA genomic data, the family Arcidae is found to be the only family of bivalves that is not monophyletic (polyphyly of Barbatia, paraphyly of Pteriida, Pteriidae, Arcidae and Arcinae)[92] [93] . 18S ribosomal DNA was used in the evaluation because mitochondrial DNA evolves more rapidly than nuclear DNA, and is less useful for earlier changes in its evolutionary history, and also, most genomic data available was of 18S rDNA[94] . This is also found to be the case in another study by Plazzi et al. in 2011[95] . However, the methodology of this study might not be the most foolproof. With consideration to the reasons stated above for the use of ribosomal DNA for this study, it might still be better if protein-encoding genes (some nuclear or mitochondrial genes) were used. This is because changes in a non-protein-encoding gene might not really reflect morphological or physiological characteristics of the organism, and therefore its evolutionary fitness and changes.
Species level
As for the species itself, there have been surprisingly little phylogenetic studies done. It was only recently in 2015 that the phylogenetic relationships below the level of Order Arcida are clearly defined using genetic data. In the 2015 paper authored by Combosch and Giribet[96] , 55 species (6 out of 7 extant families, 19 genera) in the Order Arcida was used to study phylogenetic relationships using three nuclear markers (ribosomal: 18S rRNA, 28S rRNA ; protein-coding: histone H3). Three specimen of Tegillarca granosa was used in this study, (1) from Hainan, China, (2) from Wenzhou, China and (3) Rayong, Thailand. RAxML was used for maximum likelihood analyses, and support for nodes were evaluated using the Bayesian probability model. The tree is below (Figure 27).
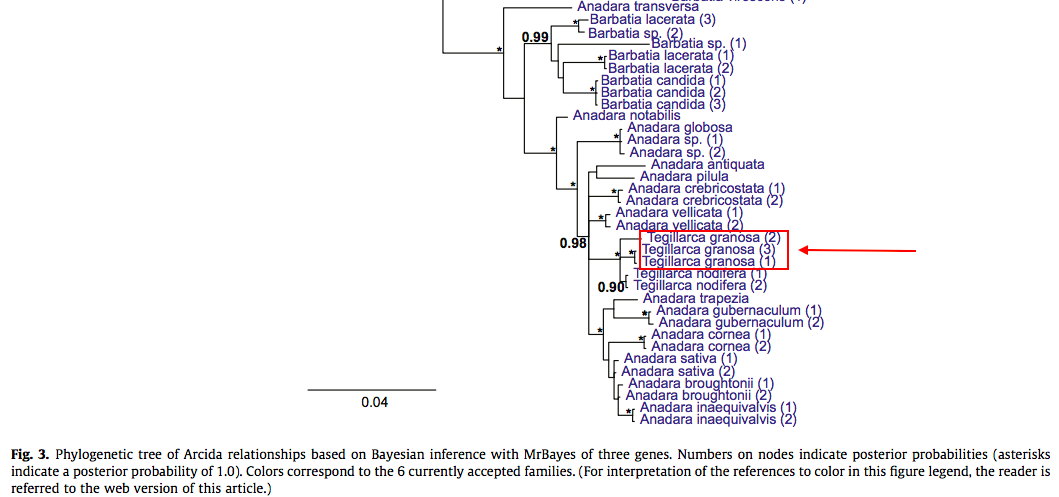
From Figure 27, we can see that the specimen from Wenzhou, China is significantly different from specimen from the other two localities for Tegillarca granosa. Since the pp values for the nodes determining relationships between the three specimen are high (pp of at least 0.9), further studies could be done to determine if there could be a different species arising from Tegillarca granosa, or possible subspecies. The two species in the genus Tegillarca (Tegillarca gransoa and Tegillarca nodifera) that was included in this study form a monophyletic clade among various other species in the Order Arcida.
Another study was one done in 2013, using 12S rDNA, 16S rDNA and Cyt b genes of 7 different species of similar-looking blood cockles. Genetic analyses are crucial in the case of these species because morphological differences (usually using the number and type of ribs on the shell) change with maturity, and also because the number of ribs on blood cockle species are often found to be the same[98] . The study yielded the phylogenetic tree in Figure 28 below. This is a good example when morphological methods fail.
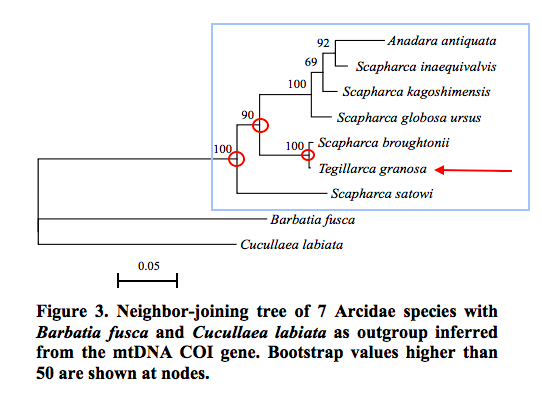
This study uses the neighbour-joining method to put together the tree. After which, bootstrap analyses were done to determine the strength of each node (points of intersection on the tree).
What is the neighbour-joining method?
It is using an algorithm that takes into account the different tree lengths, and uses them to string together different groups of organisms or species based on how long or short their lengths are on the phylogenetic tree. This is hence, a way to choose the best tree, using the tree that has the highest optimality score. This method is especially useful in identifying certain groups that are very different/ distant from other groups.
What is bootstrapping?
Bootstrapping is a statistical method to determine how well the original genomic data is explained by the resulting phylogenetic tree. In general, a bootstrap score is measured upon a 100(%). If a certain relationship is tested, and has a bootstrap score of 98 (%), this means that the relationship on the tree was 98% accurate in explaining the data, and hence has strong support.
Another method to determine support for nodes: Jackknife method
Hence, for the tree in Figure 28, the bootstrap values at the nodes preceding the output of Tegillarca granosa (circled in red in Figure 28) are rather high, this reflects that the relationships are very strongly supported. Tegillarca granosa has rather distinct rDNA and Cyt b genes for a blood cockle, as compared to other closer related species like Anadara antiquata, Scapharca inaequivalvis, S. kagoshimensis and S. globosa ursus that are clustered in a separate sister clade.
Morphological vs Genomic phylogenetic comparisons
Table 5: Morphological vs Genomic comparisons
| Morphological |
Genomic |
| Used for visibly different organisms |
Used when individuals cannot be easily differentiated by eye |
| Accuracy of comparisons might be affected by differences in morphology at different life stages |
Affected by different types of alignment softwares used for sequences. |
| Accuracy of comparisons might be affected by sexual dimorphism and intra-specific variability in morphology. |
Not affected by gender of specimen, although there might still be intra-specific variations. |
| Number of species determined depends on which part of the specimen the author decides to look for differences |
Number of species determined depends on which part of the sequences the author decides to look for differences. |
| Number of species determined depends on how many traits the author identifies as a characteristic only of the species. |
Number of species determined depends on different similarity thresholds decided by the author |
Changes in scientific name of Tegillarca granosa
Changes to the species name are as follows:- initially described and named by Linneaus in 1758 as 'Arca granosa' in his book Systema Naturæ.
- changed to be belonging to genus Anadara instead (Anadara granosa), this name is still rather frequently used. This genus was described by Gray in 1847.
- finally, changed to its present name, Tegillarca granosa. The Tegillarca genus was described more recently in 1939 by Iredale.
Links to other types of species pages
Species Page on Ark Shells: http://www.wildsingapore.com/wildfacts/mollusca/bivalvia/arcidae/arcidae.htmFAO's species sheet on Tegillarca granosa: http://www.fao.org/fishery/species/3503/en.
*All information and images are used according to Creative Commons Attribution CC BY-SA 2.0 / and Fair Use guidelines. If you are an owner and have qualms about any of your information or images shown on this page, please contact me, Ang Ju Hui at a0131255@u.nus.edu. Thank you.
- ^ Broom, M. J. (1985). The biology and culture of marine bivalve molluscs of the genus Anadara (Vol. 12). WorldFish
- ^ Broom, M. J. (1985). The biology and culture of marine bivalve molluscs of the genus Anadara (Vol. 12). WorldFish
- ^ Afiati, N. (2007). Hermaphroditism in Anadara granosa (L.) and Anadara antiquata (L.)(Bivalvia: Arcidae) from central Java. Journal of Coastal Development, 10(3),
171179. - ^ Pathansali, D., & Soong, M. K. (1958). Some aspects of cockle (Anadara granosa L.) culture in Malaya. Proceedings of the IndoPacific
Fisheries Council, 8(2), 2631. - ^
Davenport, J., & Wong, T. M. (1986). Responses of the blood cockle Anadara granosa (L.)(Bivalvia: Arcidae) to salinity, hypoxia and aerial exposure. Aquaculture,
56(2), 151162.
Narasimham, K. A. (1988). Biology of the blood clam Anadara granosa (Linnaeus) in Kakinada Bay. Journal of the Marine Biological Association of India, 30(1&2),
137150.
FAO Corporate Document Repository – Fisheries and Aquaculture Department (2017).The hatchery culture of bivalves: a practical manual. Fao.org. Retrieved from
http://www.fao.org/docrep/007/y5720e/y5720e07.htm
137150.
Fisheries Council, 8(2), 2631.
sediments. Songklanakarin Journal of Science & Technology, 31(5).
FAO Fisheries & Aquaculture (n.d.). Species Fact Sheets – Anadara granosa. Food and Agriculture Organisation of the United Nations. Retrieved from
http://www.fao.org/fishery/species/3503/en .
Dame, R. F. (1996). Ecology of marine bivalves: an ecosystem approach. CRC press.
the oyster Crassostrea virginica and the
mussel Geukensia demissa, Mar. Ecol. Prog. Ser., 58, 299310.
Jørgensen, C. B. (1990). Bivalve filter feeding: hydrodynamics, bioenergetics, physiology and ecology. Olsen & Olsen.
Dame, R. F. (1996). Ecology of marine bivalves: an ecosystem approach. CRC press.
FAO Fisheries & Aquaculture (n.d.). Species Fact Sheets – Anadara granosa. Food and Agriculture Organisation of the United Nations. Retrieved from
http://www.fao.org/fishery/species/3503/en .
Pathansali, D., & Soong, M. K. (1958). Some aspects of cockle (Anadara granosa L.) culture in Malaya. Proceedings of the IndoPacific
Fisheries Council, 8(2), 2631.
Broom, M. J. (1985). The biology and culture of marine bivalve molluscs of the genus Anadara (Vol. 12). WorldFish
Pathansali, D., & Soong, M. K. (1958). Some aspects of cockle (Anadara granosa L.) culture in Malaya. Proceedings of the IndoPacific
Fisheries Council, 8(2), 2631.
Pathansali, D., & Soong, M. K. (1958). Some aspects of cockle (Anadara granosa L.) culture in Malaya. Proceedings of the IndoPacific
Fisheries Council, 8(2), 2631.
Fisheries Council, 8(2), 2631.
http://www.fao.org/fishery/species/3503/en
Broom, M. J. (1985). The biology and culture of marine bivalve molluscs of the genus Anadara (Vol. 12). WorldFish
Mirsadeghi, S. A., Zakaria, M. P., Yap, C. K., & Gobas, F. (2013). Evaluation of the potential bioaccumulation ability of the blood cockle (Anadara granosa L.) for
assessment of environmental matrices of mudflats. Science of the Total Environment, 454, 584597.
Agri-Food and Veterinary authority of Singapore (n.d.) Commercial Food Imports. Retrieved from https://www.ava.gov.sg/explore-by-sections/food/bringing-food-into-singapore-and-exporting/commercial-food-imports
Mirsadeghi, S. A., Zakaria, M. P., Yap, C. K., & Gobas, F. (2013). Evaluation of the potential bioaccumulation ability of the blood cockle (Anadara granosa L.) for
assessment of environmental matrices of mudflats. Science of the Total Environment, 454, 584597.
Sany, S. B. T., Hashim, R., Rezayi, M., Salleh, A., Rahman, M. A., Safari, O., & Sasekumar, A. (2014). Human health risk of polycyclic aromatic hydrocarbons from
consumption of blood cockle and exposure to contaminated sediments and water along the Klang Strait, Malaysia. Marine pollution bulletin, 84(1), 268279.
Journal of hazardous materials, 172(1), 112.
Bilung, L. M., Radu, S., Bahaman, A. R., Rahim, R. A., Napis, S., Ling, M. W. C. V., ... & Nishibuchi, M. (2005). Detection of Vibrio parahaemolyticus in cockle
(Anadara granosa) by PCR. FEMS microbiology letters, 252(1), 8588.
Wiens, M., (2016). Bangkok's Blood Cockle Street Food Bar (หอยแครงป้าจิน). Thai Street Food, Restaurants, and Recipes | Eating Thai Food. Retrieved from
https://www.eatingthaifood.com/bestbloodcocklesbangkok/
Narasimham, K. A. (1988). Biology of the blood clam Anadara granosa (Linnaeus) in Kakinada Bay. Journal of the Marine Biological Association of India, 30(1&2),
137150.
Chenyze, M & Ong, S. (2016). 5 Farms You Never Knew Existed in Singapore, The Singapore Women's Weekly. Retrieved from
http://www.womensweekly.com.sg/family/5farmsneverknewexistedsingapore/
Kahler, G. A., Fisher Jr, F. M., & Sass, R. L. (1976). The chemical composition and mechanical properties of the hinge ligament in bivalve molluscs. The Biological
Bulletin, 151(1), 161181.
Kahler, G. A., Fisher Jr, F. M., & Sass, R. L. (1976). The chemical composition and mechanical properties of the hinge ligament in bivalve molluscs. The Biological
Bulletin, 151(1), 161181.
Yoloye, V. (1975). The habits and functional anatomy of the West African bloody cockle, Anadara senilis (L.). Journal of Molluscan Studies, 41(4), 277299.
Patel, B., & Eapen, J. T. (1989). Physiological evaluation of naphthalene intoxication in the tropical acrid clam Anadara granosa. Marine Biology, 103(2), 193202.
Narasimham, K. A. (1988). Biology of the blood clam Anadara granosa (Linnaeus) in Kakinada Bay. Journal of the Marine Biological Association of India, 30(1&2),
137150.
Afiati-Brotohadikusumo (2002) N. Pinnotherid Infestation in Anadara antiquata (L.) and Anadara granosa (L.)(Bivalvia: Arcidae): The Physiological Consequences of Harbouring a Symbiont. Dept. of Fisheries, Faculty of Fisheries & Marine Sciences, Diponegoro University
Afiati-Brotohadikusumo (2002) N. Pinnotherid Infestation in Anadara antiquata (L.) and Anadara granosa (L.)(Bivalvia: Arcidae): The Physiological Consequences of Harbouring a Symbiont. Dept. of Fisheries, Faculty of Fisheries & Marine Sciences, Diponegoro University
Afiati-Brotohadikusumo (2002) N. Pinnotherid Infestation in Anadara antiquata (L.) and Anadara granosa (L.)(Bivalvia: Arcidae): The Physiological Consequences of Harbouring a Symbiont. Dept. of Fisheries, Faculty of Fisheries & Marine Sciences, Diponegoro University
Tan, R. (2017a). Ark clams (Arcidae) on the Shores of Singapore. Wildsingapore.com. Retrieved from http://www.wildsingapore.com/wildfacts/mollusca/bivalvia/arcidae/arcidae.htm
LiveScience. (2014) Mussels And Clams Can Clean Up Polluted Water. Seeker. Retrieved from https://www.seeker.com/musselsandclamscancleanuppollutedwater1768972732.
html
Dame, R. F. (1996). Ecology of marine bivalves: an ecosystem approach. CRC press.
Broom, M. J. (1985). The biology and culture of marine bivalve molluscs of the genus Anadara (Vol. 12). WorldFish
(2016, May 24). A clammy situation for cockle importers. The New Paper. Retrieved from http://www.tnp.sg/news/singapore/clammysituationcockleimporters
Guo, X., Ford S. E., & Zhang, F., (1999). Molluscan Aquaculture in China. Journal of Shellfish Research 18 (1): 19312
JSPS conferences, United Nation University, Tokyo, 15–16 Dec 2009
Ocean Science Journal, 52(1), 7589.
Pathansali, D., & Soong, M. K. (1958). Some aspects of cockle (Anadara granosa L.) culture in Malaya. Proceedings of the IndoPacific Fisheries Council, 8(2), 2631.
http://www.fao.org/fishery/species/3503/en .
Ocean Science Journal, 52(1), 7589.
Ocean Science Journal, 52(1), 7589.
Fisheries Council, 8(2), 2631.
Uddin, M. J., Yasin, Z., Khalil, M., & ShauHwai, A. T. (2011). Parasites of blood cockle (Anadara granosa Linnaeus, 1758) from the Straits of Malacca. Journal of Shellfish Research, 30(3), 875880.
(2017). "Laksa". Visitsingapore.Com. Retrieved from http://www.visitsingapore.com/diningdrinkssingapore/localdishes/laksa/
Tan, B., (2017). National Library Board, S. Char kway teow | Infopedia. Eresources.nlb.gov.sg. Retrieved from http://eresources.nlb.gov.sg/infopedia/articles/SIP_20150728_
134215.html.
Pathansali, D., & Soong, M. K. (1958). Some aspects of cockle (Anadara granosa L.) culture in Malaya. Proceedings of the IndoPacific
Fisheries Council, 8(2), 2631.
Wiens, M., (2016). Bangkok's Blood Cockle Street Food Bar (หอยแครงป้าจิน). Thai Street Food, Restaurants, and Recipes | Eating Thai Food. Retrieved from
https://www.eatingthaifood.com/bestbloodcocklesbangkok/
Swanson, S., (2016). Ggomak: Blood cockles · bburi kitchen. bburi kitchen. Retrieved from http://bburikitchen.com/ggomakbloodcockles
Narasimham, K. A. (1988). Biology of the blood clam Anadara granosa (Linnaeus) in Kakinada Bay. Journal of the Marine Biological Association of India, 30(1&2),
137150.
Narasimham, K. A. (1988). Biology of the blood clam Anadara granosa (Linnaeus) in Kakinada Bay. Journal of the Marine Biological Association of India, 30(1&2),
137150.
137150.
Narasimham, K. A. (1988). Biology of the blood clam Anadara granosa (Linnaeus) in Kakinada Bay. Journal of the Marine Biological Association of India, 30(1&2),
137150.
Narasimham, K. A. (1988). Biology of the blood clam Anadara granosa (Linnaeus) in Kakinada Bay. Journal of the Marine Biological Association of India, 30(1&2),
137150.
Plazzi, F., Ceregato, A., Taviani, M., & Passamonti, M. (2011). A molecular phylogeny of bivalve mollusks: ancient radiations and divergences as revealed by
mitochondrial genes. PLoS One, 6(11), e27147.
mitochondrial genes. PLoS One, 6(11), e27147.
Campbell, D. C. (2000). Molecular evidence on the evolution of the Bivalvia. Geological Society, London, Special Publications, 177(1), 3146.
mitochondrial genes. PLoS One, 6(11), e27147.
Campbell, D. C. (2000). Molecular evidence on the evolution of the Bivalvia. Geological Society, London, Special Publications, 177(1), 3146.
mitochondrial genes. PLoS One, 6(11), e27147.
Combosch, D. J., & Giribet, G. (2016). Clarifying phylogenetic relationships and the evolutionary history of the bivalve order Arcida (Mollusca: Bivalvia: Pteriomorphia).
Molecular phylogenetics and evolution, 94, 298312.
Molecular phylogenetics and evolution, 94, 298312.
Tanaka, T., & Aranishi, F. (2013). Mitochondrial DNA markers for PCR-based phylogenetic analysis of ark shells. Open†Journal†of†Marine†Science, 3(4), 182.