Table of Contents
 |
| Photo by: Ria Tan(Attribution-NonCommercial-NoDerivs 2.0 Generic) |
Common Names: Fluted giant clam, scaled clam, squamosa clam
Introduction
Once rumored to be man-eaters for their huge size and ability to clam up , the giant clams are the largest and heaviest bivalves known on Earth today[1] . The fluted giant clams are native to the South Pacific and Indian oceans, including Singapore waters[2] . Found in the coral reef ecosystems, these giant clams play important ecological roles as they provide food and shelter, contribute to reef productivity, and help shape the reefs[3] . While many regard giant clams as sessile organisms, this webpage will present on the locomotion, aggregation, and other unexpected behaviors exhibited by the fluted giant clam. |
| Photo by: Dr Neo Mei Lin Permission granted |
Etymology
The genus 'Tridacna' came from the Latin word 'Tri' meaning three, and 'dacn' from the Greek word meaning bite or sting. The species 'squamosa' from the Latin word 'squama', meaning scales and the Latin root '-osa', meaning full of.Tridacna squamosa could thus be translated to “three bites full of scales”. The “three bites” refers to the tooth pattern of the zig-zag mating of the two shells, and “full of scales” refers to the ridge-like scales covering the exterior of the shell.
Habitat and Distribution
Tridacna squamosa lives in close association with coral reefs (typically anchored amidst Acropora corals) throughout the Indo-Pacific[4] . They are usually limited to shallow waters where they receive the most light.Worldwide
This species is relatively comospolitan in distribution[5] . The following map shows the global distribution of the Tridacna squamosa.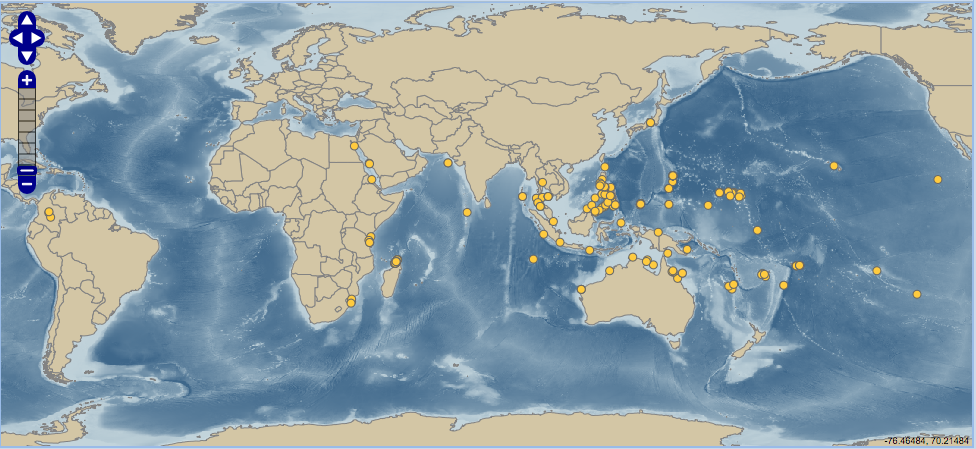 |
| Global distribution of Tridacna squamosa (provided by: iobis.org[6] ) |
This species can be found in the fringing reefs and patch reefs of Singapore's Southern Islands[7] . During sufficiently low tides, the fluted giant clam may be exposed. With the opening of the Sisters' Islands Marine Park, certified divers can have an underwater experience with the native Tridacna squamosa of Singapore.
| Markers showing reported locations of Tridacna squamosa in Singapore (Google Maps 2015) |
Description
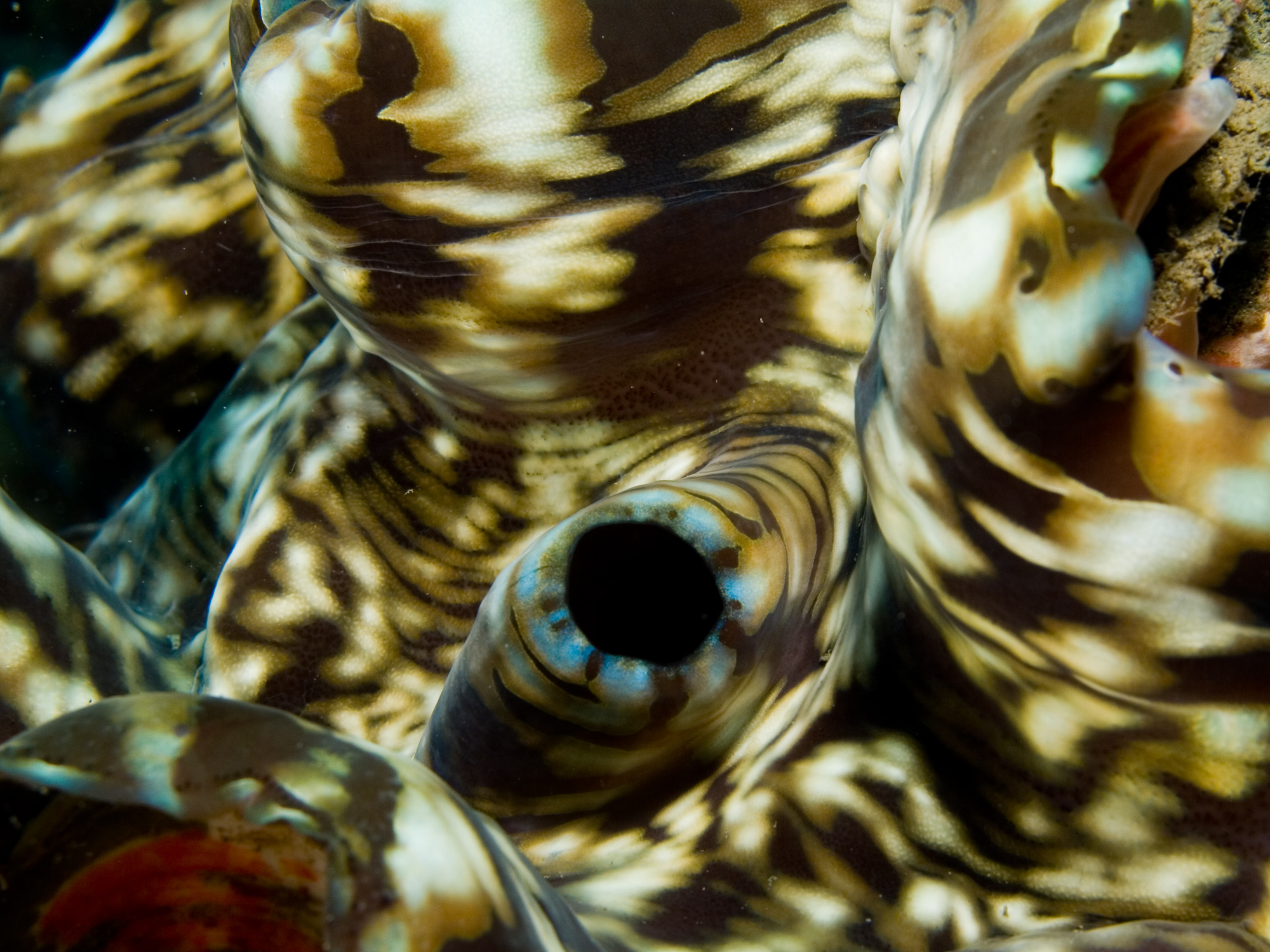
Tridacna squamosa can be identified by the large, leaf-like fluted scales on its shell, which are often used as shelter by organisms such as small crabs, clams, and other invertebrates[8] . These scales run parallel from the rim to the bottom of the shell. The mantle is normally brown, generally mottled with linear and/or spotted markings of varying colours, such as green, blue, and purple. Mantle extension can be well past the margin, completely hiding the shell and scutes[9] .General Anatomy
Like any bivalve, the giant clam has two valves of shell that may or may not entirely enclose the inner soft body. The soft body is covered by fleshy mantle which is usually pigmented. The bright and attractive color patterns are unique to each clam. The giant clams are equipped with two siphons, the incurrent siphon and the excurrent siphon, which ingest water with food and eject bodily fluids respectively[10] .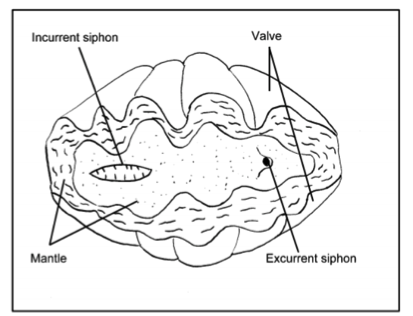 |
 |
 |
 |
| General anatomy of a giant clam(Image by Ellis 1999[11]) |
Photo by Ria Tan (Attribution-NonCommercial-NoDerivs 2.0 Generic) |
Incurrent Siphon lined with tentacles (Image by Nick Hobgood, Creative Commons Attribution-Share Alike 3.0 Unported) |
Excurrent Siphon (Image by Nick Hobgood, Creative Commons Attribution-Share Alike 3.0 Unported) |
Shell Morphology
Observable features of the shell which may serve as identification aids: Hinge, Umbo, Byssal Orifice, Upper Margins, Ribs, Folds, Scutes.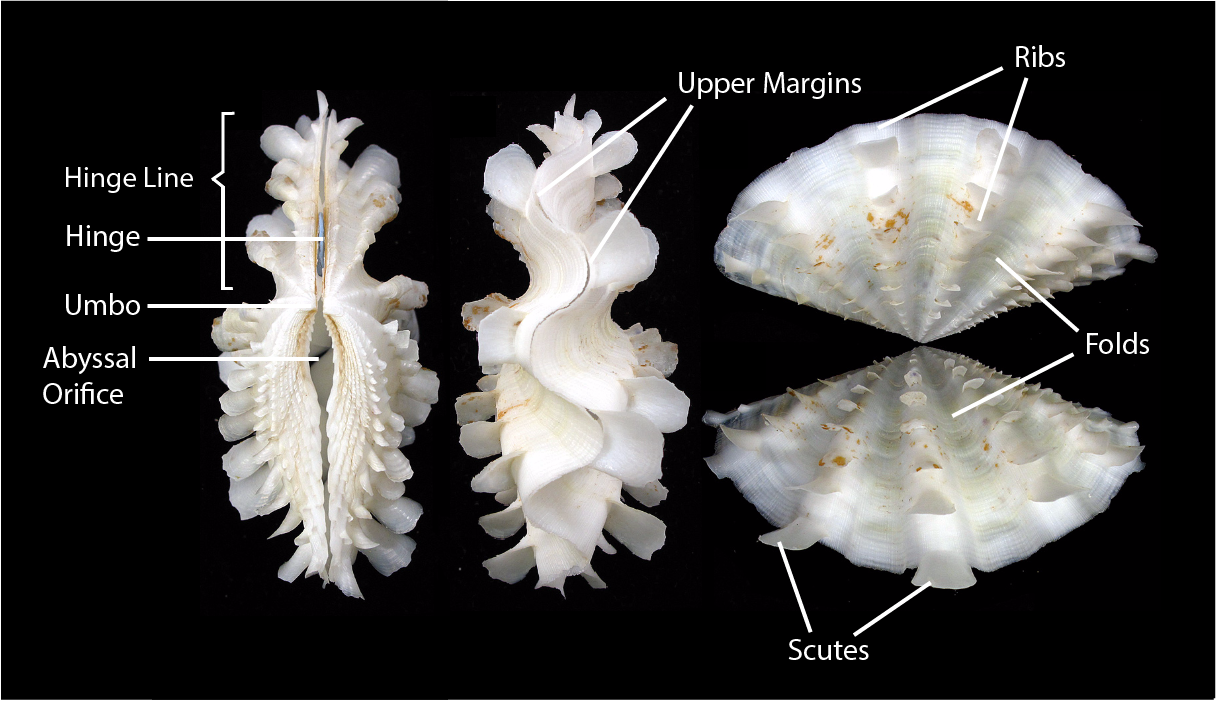 |
| Labeled shell morphology of the fluted giant clam. Photo by Dr Neo Permission granted. Edited by Tricia Poh. |
Species Diagnosis
There are five native giant clam species in Singapore, two of which (Tridacna gigas and Hippopus hippopus) were presumed to be nationally extinct[12] . In general, mantle colourations and patterns are not the diagnostic features one should look at to differentiate between giant clam species because these features exhibit large variations within species as well as similarities between species[13] . The following morphological characteristics can help in distinguishing the three Tridacna species (Tridacna squamosa, Tridacna maxima, and Tridacna crocea) found in Singapore.Table 1. Morphology characteristics distinguishing between Tridacna squamosa, Tridacna maxima, and Tridacna crocea. (Information from Knop 1996[14] , Rosewater 1965[15] , and Fatherree 2002[16] . Illustration by Bradley 1987[17] . Photos by Dr Neo Mei Lin – Permission granted)
| Distinguishing Characteristics |
Tridacna squamosaFluted giant clam |
Tridacna maximaMaxima clam |
Tridacna croceaBoring clam |
| Maximum shell length |
41 cm |
35 cm |
15 cm |
| Byssal orifice |
Medium to small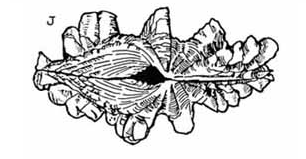 |
Large (relative shorter than that of T. crocea)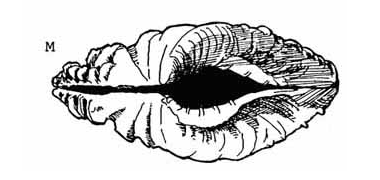 |
Large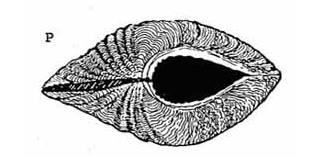 |
| Ribs |
4 - 6 large, well spaced distinct ribs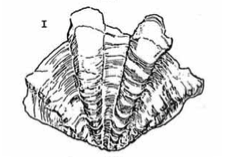 |
5 distinct ribs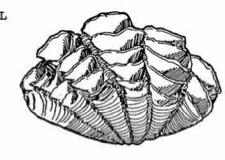 |
5 - 6 low ribs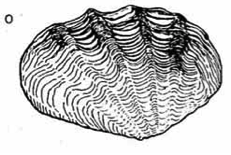 |
| Scutes |
Broad, well-spaced leaf-like projecting scutes |
Smaller closely-spaced scutes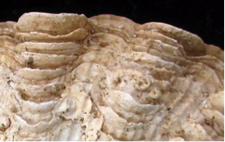 |
Tightly-spaced light scutes usually eroded away bythe natural burrrowing activities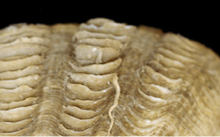 |
| Upper margin |
Strongly curved |
Strongly curved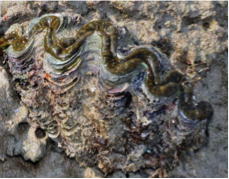 |
Moderately curved |
| Hinge line |
Half of shell length |
Less than half of shell length |
Less than half of shelf length |
| Symmetry |
Strongly symmetrical |
Asymmetrical |
Asymmetrical |
Back to top
Biology and Behavior
Symbiosis
Giant clams share a symbiotic relationship with zooxanthellae (Symbionidium spp.), a type of photosynthetic microalgae, living in their fleshy mantle[18] [19] . The clam host the zooxanthellae, providing shelter and protection from UV, while the zooxanthellae provides food to their clam hosts.Zooxanthellae are introduced into juvenile clams through their feeding organs and move from the stomach of the clams into the mantle through the tubule system (zooxanthellal tubules)[20] . These zooxanthellae do not pass from one generation to another[21] .
Feeding
Tridacna squamosa is a filter feeder that strains food from the surrounding water brought across their enlarged gills by ciliated cells[22] . Visible branching tentacles line the incurrent siphon and are used for filter feeding. Most clams fulfill their nutritional requirements by filter feeding and absorbing dissolved organic compounds from the water, but tridacnid clams have gone further than this by using zooxanthellae algae in their tissue to manufacture food for them[23] . The zooxanthellae transforms carbon dioxide and dissolved nitrogen, such as ammonium, into carbohydrates and other nutrients for their hosts.Reproduction and Life Cycle
Giant clams first attain sexual maturity as males and become hermaphrodites about a year later[24] . Having both male and female reproductive organs, the giant clam spawn sperms first followed by eggs. Producing the gametes separately helps prevent self-fertilisation, although self-fertilisation can occur. During the spawning season, the egg spawning of one individual will chemically trigger sperm spawning of nearby clams, ensuring that gametes meet . Millions of sperms and eggs are produced and released each time, but few of them will survive to adulthood[25] .The fertilised eggs quickly enter a swimming stage, as trochophores, before entering a planktonic stage. During this stage, the larvae, known as veligers, inhabit the open ocean before settling in the subtrate[26] . As veligers, dispersal by currents and the search for optimal settlement sites are key factors of early-life survival[27] . During the transition to the pediveliger stage, the free-swimming larvae leaves the water column to metamorphose into benthic juvenile [28] . In giant clams, pediveligers generally develop 6 - 14 days after fertilisation. They have a functionally active foot which allows for benthic locomotion activities and active exploration for suitable substrates. Giant clam larvae have the tendency to settle on substrates which provide shelter in the form of grooves or crevices [29] . Additionally, it has been found that giant clam larval settlement can be elicited by chemicals associated with crustose coralline algae, a well-established inducer of settlement and metamorphosis in many other reef invertebrate larvae[30] .
The transition from giant clam pediveligers into juveniles is often assumed to result in the immobility of the later; this was however proven to be an invalid assumption (refer to Aggregation). Tridacna squamosa reaches maturity in 4 years as a male, at sizes of 6 - 16 cm.
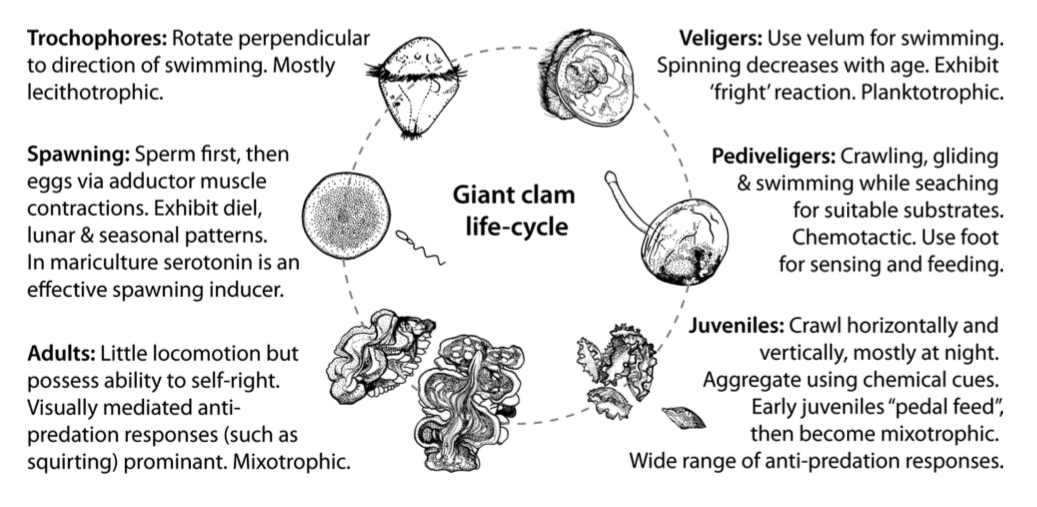 |
| The behaviors associated with the various life stages of a giant clam (Adapted from Soo and Todd, 2014). |
Locomotion: Righting
Righting is a behavior than can be observed throughout the lifetime of a giant clam. In the coral reef environment, strong waves and swimming organisms could knock off the fluted giant clam even though it is a species that can anchor onto the substrate. With this ability to regain their original upright position, the symbiotic zooxanthellae are able to obtain maximum light for photosynthesis[31] . A young Tridacna squamosa lying on its side will reach its foot out to sense the substract before contracting their adductor muscles to position themselves upright. In adults, the contraction of the adductor muscles alone is enough to upright the clam.Giant clams can regain their upright position when knocked off (obtained fromYoutube)
Aggregation
Juvenile Tridacna squamosa clams have been recorded to have a tendency to aggregate with their conspecifics when distributed evenly in a tank[32] . Such a behaviour could serve ecological functions, such as defence against predation (decreasing individual risk of predation) and reproduction facilitation. For instance, the aggregation of juvenile fluted giant clams will result in the decrease in shell area that is freely exposed to predators (crab's claw) as compared to a single standing giant clam. Additionally, the close proximity of the clams meant that during spawning season (at a later age) there will be higher chance of gametes mixing.The aggregation behavior of juvenile giant clams (obtained from Youtube).
Predation & Parasitism
Young giant clams are vulnerable to crabs (e.g. Thalamita spp., Demania ssp.), which are capable of crushing the shell valves with their chelae[33] .Potential predators of older clams includes turtles, eagle rays and large benthivorous fish. Wrasse, triggerfish, and pufferfish are known predators to both juvenile and adult giant clams. These fishes are responsible for the bite marks on the mantle edges of wild giant clams.
Tridacna squamosa is known to be parasitized by Pyramidellidae and Ranellidae snails, micro-gastropods that can reside under the scutes of the shells[34] .
Defence mechanisms
| 1) Scutes (sharp shell projections) were found to aid in deterring crab predator by increasing the risk of claw damage and/or handling time[35] . 2) Camouflaging mantle colours allows for background matching which decreases the frequency of attack from visual predators[36]. 3) The sudden retraction of mantle minimizes mantle exposure and startle their predators[37] . This fast response is made possible due to the numerous pinhole eyes found along the mantle edges of the giant clam. 4) Squirting a sufficiently strong jet of water could potentially drive away small fishes and deter larger predators[38] . |
 Pinhole eyes at mantle edges. (Image by Nick Hobgood, Creative Commons Attribution-Share Alike 3.0 Unported) |
The squirting behavior of the fluted giant clam (obtained from Youtube).
Back to top
Status and Conservation
IUCN
This IUCN lists Tridacna squamosa under lower risk, but dependent on conservation efforts[39] .CITES
All nine clam species of the Family Tridacnidae are protected by the CITES (Appendix II).– Tridacna gigas
– Tridacna derasa
– Tridacna squamosa
– Tridacna maxima
– Tridacna crocea
– Tridacna rosewateri
– Tridacna tevoroa
– Hippopus hippopus
– Hippopus porcellanus
Singapore Red Data Book
Tridacna squamosa is listed as 'Endangered' on the Red List of threatened of Singapore[40] . According to the Singapore Red Data Book: "Large specimens have virtually disappeared from our shores. Young specimens are occasionally but infrequently seen".Threats
Globally, giant clam populations are declining at an alarming rate due to anthropogenic impacts.1) Overexploitation for Food and Shell
The adductor muscle of the giant clam is considered a delicacy in the Southeast Asia and serve as a protein food in the rural diets of Solomon islands[41] . Overexploitation of giant clams for food was also recorded. The large valves of the giant clams are often collected and used as containers or to make ornaments. The overcollection of giant clams for food and ornaments is one of the greatest threat to the global giant clam population.
2) Pollution and Habitat Loss
Coastal developments continue to deleteriously affect the existing reefs in Singapore, resulting in the deterioration of habitats for the giant clams[42] . As giant clams are filter feeders, high turbidity in Singapore could deteriorate the feeding ability of the giant clams. Additionally, sediment pollution and the associated tubidity reduces the amount of sunlight received by the giant clam, thus affecting the photosynthetic efficiency of the zooxanthellae critical of the giant clam's nutritional needs[43] .
3) Aquarium trade
In recent years, the demand for live giant clams for aquaria has also grown considerably; this resulted in the exploitation of wild giant clams[44] . Many of the giant clams are now captive raised at clam farms to supply for aquarium trades. Advances in selective breeding techniques have also resulted in clams that are bred for brighter mantle colours.
Back to top
Taxonomy and Systematics
Taxonomic Classification
The following is the hierarchical summary of the taxa whereby Tridacna squamosa is placed under:| Animalia Mollusca Bivalvia Veneroida Cardiidae Tridacninae Tridacna |
Original Description
This species was originally described by Lamarck in 1816 based on a specimen from the Indian Ocean[45] . |
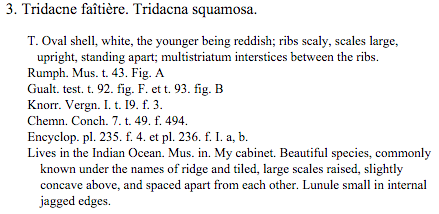 |
| Original description of Tridacna squamosa in Latin and French (Retrieved from the Biodiversity Heritage Library) |
English translation (using Google Translate and Wikitionary) |
Type Specimen
The locality of the holotype specimen is currently unknown. It is not present in the French National Museum of Natural History nor in the Museum of the Natural History of the City of Geneva, Switzerland[46] .A lectotype (Rumphius, 1705: pl. 43 [42], fig. A) is thus designated by Rosewater (1965). The type locality is restricted to Ambon, Indondesia [47] .
Phylogeny
Giant clams, traditionally regarded as a distinct family Tridacnidae, were re-examined and placed under the family Cardiidae (true cockles)[48] [49] . Tridacnidae is now regarded as Tridacninae, a subfamily nested within Cardiidae, and not worthy of being placed in a separated superfamily.According to the phylogenetic analysis of the mitochondrial subunit 16S gene sequence, the genus Hippopus and Tridacna are monophyletic sister taxa belonging to the subfamily Tridacninae (demarcated by red box in the following phylogenetic tree). Additionally, Tridacna squamosa, Tridacna crocea, and Tridacna maxima formed a monophyletic group,[50] [51] Tridacna (Chametrachea)[52] .
The following two phylogenetic trees are based on three gene sequences (H3, 16S, 28S) and one gene sequences (16S) respectively.
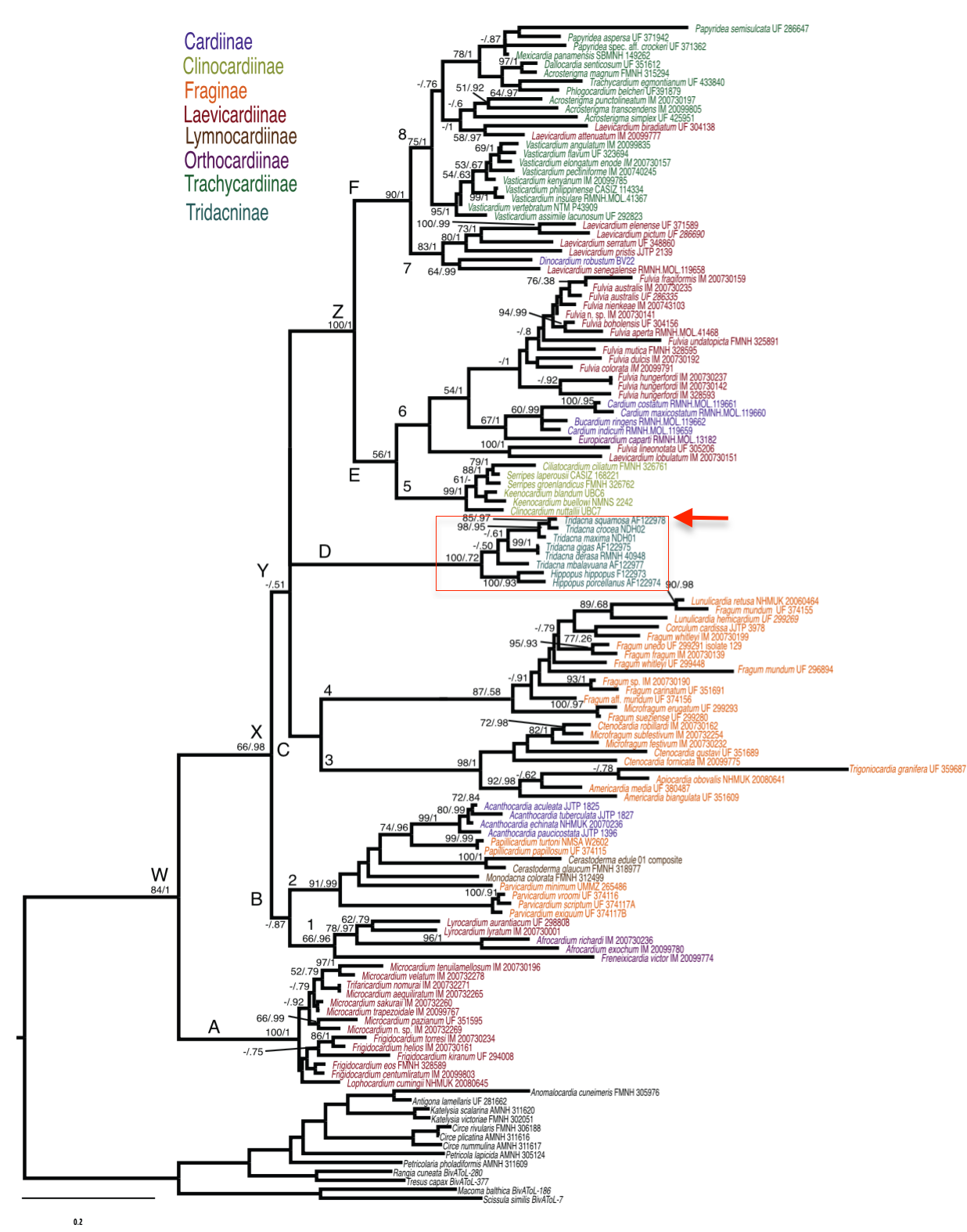 |
| Maximum-likelihood phylogram of the Family Cardiidae (True Cockles) based on the concatenated dataset for three genes (H3, 16S, 28S). ML booststrap and Bayesian posterior probabilities are indicated above branches. (indicated by red arrow) is nested in the monophyletic subfamily Tridacninae. Phylogram obtained from the study by Herrera et al. (2015)[53] . |
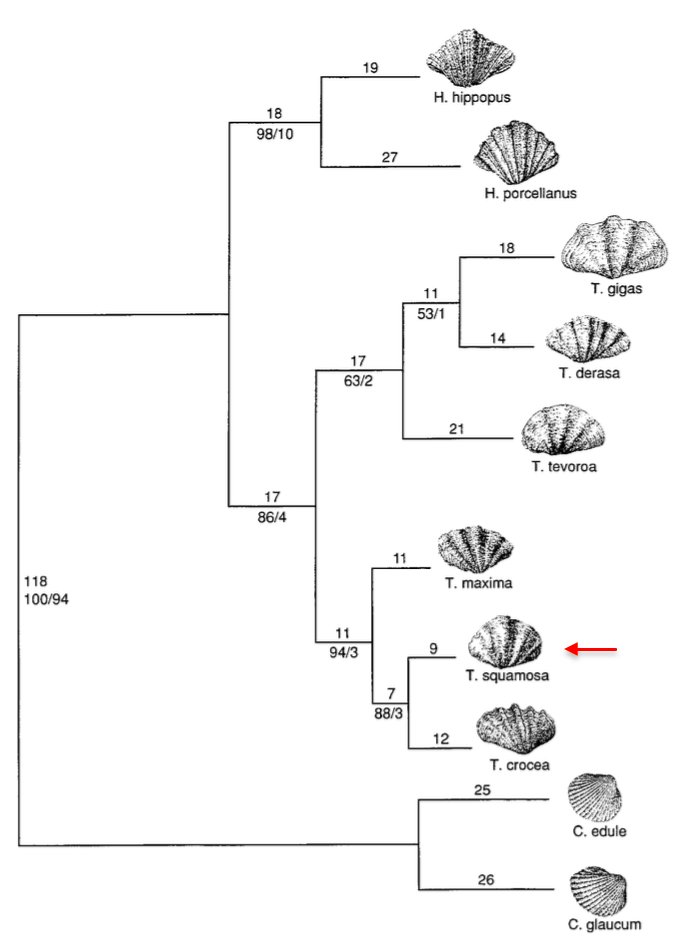 |
| The single most parsimonious tree based on the 16S mitochondrial gene fragment (PAUP analysis, 462 nucleotides), showing the relationship within the Subfamily Tridacninae. Both species of Ceratoderma were designated as outgroups. Tree obtained from the study by Schneider & Foighil (1999). |
Back to top
References
Popular Mechanics, 1924. Giant Clams Trap Sea Divers in Grip of Shells. Hearst Magazines, UK, 41 (5): 685
Lucas, J.S.,1988. Giant clams: Description, distribution and life history. In: Copland JW, Lucas JS (eds) Giant clams in Asia and the Pacific. Australian Centre for International Agricultural Research, Canberra, pp 21–32
Wells, S., 1996. Tridacna squamosa. The IUCN Red List of Threatened Species 1996: e.T22140A9362870. http://dx.doi.org/10.2305/IUCN.UK.1996.RLTS.T22140A9362870.en. Downloaded on 08 November 2015.
Neo, M.L. & Todd, P.A., 2013. Conservation Status Reassessment of Giant Clams (Mollusca: Bivalvia: Tridacninae) in Singapore. Nature in Singapore, 6: 125-133.
Bradley, R. D., 1989. A Giant Clam Stock Survey and Preliminary Investigation of Pearl Oyster Resources in the Tokelau Islands. FAO Corporate Document Repository. http://www.fao.org/docrep/field/003/ac293e/AC293E01.htm (Retrieved 20 October 2015).
Faterree, J. W., 2002. Identifying the Tridacnid Clams. Reef Central Website. http://www.reefkeeping.com/issues/2002-07/jf/feature/ (Retrieved 20 October 2015).
Neo, M.L. & Todd, P.A., 2013. Conservation Status Reassessment of Giant Clams (Mollusca: Bivalvia: Tridacninae) in Singapore. Nature in Singapore, 6: 125-133.
Knop, D., 1996. Giant Clams: A Comprehensive Guide to the Identification and Care of Tridacnid Clams. Dahne Verlag, Ettlingen, Germany. 255 p.
Trench, R.K., Wethey D.S., Porter J.W., 1981. Observations on the symbiosis with zooxanthellae among the Tridacnidae (Mollusca: Bivalvia). The Biological Bulletin, 161(1): 180-198.
Fankboner, P.V. 1971. Intracellular digestion of symbiontic zooxanthellae by host amoebocytes in giant clams (Bivalvia: Tridacnidae), with a note on the nutritional role of the hypertrophied siphonal epidermis. The Biological Bulletin, 141: 222-234.
Hardy, J.T., Hardy, S.A., 1969. Ecology of Tridacna in Palau. Pacific Science, 23:467–472.
Wada, S.K., 1952. Protandric functional hermaphroditism in the tridacnid clams. Oceanographical Magazine, 4:23–30.
Ellis, S., 1999. Spawning and Early Larval Rearing of Giant Clams (Bivalvia: Tridacnidae). Center for Tropical and Subtropical Aquaculture, Hawaii.
Peck, L.S., Webb, K.E., Bailey, D.M., 2004. Extreme sensitivity of biological function to temperature in Antarctic marine species. Functional Ecology, 18:625–630
Huang, D., Todd, P.A., Guest, J.R., 2007. Movement and aggregation in the fluted giant clam (Tridacna squamosa L.). Journal of Experimental Marine Biology and Ecology, 342:269–281.
Soo, P., Todd, P.A., 2014. The behaviour of giant clams (Bivalvia: Cardiidae: Tridacninae). Marine Biology, 161: 2699–2717.
Cumming, R.L., 1988. Pyramidellid parasites in giant clam mariculture systems. In: Copeland, J.W. and J.S. Lucas (eds.) Giant Clams in Asia and the Pacific. ACIAR Monograph Number 9, Canberra. 274 pp.
Morton, J.E., 1967. Molluscs, 4th edn. Hutchinson University Library, London. 244 pp.
Stasek, C.R., 1965. Behavioral adaptation of the giant clam Tridacna maxima to the presence of grazing fishes. Veliger, 8:29–35
Wells, S., 1996. Tridacna squamosa. The IUCN Red List of Threatened Species 1996: e.T22140A9362870. http://dx.doi.org/10.2305/IUCN.UK.1996.RLTS.T22140A9362870.en. Downloaded on 08 November 2015.
Ng, P.K.L. & Wee Y.C., 1994. The Singapore Red Data Book: Threatened Plants and Animals of Singapore. The Nature Society (Singapore). 343 pp.
Tisdell, C., 1986. The Economic and Socio-Economic Potential of Giant Clam (Tridacnid) Culture: A Review. Research Report or Occasional Paper No. 128. Department of Economics, The University of Newcastle, N.S.W., Australia. 115 pp.
Chou, L.M., 1999. Coral reefs. In: Briffett, C. & H. H. Chew (eds.), State of the Natural Environment in Singapore. Nature Society (Singapore), Singapore. Pp. 33–45.
Wabnitz, C., 2003. From Ocean to Aquarium: The Global Trade in Marine Ornamental Species. Issue 17 of UNEP-WCMC biodiversity series, World Conservation Montoring Centre. 64 pp.
Lamarck J.-B. M. de., 1816. Histoire naturelle des animaux sans vertèbres. Tome sixième, 1re partie. Paris: published by the Author, vi + 343 pp., available online at http://www.biodiversitylibrary.org/item/47441 (page 106).
ter Poorten, J.J, Bouchet, P., Rosenberg, G., 2015. Tridacna squamosa Lamarck, 1819. In: MolluscaBase (2015). Accessed through: World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id=207674 on 14 October 2015
Rosewater, J., 1965. The Family Tridacnidae in the Indo-Pacific. Indo-Pacific Mollusca, 1 (6): 347-396.
Harper, E.M., Taylor, J.D., Crame, J.A., 2001. The Evolutionary Biology of the Bivalvia (Geological Society of London Special Publications Book 177). Geological Soceity of London, London. 494 pp.
Herrera, N.D., ter Poorten, J.J., Bieler, R., Mikkelsen, P.M., Strong, E.E., Jablonski, D., Steppan, S.J., 2015. Molecular phylogenetics and historical biogeography amid shifting continents in the cockles and giant clams (Bivalvia: Cardiidae). Molecular Phylogenetics and Evolution, 93: 94-106.